Mechanical Model for Catch-Bond-Mediated Cell Adhesion in Shear Flow
Abstract
1. Introduction
2. Theoretical Model
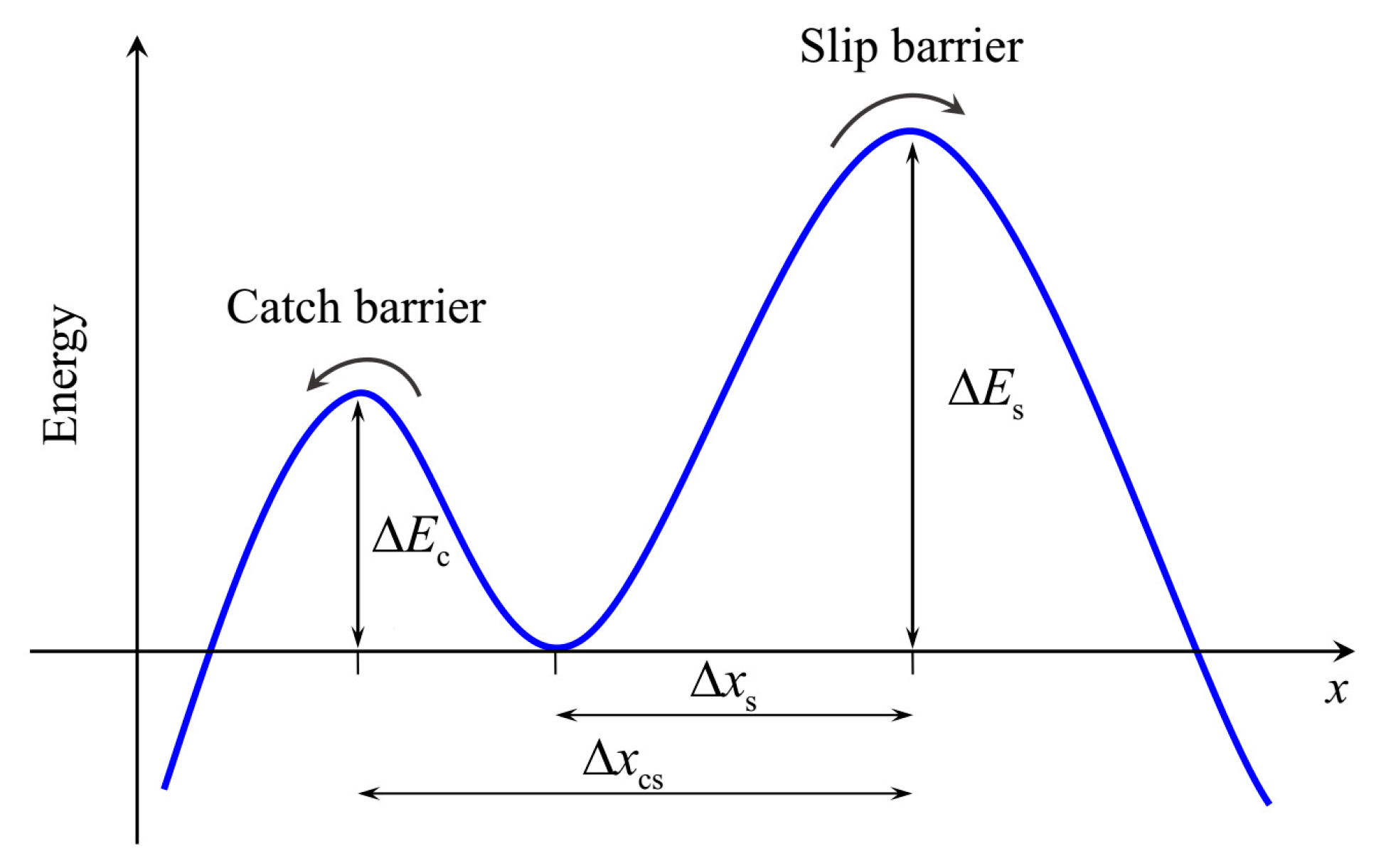
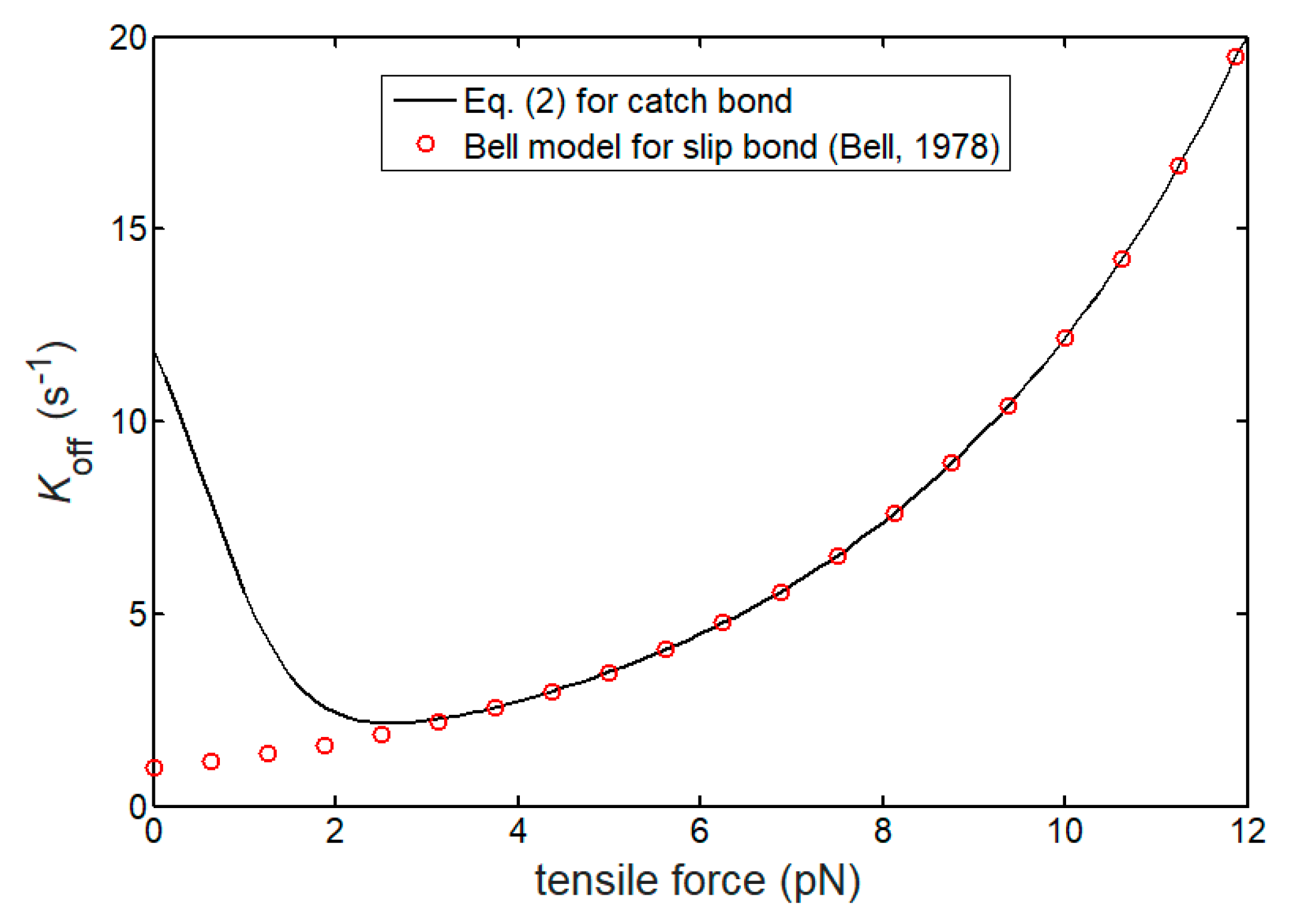
2.1. Lifetime of Catch Bonds
2.2. Effective Works of Adhesion for Cell Adhesion
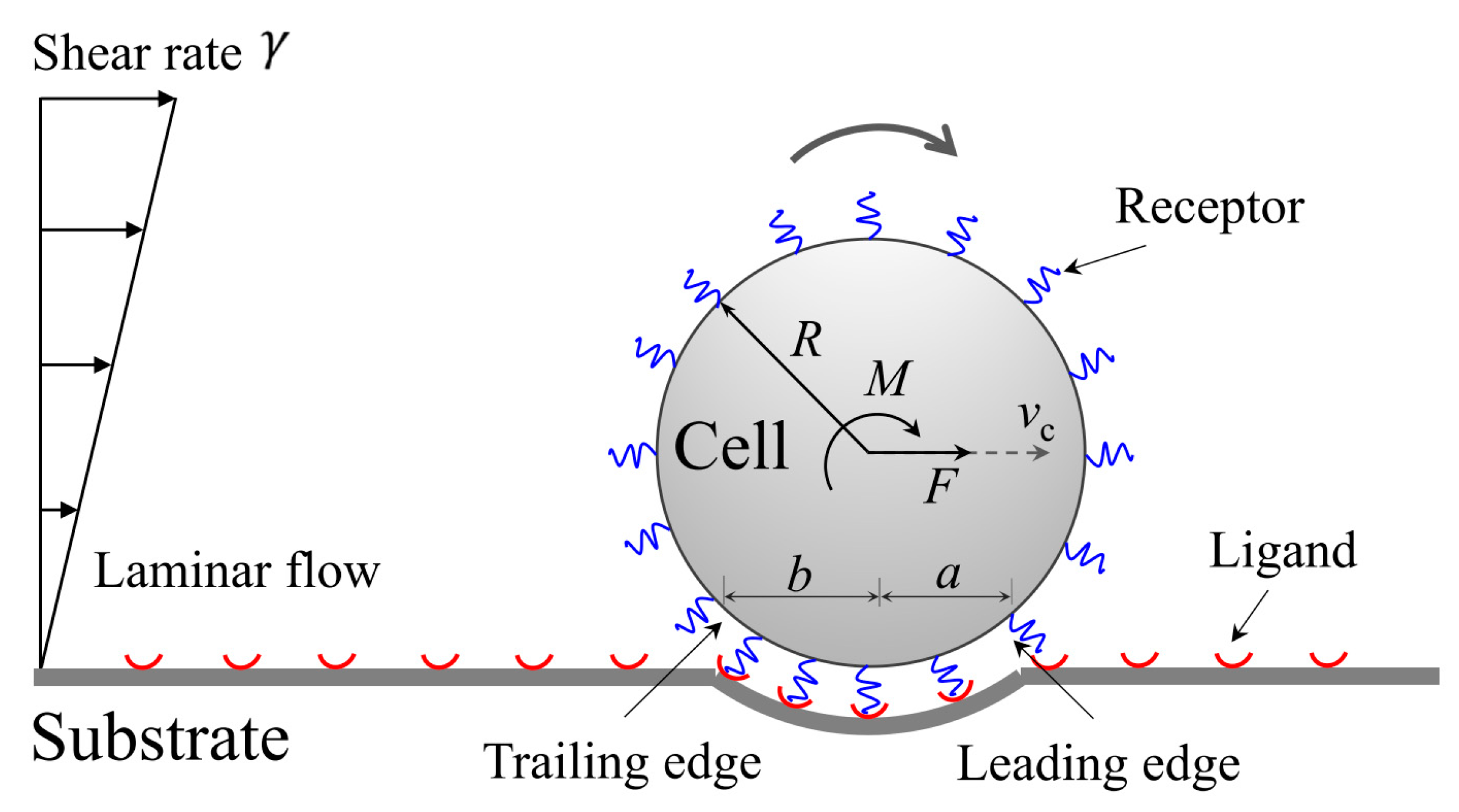
2.3. Kinetics of Cell Rolling in Shear Flow
3. Results and Discussion
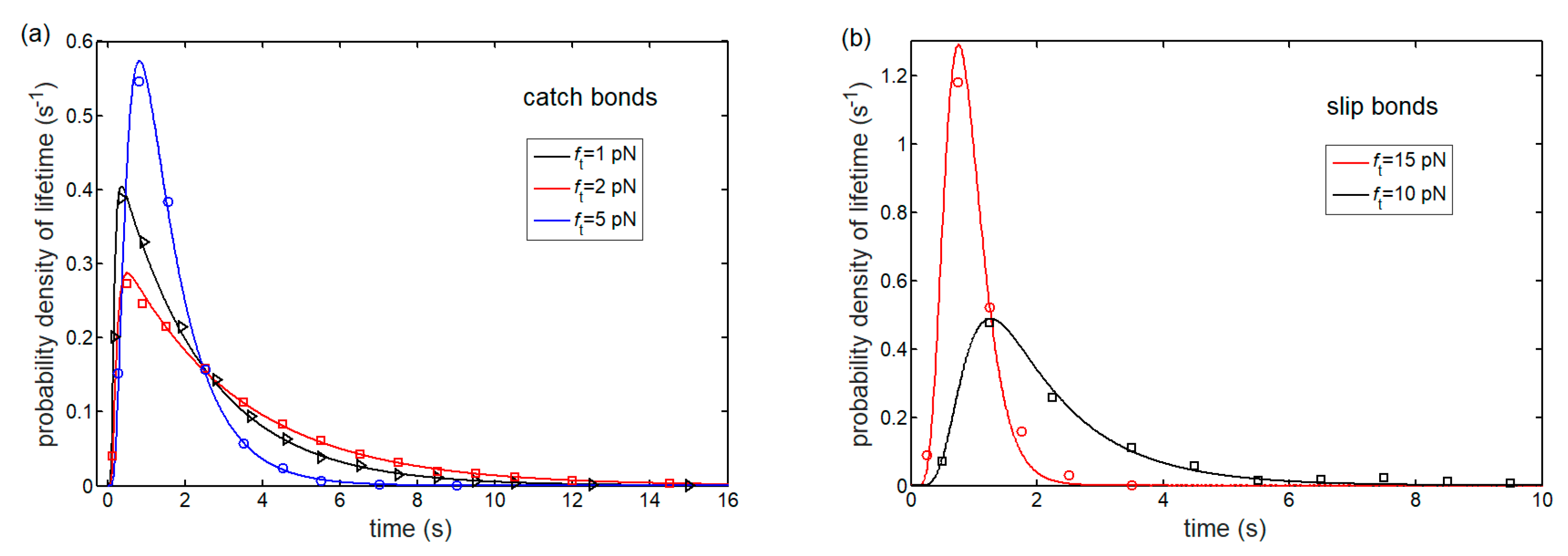
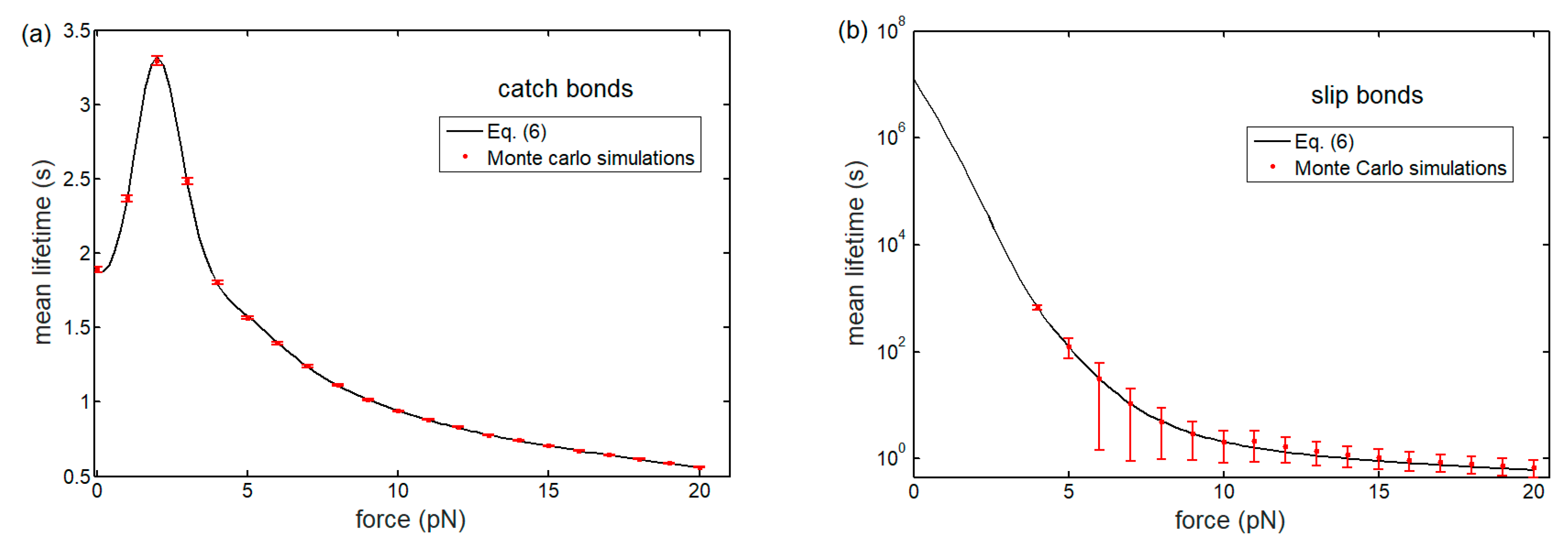
3.1. Lifetime of Catch-Bond Cluster
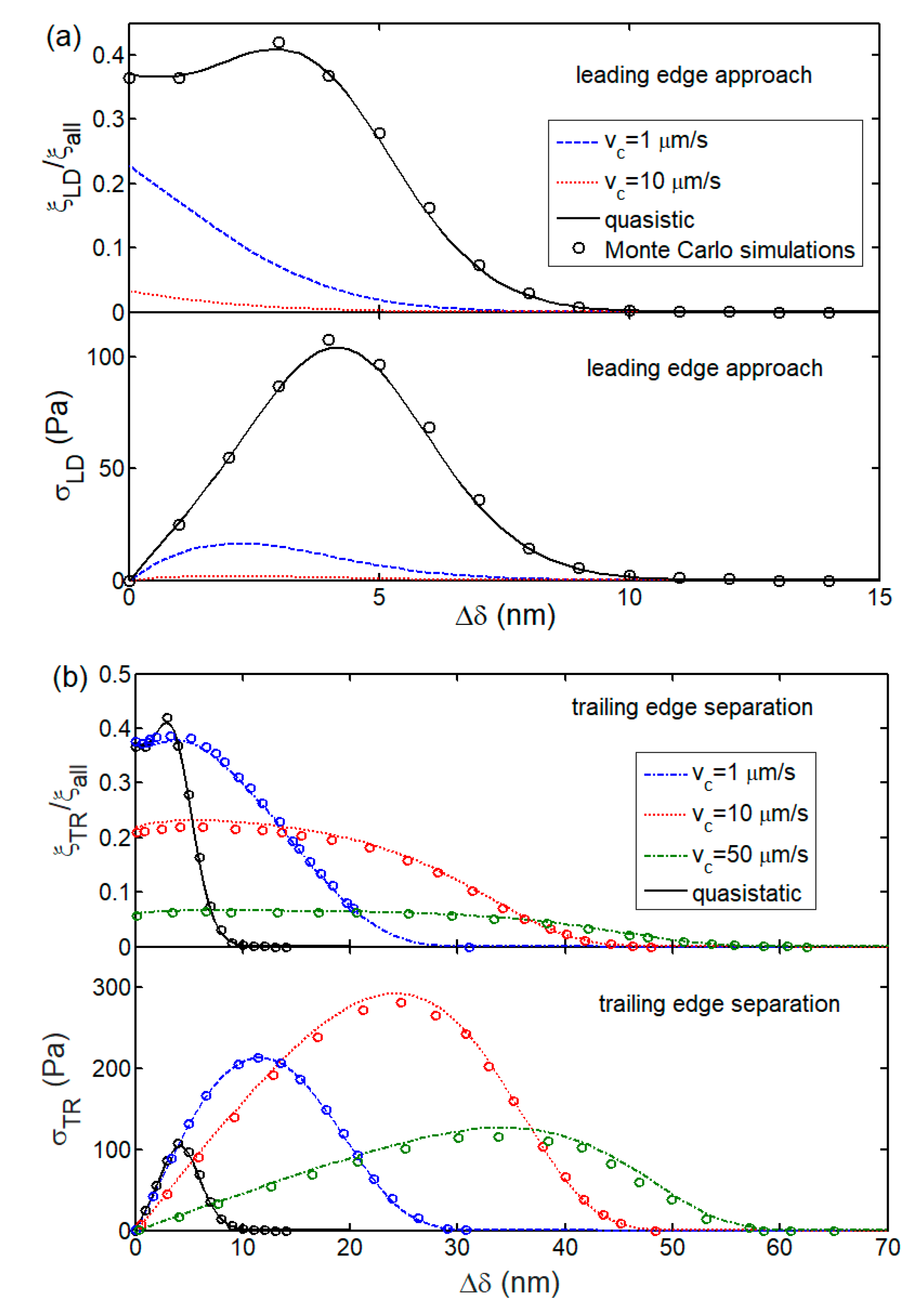
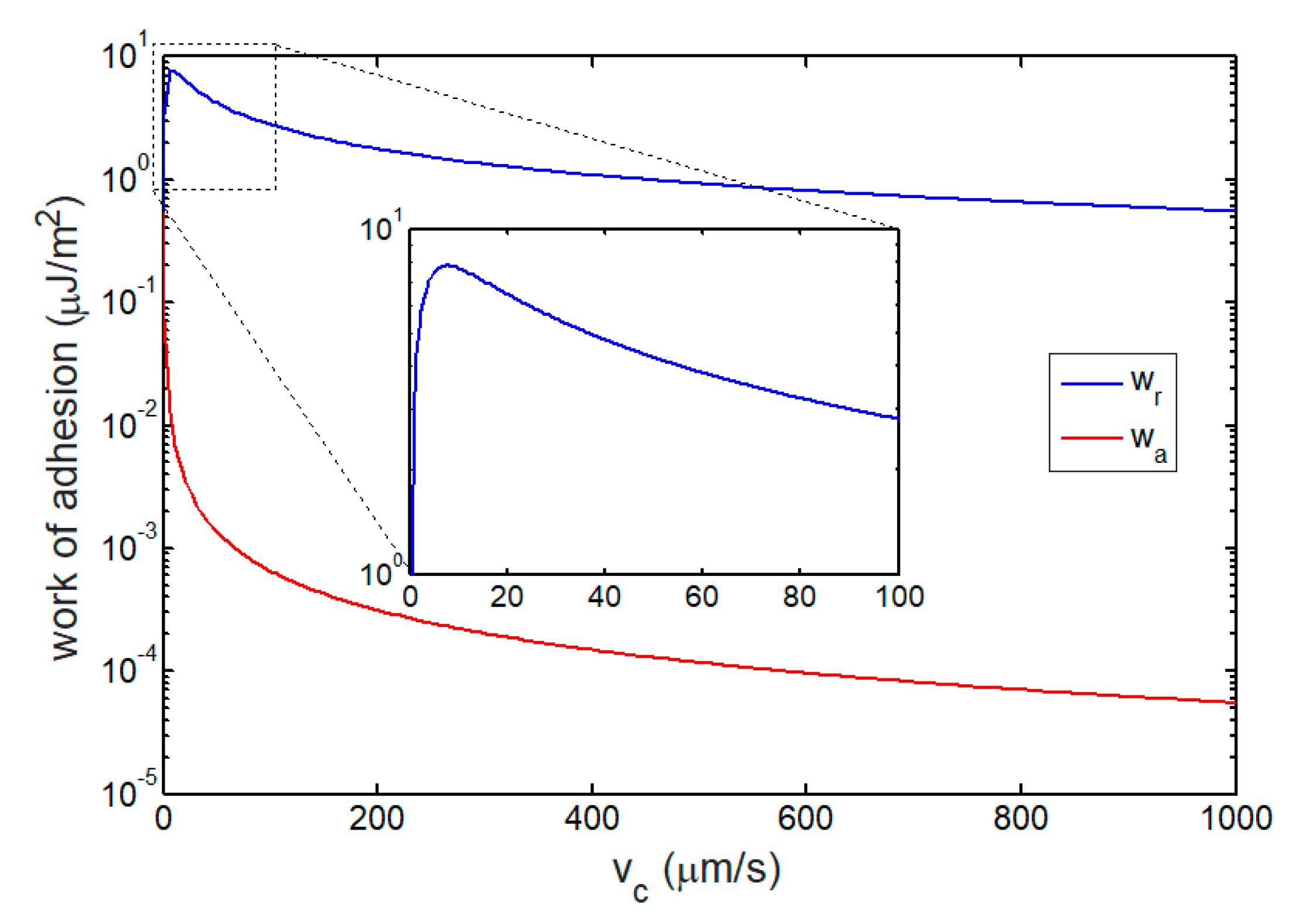
3.2. Works of Adhesion
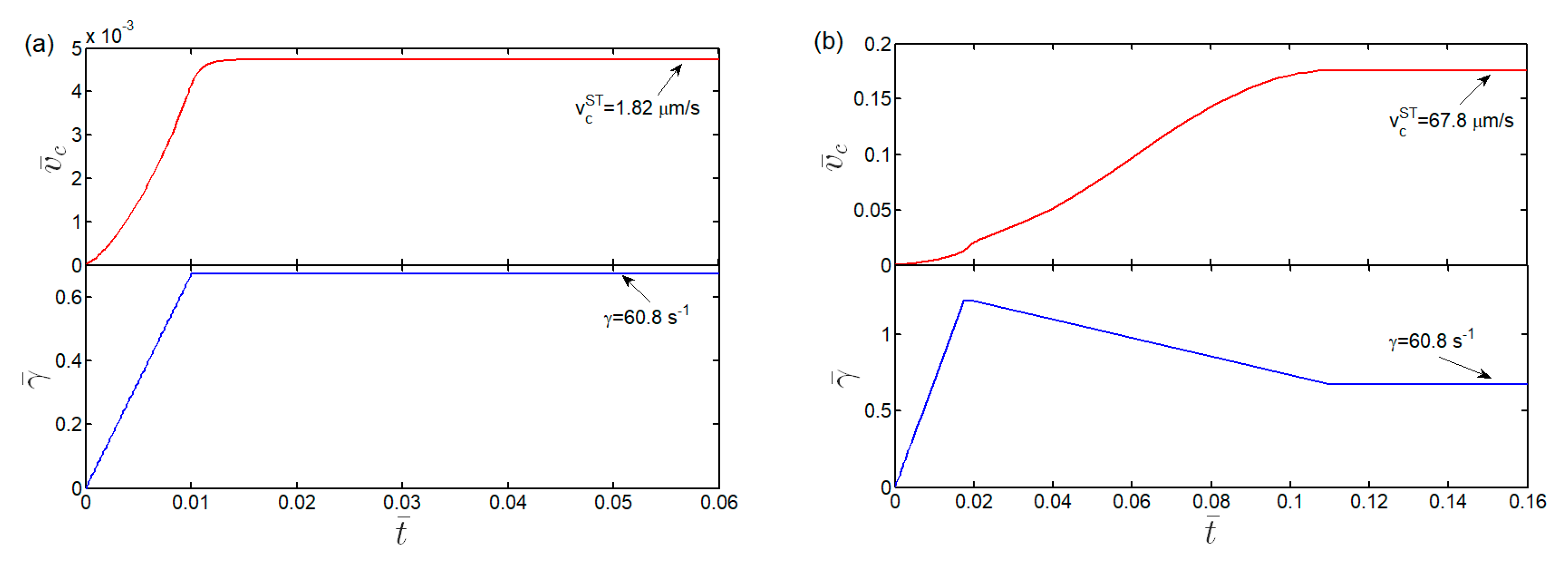
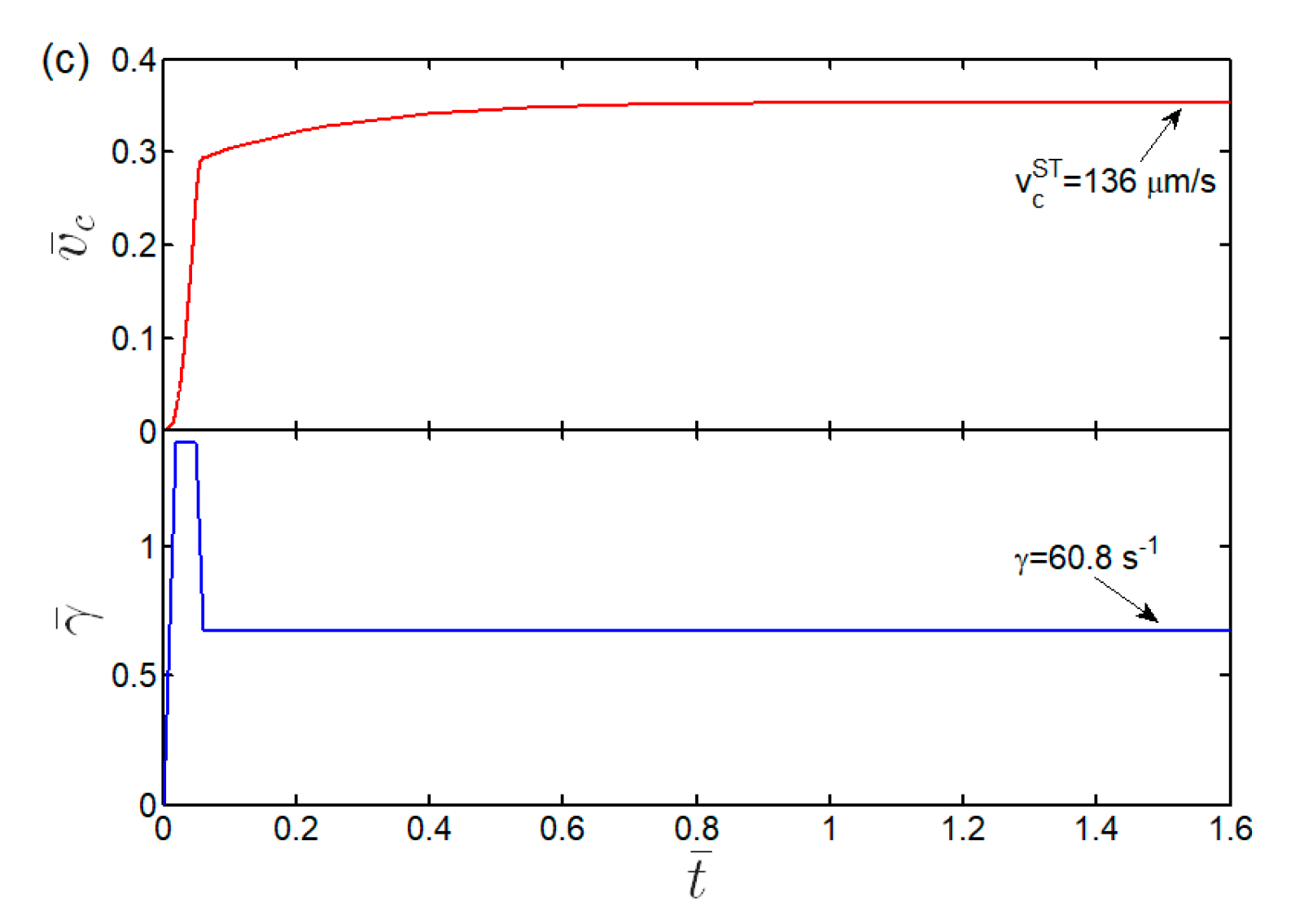
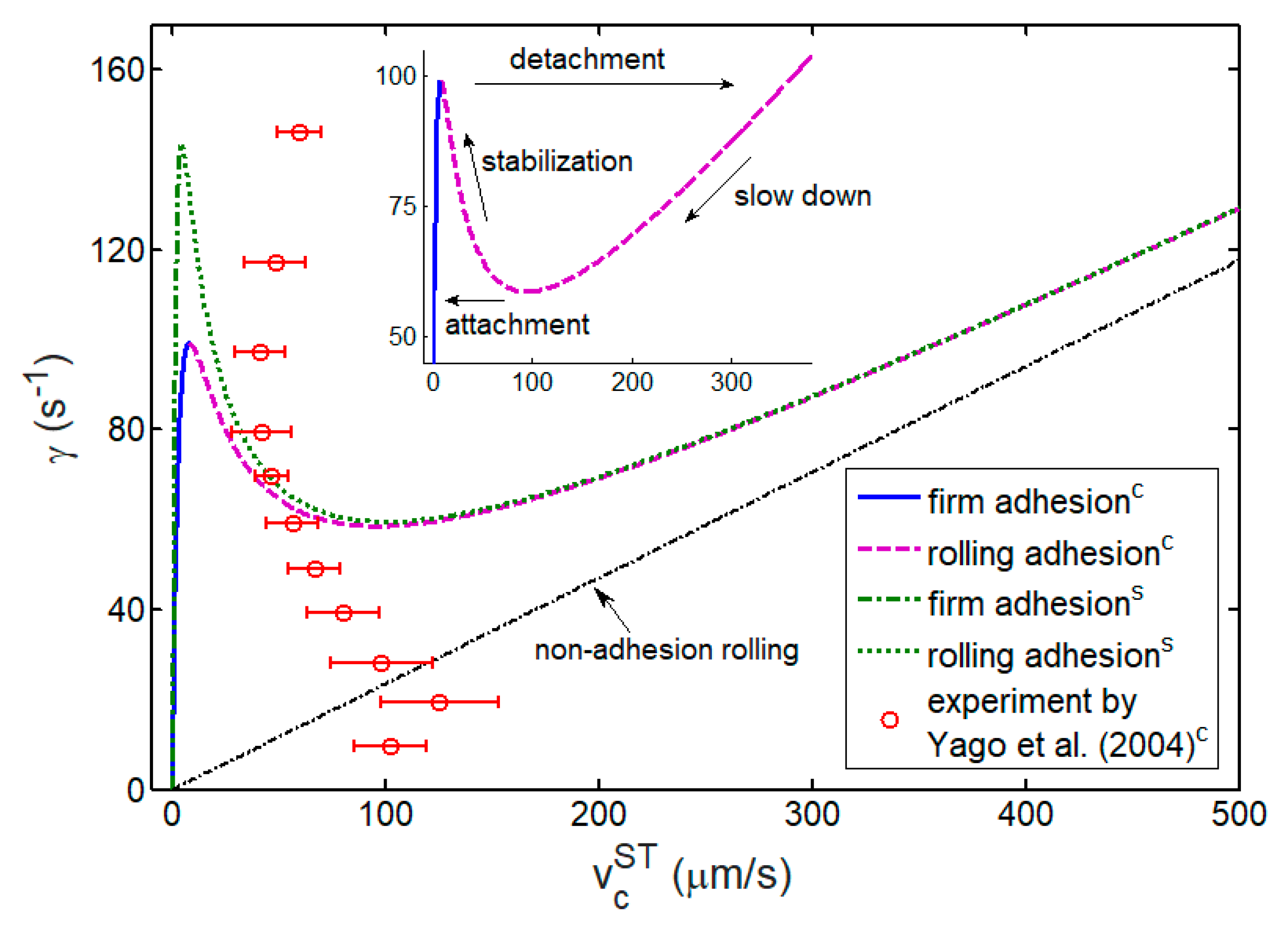
3.3. Kinetics and Shear Threshold of Cell Rolling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Monte Carlo Simulations
Appendix B. Effect of Flow’s Shear Rate History on Steady Speed of Cell
References
- Anderson, D.C.; Springer, T.A. Leukocyte adhesion deficiency: An inherited defect in the Mac-1, LFA-1, and p150, 95 glycoproteins. Ann. Rev. Med. 1987, 38, 175–194. [Google Scholar] [CrossRef]
- Yeung, L.; Hickey, M.J.; Wrigh, M.D. The many and varied roles of Tetraspanins in immune cell recruitment and migration. Front. Immunol. 2018, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Eniola, A.O.; Hammer, D.A. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials 2005, 26, 7136–7144. [Google Scholar] [CrossRef] [PubMed]
- Muzykantov, M.D.; Silvia, M. Targeting delivery of drugs in the vascular system. Int. J. Transp. Phenom. 2011, 12, 41–49. [Google Scholar] [PubMed]
- Dembo, M.; Torney, D.C.; Saxman, K.; Hammer, D.A. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc. R. Soc. B-Biol. 1988, 234, 55–83. [Google Scholar]
- Bell, G.I.; Dembo, M.; Bongrand, P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys. J. 1984, 45, 1051–1064. [Google Scholar] [CrossRef]
- Bell, G.I. Models for the specific adhesion of cells to cells. Science 1978, 200, 618–627. [Google Scholar] [CrossRef]
- Marshall, B.T.; Long, M.; Piper, J.W.; Yago, T.; McEver, R.C.; Zhu, C. Direct observation of catch bonds involving cell-adhesion molecules. Nature 2003, 423, 190–193. [Google Scholar] [CrossRef]
- Isberg, R.R.; Barnes, P. Dancing with the host: Flow-dependent bacterial adhesion. Cell 2002, 110, 1–4. [Google Scholar] [CrossRef]
- Evans, E.; Leung, A.; Heinrich, V.; Zhu, C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc. Natl. Acad. Sci. USA 2004, 101, 11281–11286. [Google Scholar] [CrossRef] [PubMed]
- Bullerjahn, J.T.; Kroy, K. Analytical catch-slip bond model for arbitrary forces and loading rates. Phys. Rev. E 2016, 93, 012404. [Google Scholar] [CrossRef] [PubMed]
- Pereverzev, Y.V.; Prezhdo, O.V.; Forero, M.; Sokurenko, E.V.; Thomas, W.E. The two-pathway model for the catch-slip transition in biological adhesion. Biophys. J. 2005, 89, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.K.; Li, B.; Feng, X.Q.; Gao, H.J. A tensegrity model of cell reorientation on cyclically stretched substrates. Biophys. J. 2016, 111, 1478–1486. [Google Scholar] [CrossRef]
- Li, L.; Yao, H.M.; Wang, J.Z. Dynamic strength of molecular bond clusters under displacement-and force-controlled loading conditions. J. Appl. Mech. Trans. 2016, 83, 021004. [Google Scholar] [CrossRef]
- Chen, B.; Kemkemer, R.; Deibler, M.; Spatz, J.; Gao, H.J. Cyclic stretch induces cell reorientation on substrates by destabilizing catch bonds in focal adhesions. PLoS ONE 2012, 7, e48346. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, G.Y.; Lu, S.Q.; Li, N.; Long, M. Low spring constant regulates P-selectin-PSGL-1 bond rupture. Biophys. J. 2008, 95, 5439–5448. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Qian, J.; Wang, J.Z.; Gao, H.J. Lifetime and strength of adhesive molecular bond clusters between elastic media. Langmuir 2008, 24, 1262–1270. [Google Scholar] [CrossRef]
- Qian, J.; Wang, J.Z.; Lin, Y.; Gao, H.J. Lifetime and strength of periodic bond clusters between elastic media under inclined loading. Biophys. J. 2015, 97, 2438–2445. [Google Scholar] [CrossRef]
- Wang, J.Z.; Gao, H.J. Clustering instability in adhesive contact between elastic solids via diffusive molecular bonds. J. Mech. Phys. Solids 2008, 56, 251–266. [Google Scholar] [CrossRef]
- Zhang, W.L.; Lin, Y.; Qian, J.; Chen, W.Q.; Gao, H.J. Tuning molecular adhesion via material anisotropy. Adv. Funct. Mater. 2013, 23, 4729–4738. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6364. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, W.Y.; Wang, J.Z. A viscoelastic–stochastic model of the effects of cytoskeleton remodelling on cell adhesion. R. Soc. Open Sci. 2016, 3, 160539. [Google Scholar] [CrossRef] [PubMed]
- He, K.C.; Li, L.; Wang, J.Z. Diffusive–Stochastic–Viscoelastic model for specific adhesion of viscoelastic solids via molecular bonds. Acta Mech. Sin. 2019, 35, 343–354. [Google Scholar] [CrossRef]
- Bihr, T.; Seifert, U.; Smith, A.S. Multiscale approaches to protein-mediated interactions between membranes—Relating microscopic and macroscopic dynamics in radially growing adhesions. New J. Phys. 2015, 17, 083016. [Google Scholar] [CrossRef]
- Fenz, S.F.; Bihr, T.; Schmidt, D.; Merkel, R.; Seifert, U.; Sengupta, K.; Smith, A.S. Membrane fluctuations mediate lateral interaction between cadherin bonds. Nat. Phys. 2017, 13, 906–913. [Google Scholar] [CrossRef]
- Krobath, H.; Różycki, B.; Lipowsky, R.; Weikl, T.R. Binding cooperativity of membrane adhesion receptors. Soft Matter 2009, 5, 3354–3361. [Google Scholar] [CrossRef]
- Rozycki, B.; Lipowsky, R.; Weikl, T.R. Segregation of receptor–ligand complexes in cell adhesion zones: Phase diagrams and the role of thermal membrane roughness. New J. Phys. 2010, 12, 095003. [Google Scholar] [CrossRef]
- Li, N.; Lu, S.Q.; Zhang, Y.; Long, M. Mechanokinetics of receptor–ligand interactions in cell adhesion. Acta Mech. Sin. 2015, 31, 248–258. [Google Scholar] [CrossRef]
- Robin, T.; Sokolov, I.M.; Urbakh, M. Life time of catch bond clusters. Phys. A 2018, 507, 398–405. [Google Scholar] [CrossRef]
- Wang, J.Z.; Gao, H.J. Size and shape dependent steady-state pull-off force in molecular adhesion between soft elastic materials. Int. J. Fract. 2010, 166, 13–19. [Google Scholar] [CrossRef]
- Chen, B.; Gao, H.J. Mechanical principle of enhancing cell-substrate adhesion via pre-tension in the cytoskeleton. Biophys. J. 2010, 98, 2154–2162. [Google Scholar] [CrossRef]
- He, S.J.; Su, Y.W.; Ji, B.H.; Gao, H.J. Some basic questions on mechanosensing in cell–substrate interaction. J. Mech. Phys. Solids 2014, 70, 116–135. [Google Scholar] [CrossRef]
- Li, L.; Tang, H.; Wang, J.Z.; Lin, J.; Yao, H.M. Rolling adhesion of cell in shear flow: A theoretical model. J. Mech. Phys. Solids 2018, 119, 369–381. [Google Scholar] [CrossRef]
- Du, Y.; Peng, S.; Cui, Y.H.; Lv, S.Q.; Zhang, Y.; Long, M. Combined modeling of cell aggregation and adhesion mediated by receptor–ligand interactions under shear flow. Theor. Appl. Mech. Lett. 2015, 5, 216–221. [Google Scholar] [CrossRef][Green Version]
- Long, M.; Goldsmith, H.L.; Tees, D.F.J.; Zhu, C. Probabilistic modeling of shear-induced formation and breakage of doublets cross-linked by receptor-ligand bonds. Biophys. J. 1999, 76, 1112–1128. [Google Scholar] [CrossRef][Green Version]
- Dong, C.; Lei, X.X. Biomechanics of cell rolling: Shear flow, cell-surface adhesion, and cell deformability. J. Biomech. 2000, 33, 35–43. [Google Scholar] [CrossRef]
- Reboux, S.; Richardson, G.; Jensen, O.E. Bond tilting and sliding friction in a model of cell adhesion. Proc. R. Soc. A-Math. Phys. Eng. Sci. 2007, 464, 447–467. [Google Scholar] [CrossRef]
- Xu, X.F.; Efremov, A.K.; Li, A.; Lai, L.P.; Dao, M.; Lim, C.T.; Cao, J.S. Probing the cytoadherence of malaria infected red blood cells under flow. PLoS ONE 2013, 8, e64763. [Google Scholar] [CrossRef]
- Sircar, S.; Bortz, D.M. Impact of flow on ligand-mediated bacterial flocculation. Math. Biosci. 2013, 245, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.J.; Zhang, R.B.; Zhang, F.; Zhang, Y.C.; Li, G.X.; Miao, R.J.; Shao, S.H. An Evaluation Approach of Cell Viability Based on Cell Detachment Assay in a Single-Channel Integrated Microfluidic Chip. ACS Sens. 2019, 4, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.F.; Ding, Z.Y.; Hood, M.; Li, W. Simulation of circulating tumor cell transport and adhesion in cell suspensions in microfluidic devices. Biomicrofluidics 2019, 13, 064105. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.B.; Li, Y.Q.; Gao, Q.C.; Cheng, B.B.; Shen, B.R.; Yan, Z.Q.; Jiang, Z.L. Adhesion behavior of endothelial progenitor cells to endothelial cells in simple shear flow. Acta Mech. Sin. 2011, 27, 1071–1080. [Google Scholar] [CrossRef]
- Efremov, A.; Cao, J.S. Bistability of cell adhesion in shear flow. Biophys. J. 2011, 101, 1032–1040. [Google Scholar] [CrossRef][Green Version]
- Yago, T.; Wu, J.H.; Wey, C.D.; Klopocki, A.G.; Zhu, C.; McEver, R.P. Catch bonds govern adhesion through L-selectin at threshold shear. J. Cell Biol. 2004, 166, 913–923. [Google Scholar] [CrossRef]
- Sarangapani, K.K.; Yago, T.; Klopocki, A.G.; Lawrence, M.B.; Fieger, C.B.; Rosen, S.D.; McEver, R.P.; Zhu, C. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J. Biol. Chem. 2004, 279, 2291–2298. [Google Scholar] [CrossRef]
- Alon, R.; Chen, S.Q.; Puri, K.D.; Finger, E.B.; Springer, T.A. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J. Cell Biol. 1997, 138, 1169–1180. [Google Scholar] [CrossRef]
- Beste, M.T.; Hammer, D.A. Selectin catch–slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc. Natl. Acad. Sci. USA 2008, 105, 20716–20721. [Google Scholar] [CrossRef]
- Caputo, K.E.; Lee, D.; King, M.R.; Hammer, D.A. Adhesive dynamics simulations of the shear threshold effect for leukocytes. Biophys. J. 2007, 92, 787–797. [Google Scholar] [CrossRef]
- Whitfield, M.; Ghose, T.; Thomas, W. Shear-Stabilized Rolling Behavior of E. Coli Examined with Simulations. Biophys. J. 2010, 99, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Kampen, V.; Godfried, N. Stochastic Processes in Physics and Chemistry; North-Holland Personal Library: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Erdmann, T.; Schwarz, U.S. Impact of receptor-ligand distance on adhesion cluster stability. Eur. Phys. J. E 2007, 22, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, N.F.; Therien, M.J.; Beratan, D.N. Mean First-Passage Times in Biology. Isr. J. Chem. 2016, 56, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, T.; Schwarz, U.S. Stability of adhesion clusters under constant force. Phys. Rev. Lett. 2004, 92, 108102. [Google Scholar] [CrossRef]
- Schubert, G. Viscous flow near a cusped corner. J. Fluid Mech. 1967, 27, 647–656. [Google Scholar] [CrossRef]
- Yao, H.M. A generalized model for adhesive contact between a rigid cylinder and a transversely isotropic substrate. J. Appl. Mech. 2013, 80, 011027. [Google Scholar] [CrossRef]
- Lawrence, M.B.; Springer, T.A. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell 1991, 65, 859–873. [Google Scholar] [CrossRef]
- Schmid-Schoenbein, G.W.; Fung, Y.C.; Zweifach, B.W. Vascular endothelium-leukocyte interaction; sticking shear force in venules. Circ. Res. 1975, 36, 173–184. [Google Scholar] [CrossRef]
- Grover, W.H.; Bryan, A.K.; Diez-Silva, M.; Suresh, S.; Higgins, J.M.; Manalis, S.R. Measuring single-cell density. Proc. Natl. Acad. Sci. USA 2011, 108, 10992–10996. [Google Scholar] [CrossRef]
- Evans, E.A.; Calderwood, D.A. Forces and bond dynamics in cell adhesion. Science 2007, 316, 1148–1153. [Google Scholar] [CrossRef]
- Kong, F.; Garcia, A.J.; Mould, A.P.; Humphries, M.J.; Zhu, C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 2009, 185, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Popescu, G.; Auerbach, A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat. Neurosci. 2003, 6, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cheng, Q.H.; Gao, H.J.; Zhang, Y.W. Effect of loading conditions on the dissociation behaviour of catch bond clusters. J. R. Soc. Interface 2012, 9, 928–937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rakshit, S.; Zhang, Y.X.; Manibog, K.; Shafraz, O.; Sivasankar, S. Ideal, catch, and slip bonds in cadherin adhesion. Proc. Natl. Acad. Sci. USA 2012, 109, 18815–18820. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Tees, D.F.; Hammer, D.A. The state diagram for cell adhesion under flow: Leukocyte rolling and firm adhesion. Proc. Natl. Acad. Sci. USA 2000, 97, 11262–11267. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.D.; Chen, S.Q.; Springer, T.A. Modifying the mechanical property and shear threshold of L-selectin adhesion independently of equilibrium properties. Nature 1998, 392, 930–933. [Google Scholar] [CrossRef]
- Zhu, C.; Yago, T.; Lou, J.Z.; Zarnitsyna, V.I.; McEver, R.P. Mechanisms for flow-enhanced cell adhesion. Ann. Biomed. Eng. 2008, 36, 604–621. [Google Scholar] [CrossRef]
- Finger, E.B.; Puri, K.D.; Alon, R.; Lawrence, M.B.; von Andrian, U.H.; Springer, T.A. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature 1996, 379, 266–269. [Google Scholar] [CrossRef]
- Alon, R.; Hammer, D.A.; Springer, T.A. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature 1995, 374, 539–542. [Google Scholar] [CrossRef]
- Thomas, W.E.; Trintchina, E.; Forero, M. Bacterial adhesion to target cells enhanced by shear force. Cell 2002, 109, 913–923. [Google Scholar] [CrossRef]
- Gillespie, D.T. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 1976, 22, 403–434. [Google Scholar] [CrossRef]
- Gillespie, D.T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. C 1977, 81, 2340–2361. [Google Scholar] [CrossRef]
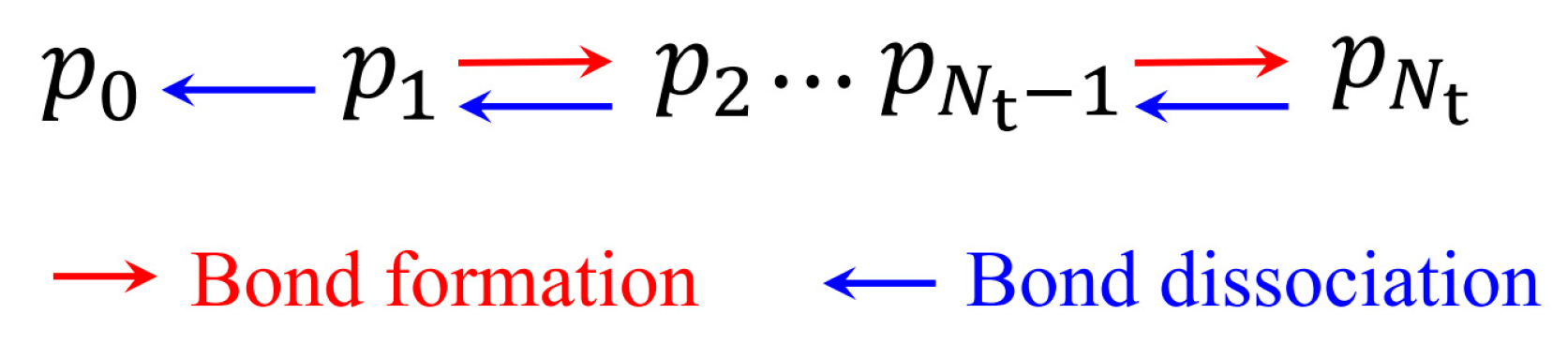
| Quantity | Symbol | Value | Reference |
|---|---|---|---|
| Cell radius | R | 4.25 μm | [58,59] |
| Cell density | 1.1 g/cm3 | [60] | |
| Flow viscosity | 0.001 Pa·s | [58] | |
| Density of total bonds | 140 μm−2 | [7,58] | |
| Rest length of bond | 11 nm | [19] | |
| Reacting radius | 1 nm | [61] | |
| Bond stiffness | 0.5 pN/nm | [62] | |
| Spontaneous association rate | 25 s−1 | [45,63] | |
| Spontaneous dissociation rate of slip barrier | 1 s−1 | [61] | |
| Spontaneous dissociation rate of catch barrier | 15 s−1 | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Kang, W.; Wang, J. Mechanical Model for Catch-Bond-Mediated Cell Adhesion in Shear Flow. Int. J. Mol. Sci. 2020, 21, 584. https://doi.org/10.3390/ijms21020584
Li L, Kang W, Wang J. Mechanical Model for Catch-Bond-Mediated Cell Adhesion in Shear Flow. International Journal of Molecular Sciences. 2020; 21(2):584. https://doi.org/10.3390/ijms21020584
Chicago/Turabian StyleLi, Long, Wei Kang, and Jizeng Wang. 2020. "Mechanical Model for Catch-Bond-Mediated Cell Adhesion in Shear Flow" International Journal of Molecular Sciences 21, no. 2: 584. https://doi.org/10.3390/ijms21020584
APA StyleLi, L., Kang, W., & Wang, J. (2020). Mechanical Model for Catch-Bond-Mediated Cell Adhesion in Shear Flow. International Journal of Molecular Sciences, 21(2), 584. https://doi.org/10.3390/ijms21020584






