Platelet-Rich Fibrin Facilitates One-Stage Cartilage Repair by Promoting Chondrocytes Viability, Migration, and Matrix Synthesis
Abstract
1. Introduction
2. Results
2.1. In Vitro
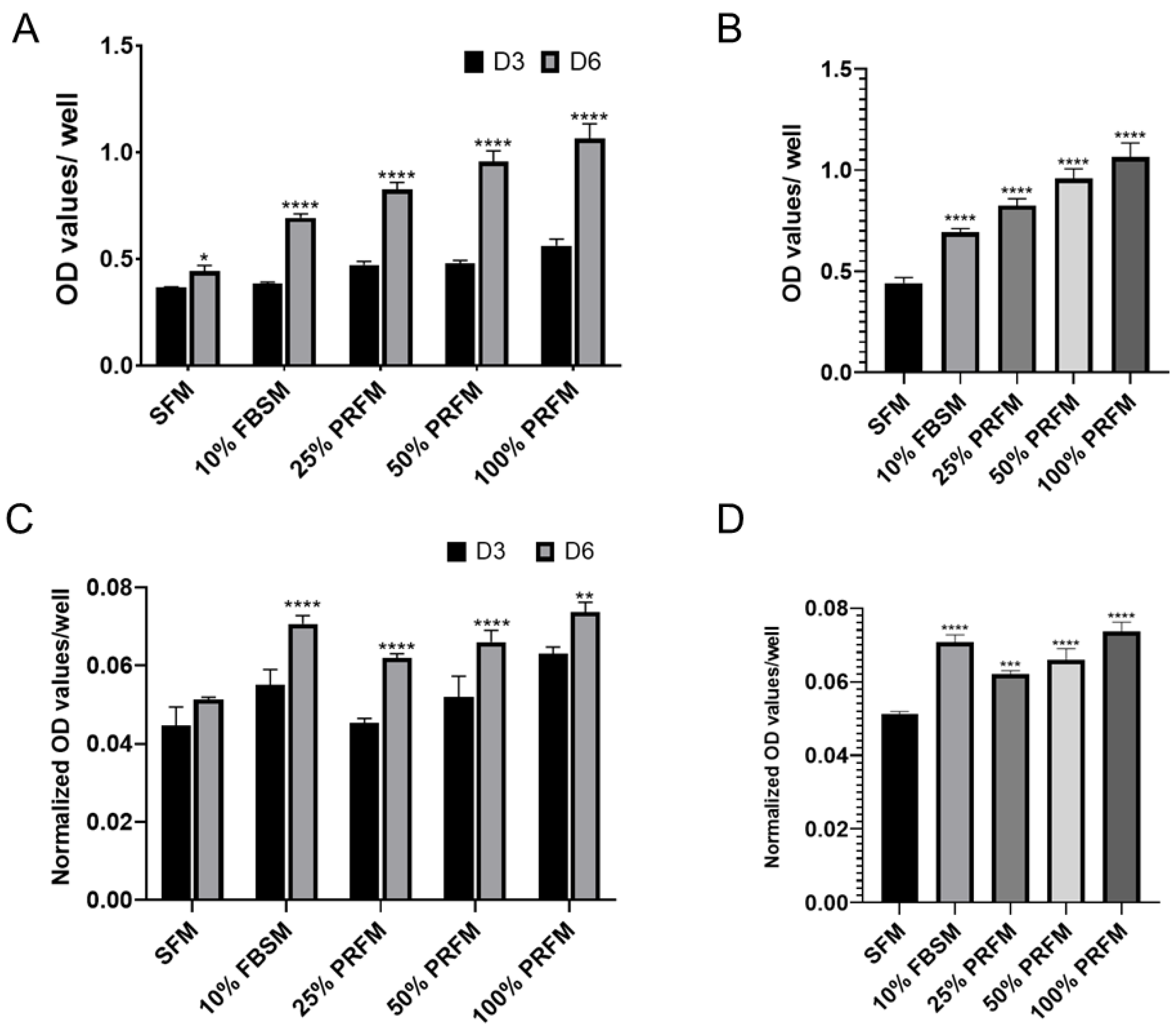
2.1.1. Dose-Dependent Effect of PRF on Proliferation of Porcine Chondrocytes
2.1.2. The Effects of PRF on Glycosaminoglycan Matrix Syntheses of Cultured Chondrocytes
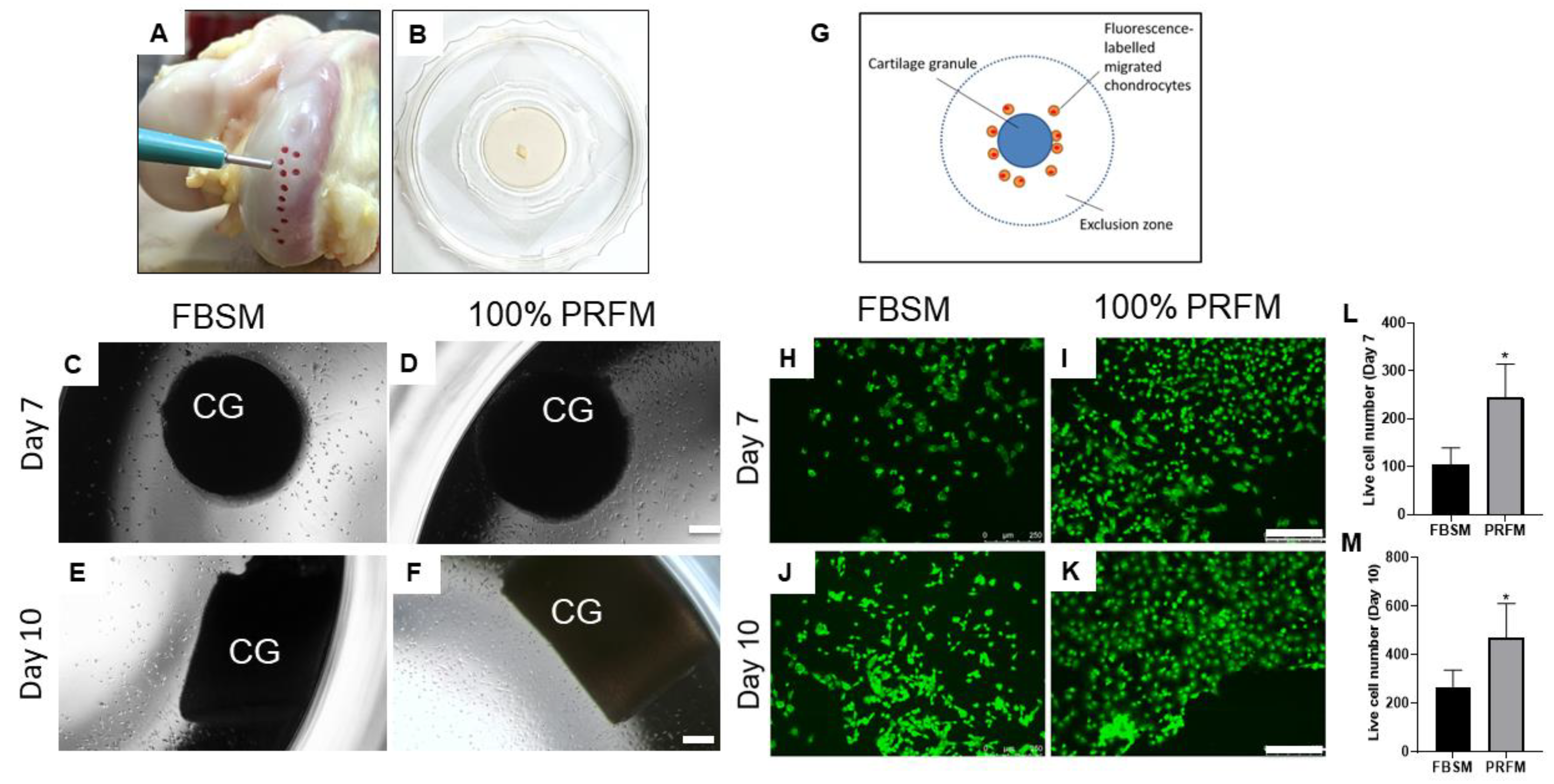
2.1.3. Chemotactic Effects of PRF on Cartilage Explants
2.1.4. Cellular Outgrowth from Cartilage Grafts on PRF Scaffolds
2.2. In Vivo
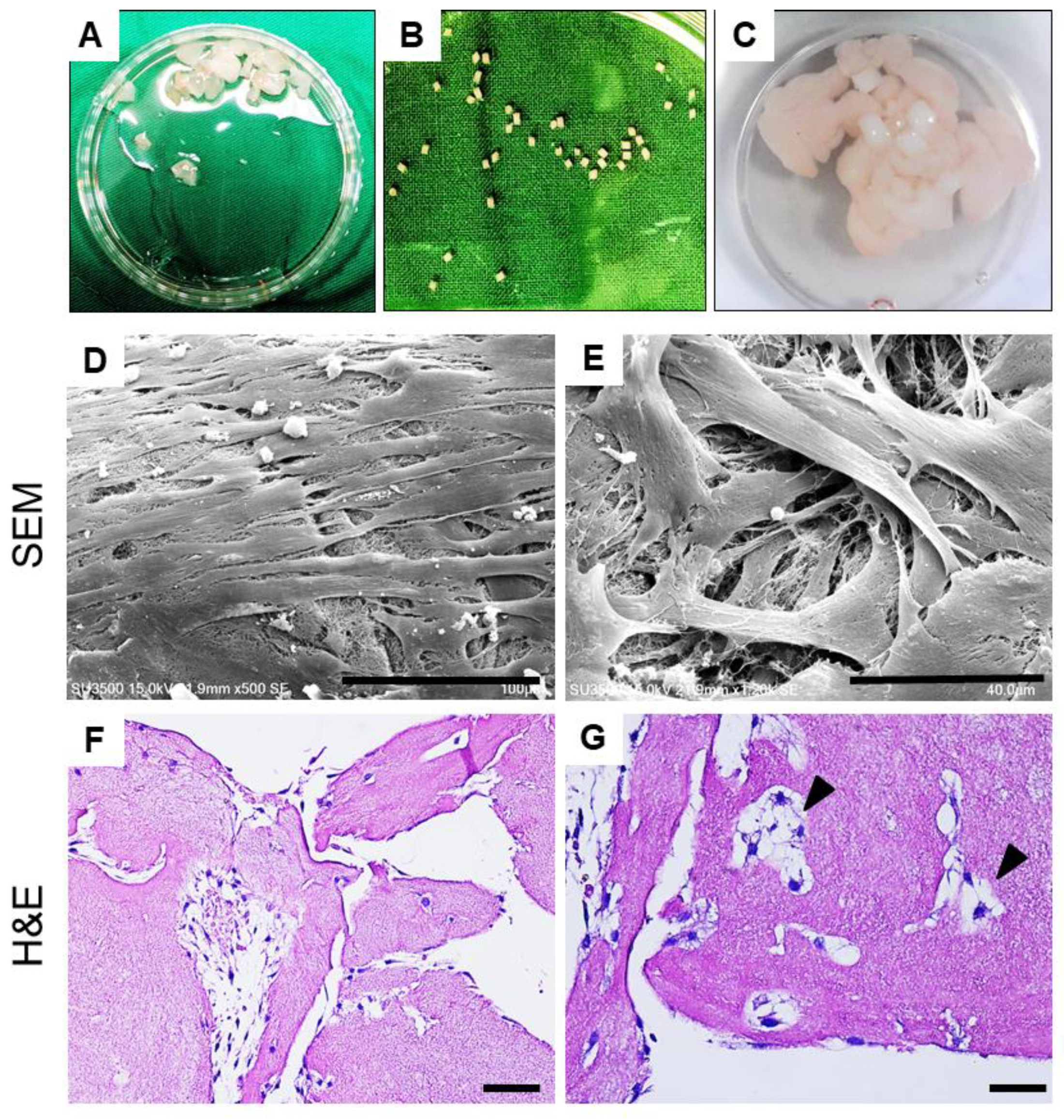
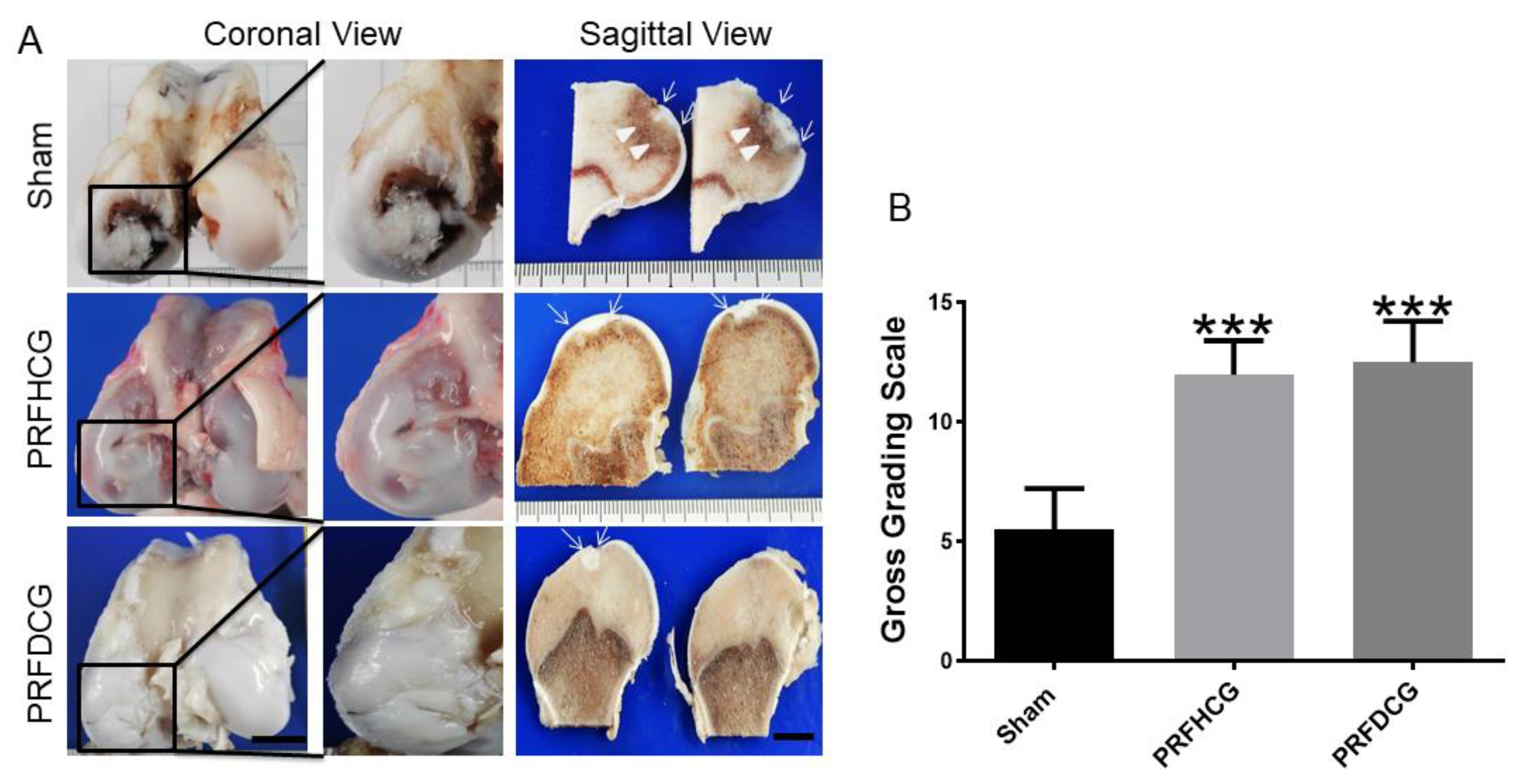
2.2.1. Macroscopic Assessments of Specimens
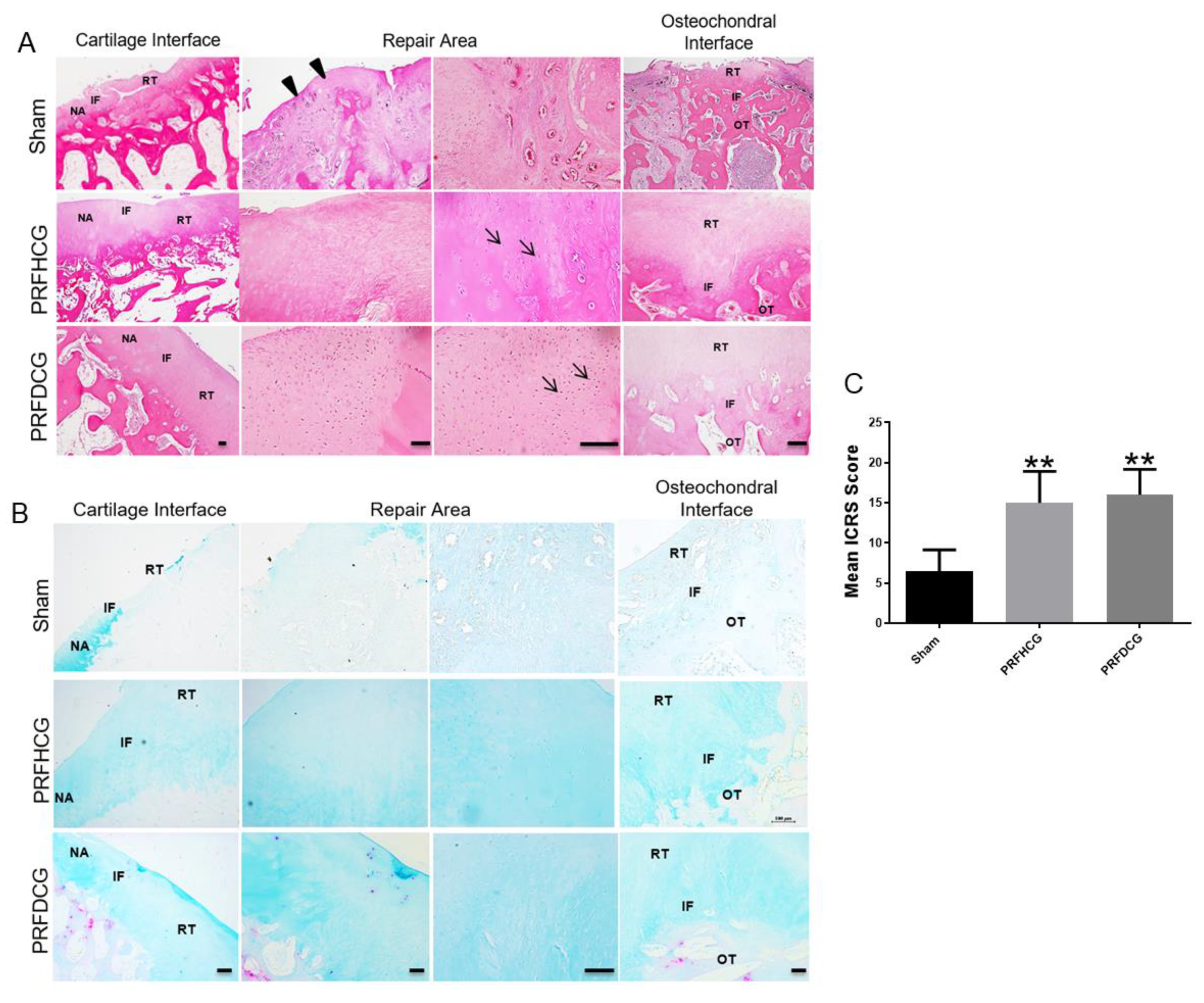
2.2.2. Histological Evaluation of Defect Healing
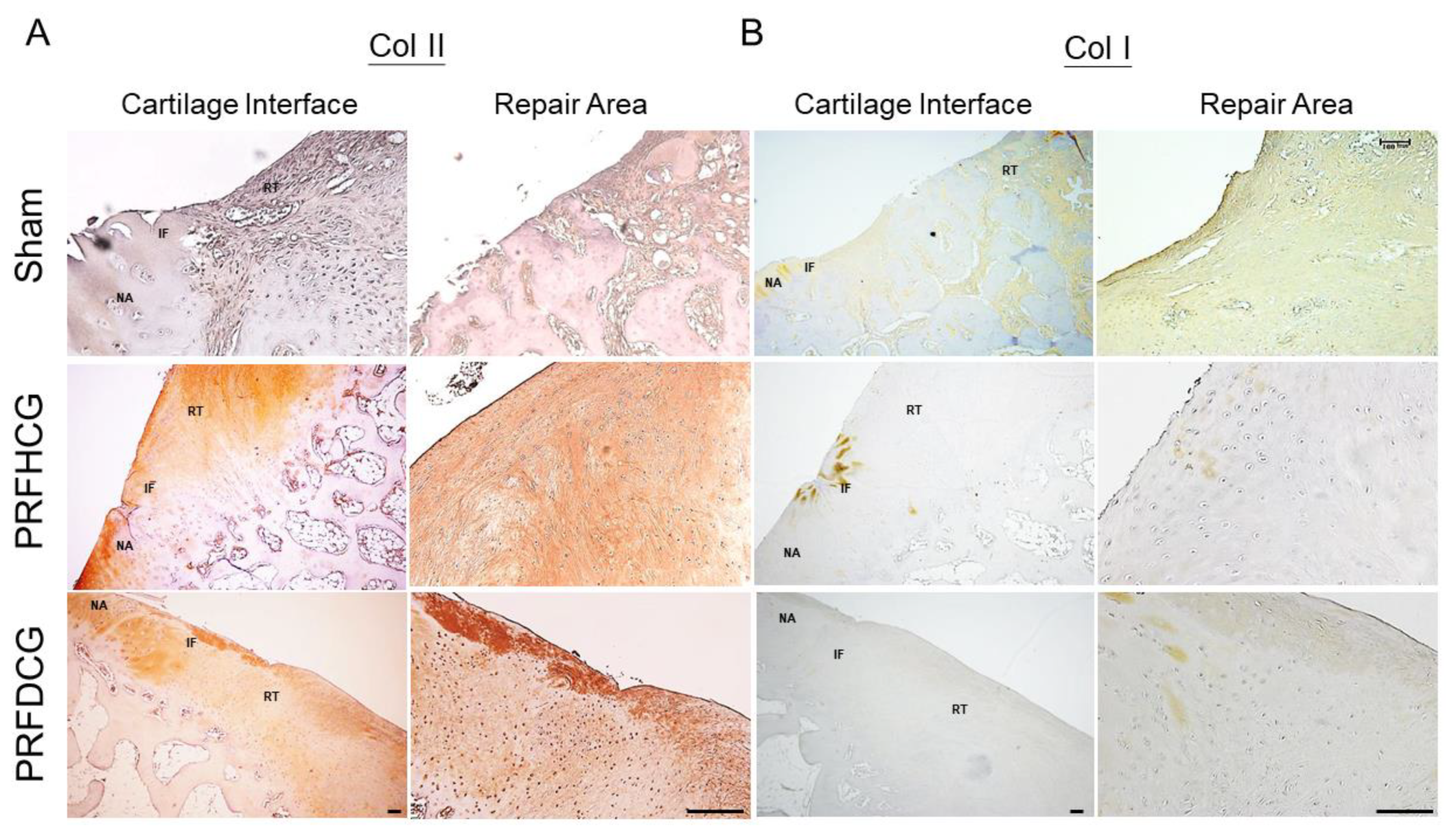
2.2.3. Chondrogenic Immunohistochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethics Statement
4.2. Preparation of PRF and PRF-Conditioned Medium
4.3. Harvest of Porcine Cartilage Explants
4.4. Isolation and Cultivation of Porcine Chondrocytes
4.5. Effects of PRF on Chondrocytes Viability
4.6. PRF on Chondrocytes Glycosaminoglycan Synthesis
4.7. Ex Vivo Model of Chondrocytes Chemotaxis
4.8. PRF Chemotactic Effects on Chondrocytes Outgrowth and Viability
4.9. Co-Culture of PRF Scaffolds with Cartilage Grafts
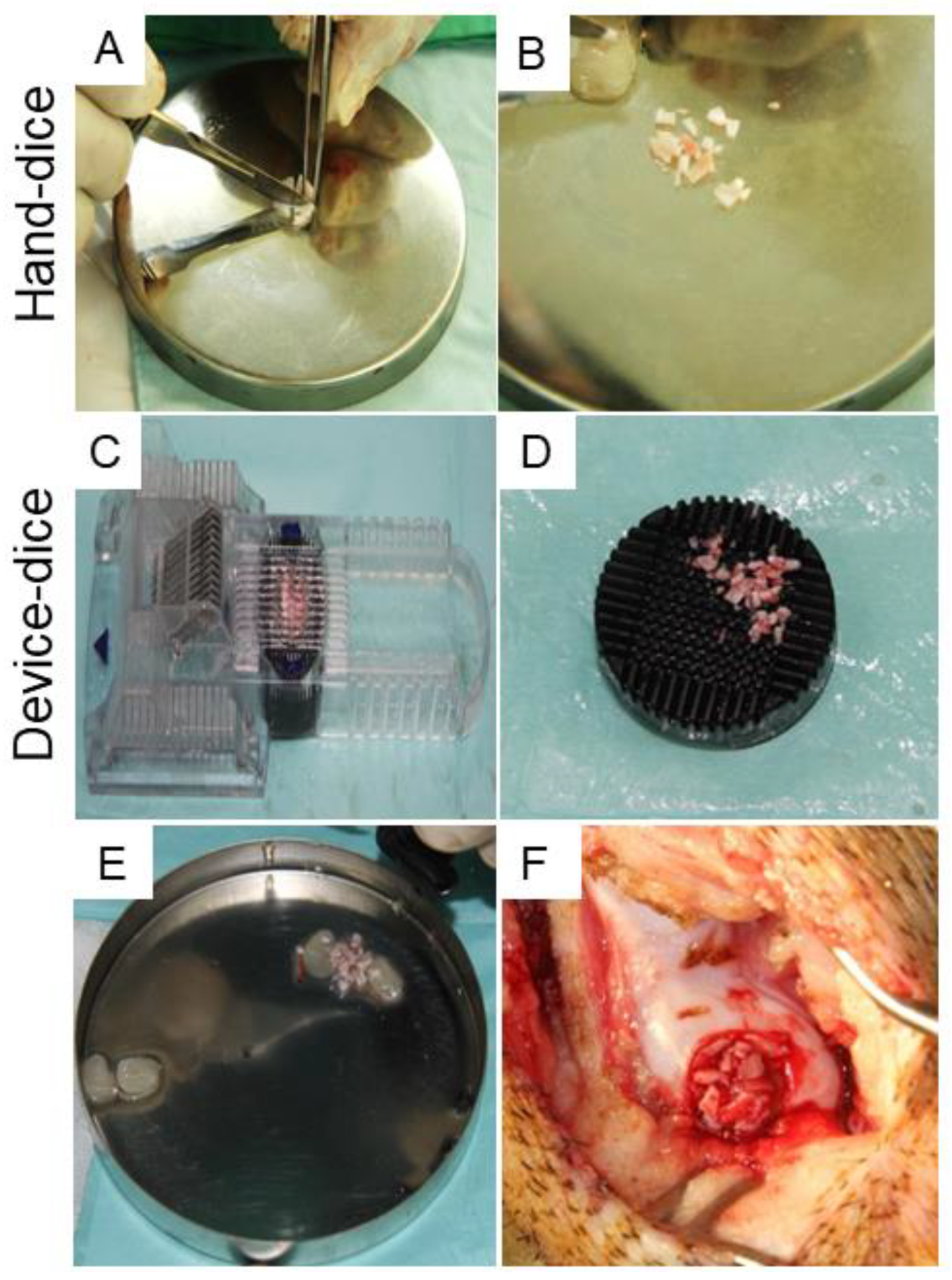
4.10. Surgical Procedure and Cartilage Dicing Process
4.11. Outcome Measurement and Gross Grading
4.12. Histological and Immunohistochemical Staining
4.13. Histological Assessment
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hjelle, K.; Solheim, E.; Strand, T.; Muri, R.; Brittberg, M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy 2002, 18, 730–734. [Google Scholar] [CrossRef]
- Cole, B.J.; Pascual-Garrido, C.; Grumet, R.C. Surgical management of articular cartilage defects in the knee. Instr. Course Lect. 2010, 59, 181–204. [Google Scholar] [PubMed]
- Gomoll, A.H.; Farr, J.; Gillogly, S.D.; Kercher, J.S.; Minas, T. Surgical management of articular cartilage defects of the knee. Instr. Course Lect. 2011, 60, 461–483. [Google Scholar] [PubMed]
- Hunziker, E.B. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr. Cartil. 1999, 7, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Joint. Surg. Am. 1993, 75, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Marmotti, A.; Bruzzone, M.; Bonasia, D.E.; Castoldi, F.; Von Degerfeld, M.M.; Bignardi, C.; Mattia, S.; Maiello, A.; Rossi, R.; Peretti, G.M. Autologous cartilage fragments in a composite scaffold for one stage osteochondral repair in a goat model. Eur. Cell Mater. 2013, 26, 15–31, discussion 31-2. [Google Scholar] [CrossRef]
- Sherman, S.L.; Thyssen, E.; Nuelle, C.W. Osteochondral Autologous Transplantation. Clin. Sports Med. 2017, 36, 489–500. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Brittberg, M.; Tallheden, T.; Sjogren-Jansson, B.; Lindahl, A.; Peterson, L. Autologous chondrocytes used for articular cartilage repair: An update. Clin. Orthop. Relat. Res. 2001, 391, S337–S348. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Belk, J.W.; Purcell, J.M.; McCarty, E.C. Microfracture Versus Autologous Chondrocyte Implantation for Articular Cartilage Lesions in the Knee: A Systematic Review of 5-Year Outcomes. Am. J. Sports Med. 2018, 46, 995–999. [Google Scholar] [CrossRef]
- Ossendorff, R.; Franke, K.; Erdle, B.; Uhl, M.; Sudkamp, N.P.; Salzmann, G.M. Clinical and radiographical ten years long-term outcome of microfracture vs. autologous chondrocyte implantation: A matched-pair analysis. Int. Orthop. 2018, 43, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anat. 2009, 191, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Bianchessi, M.; Chen, Y.; Durgam, S.; Pondenis, H.; Stewart, M. Effect of Fibroblast Growth Factor 2 on Equine Synovial Fluid Chondroprogenitor Expansion and Chondrogenesis. Stem. Cells Int. 2016, 2016, 9364974. [Google Scholar] [CrossRef]
- Brandl, A.; Angele, P.; Roll, C.; Prantl, L.; Kujat, R.; Kinner, B. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J. Orthop. Res. 2010, 28, 354–360. [Google Scholar]
- Fortier, L.A.; Mohammed, H.O.; Lust, G.; Nixon, A.J. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J. Bone Jt. Surg. Br. 2002, 84, 276–288. [Google Scholar] [CrossRef]
- Stewart, A.A.; Byron, C.R.; Pondenis, H.; Stewart, M.C. Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am. J. Vet. Res. 2007, 68, 941–945. [Google Scholar] [CrossRef]
- Wong, C.C.; Chiu, L.H.; Lai, W.F.; Tsai, T.T.; Fang, C.L.; Chen, S.C.; Tsai, Y.H. Phenotypic re-expression of near quiescent chondrocytes: The effects of type II collagen and growth factors. J. Biomater. Appl. 2010, 25, 75–95. [Google Scholar] [CrossRef]
- Macaulay, I.C.; Carr, P.; Gusnanto, A.; Ouwehand, W.H.; Fitzgerald, D.; Watkins, N.A. Platelet genomics and proteomics in human health and disease. J. Clin. Investig. 2005, 115, 3370–3377. [Google Scholar] [CrossRef]
- McRedmond, J.P.; Park, S.D.; Reilly, D.F.; Coppinger, J.A.; Maguire, P.B.; Shields, D.C.; Fitzgerald, D.J. Integration of proteomics and genomics in platelets: A profile of platelet proteins and platelet-specific genes. Mol. Cell. Proteom. 2004, 3, 133–144. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral. Surg. Oral. Med. Oral. Patho.l Oral. Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Wang, C.W.; Wang, J.Y.; Lin, M.F.; Chan, W.P. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clinics 2017, 72, 116–124. [Google Scholar] [CrossRef]
- Wong, C.C.; Kuo, T.F.; Yang, T.L.; Tsuang, Y.H.; Lin, M.F.; Chang, C.H.; Lin, Y.H.; Chan, W.P. Platelet-Rich Fibrin Facilitates Rabbit Meniscal Repair by Promoting Meniscocytes Proliferation, Migration, and Extracellular Matrix Synthesis. Int. J. Mol. Sci. 2017, 18, 1722. [Google Scholar] [CrossRef]
- Kuo, T.F.; Lin, M.F.; Lin, Y.H.; Lin, Y.C.; Su, R.J.; Lin, H.W.; Chan, W.P. Implantation of platelet-rich fibrin and cartilage grafts facilitates cartilage repair in the injured rabbit knee: Preliminary report. Clinics 2011, 66, 1835–1838. [Google Scholar] [CrossRef]
- Wong, C.C.; Chen, C.H.; Chan, W.P.; Chiu, L.H.; Ho, W.P.; Hsieh, F.J.; Chen, Y.T.; Yang, T.L. Single-Stage Cartilage Repair Using Platelet-Rich Fibrin Scaffolds with Autologous Cartilaginous Grafts. Am. J. Sports Med. 2017, 45, 3128–3142. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.S.; Okuma, M.; Attawia, M.; Miyamoto, K.; Thonar, E.J.; Lenz, M.E.; Sah, R.L.; Masuda, K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr. Cartil. 2006, 14, 1272–1280. [Google Scholar] [CrossRef]
- Drengk, A.; Zapf, A.; Sturmer, E.K.; Sturmer, K.M.; Frosch, K.H. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs 2009, 189, 317–326. [Google Scholar] [CrossRef]
- Kaps, C.; Loch, A.; Haisch, A.; Smolian, H.; Burmester, G.R.; Haupl, T.; Sittinger, M. Human platelet supernatant promotes proliferation but not differentiation of articular chondrocytes. Med. Biol. Eng. Comput. 2002, 40, 485–490. [Google Scholar] [CrossRef]
- Spreafico, A.; Chellini, F.; Frediani, B.; Bernardini, G.; Niccolini, S.; Serchi, T.; Collodel, G.; Paffetti, A.; Fossombroni, V.; Galeazzi, M.; et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J. Cell. Biochem. 2009, 108, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M.; Hidalgo, R.; Sanz, A.; Martinez, J.; Riera, P.; Smith, P.C. Effect of platelet-rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J. Periodontol. 2008, 79, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Marmotti, A.; Bruzzone, M.; Bonasia, D.E.; Castoldi, F.; Rossi, R.; Piras, L.; Maiello, A.; Realmuto, C.; Peretti, G.M. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, D.E.; Marmotti, A.; Mattia, S.; Cosentino, A.; Spolaore, S.; Governale, G.; Castoldi, F.; Rossi, R. The Degree of Chondral Fragmentation Affects Extracellular Matrix Production in Cartilage Autograft Implantation: An In Vitro Study. Arthroscopy 2015, 31, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Wayne, J.S.; McDowell, C.L.; Shields, K.J.; Tuan, R.S. In Vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005, 11, 953–963. [Google Scholar] [CrossRef]
- van den Borne, M.P.; Raijmakers, N.J.; Vanlauwe, J.; Victor, J.; de Jong, S.N.; Bellemans, J.; Saris, D.B. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthr. Cartil. 2007, 15, 1397–1402. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.-C.; Ou, K.-L.; Lin, Y.-H.; Lin, M.-F.; Yang, T.-L.; Chen, C.-H.; Chan, W.P. Platelet-Rich Fibrin Facilitates One-Stage Cartilage Repair by Promoting Chondrocytes Viability, Migration, and Matrix Synthesis. Int. J. Mol. Sci. 2020, 21, 577. https://doi.org/10.3390/ijms21020577
Wong C-C, Ou K-L, Lin Y-H, Lin M-F, Yang T-L, Chen C-H, Chan WP. Platelet-Rich Fibrin Facilitates One-Stage Cartilage Repair by Promoting Chondrocytes Viability, Migration, and Matrix Synthesis. International Journal of Molecular Sciences. 2020; 21(2):577. https://doi.org/10.3390/ijms21020577
Chicago/Turabian StyleWong, Chin-Chean, Keng-Liang Ou, Yun-Ho Lin, Ming-Fang Lin, Tsung-Lin Yang, Chih-Hwa Chen, and Wing P. Chan. 2020. "Platelet-Rich Fibrin Facilitates One-Stage Cartilage Repair by Promoting Chondrocytes Viability, Migration, and Matrix Synthesis" International Journal of Molecular Sciences 21, no. 2: 577. https://doi.org/10.3390/ijms21020577
APA StyleWong, C.-C., Ou, K.-L., Lin, Y.-H., Lin, M.-F., Yang, T.-L., Chen, C.-H., & Chan, W. P. (2020). Platelet-Rich Fibrin Facilitates One-Stage Cartilage Repair by Promoting Chondrocytes Viability, Migration, and Matrix Synthesis. International Journal of Molecular Sciences, 21(2), 577. https://doi.org/10.3390/ijms21020577




