MCM-41/PVA Composite as a Separator for Zinc–Air Batteries
Abstract
1. Introduction
2. Results & Discussion
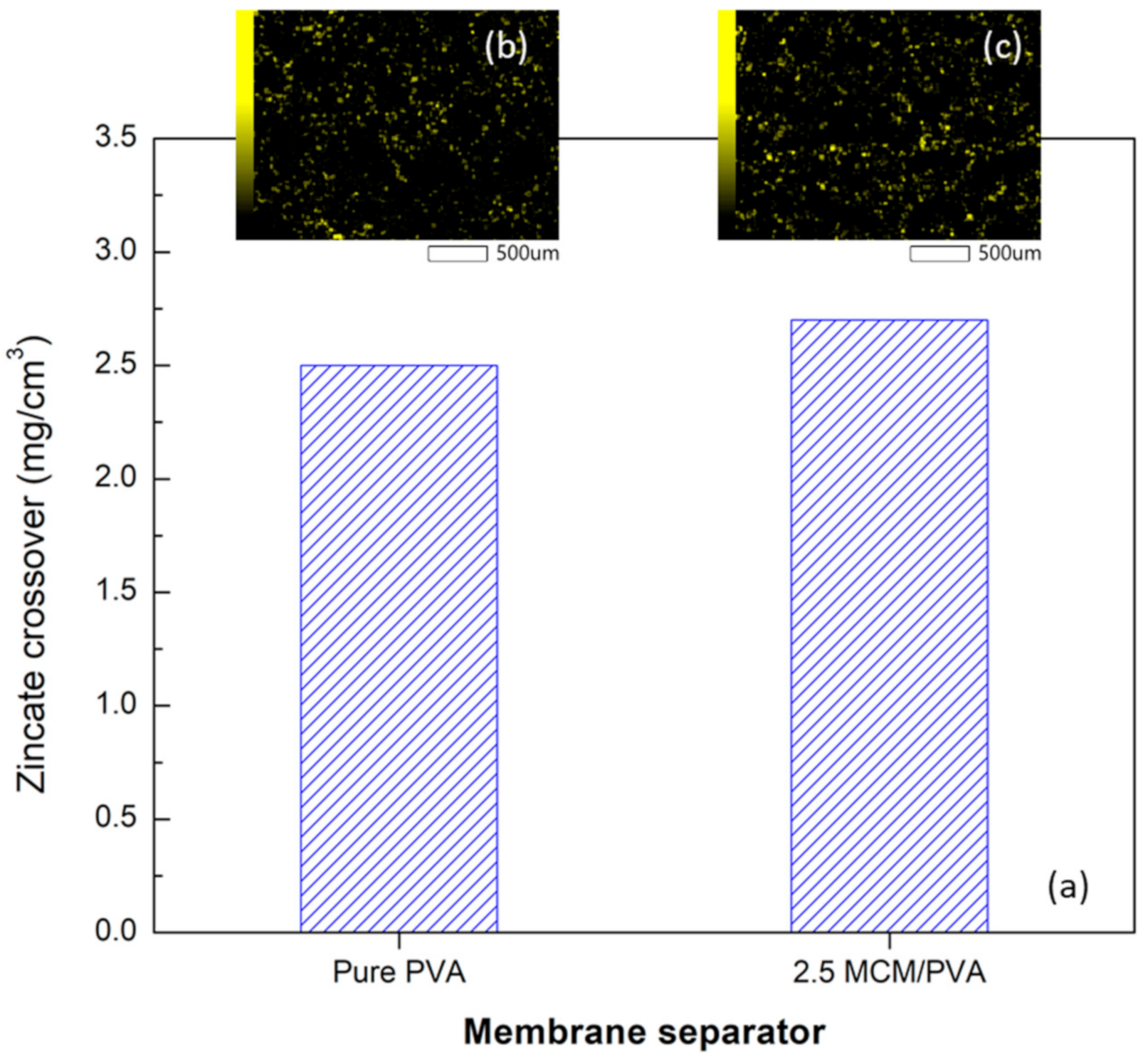
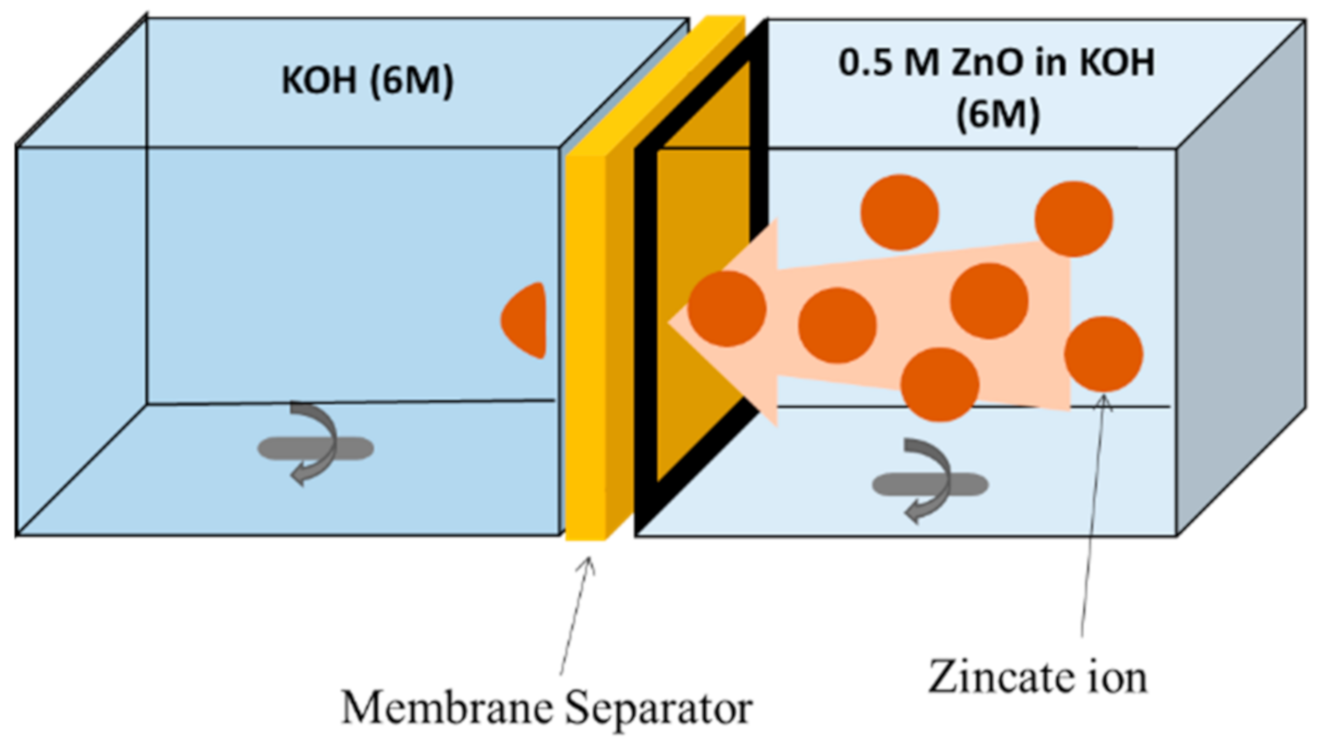
2.1. Effect of Filler Loading on Membrane Property
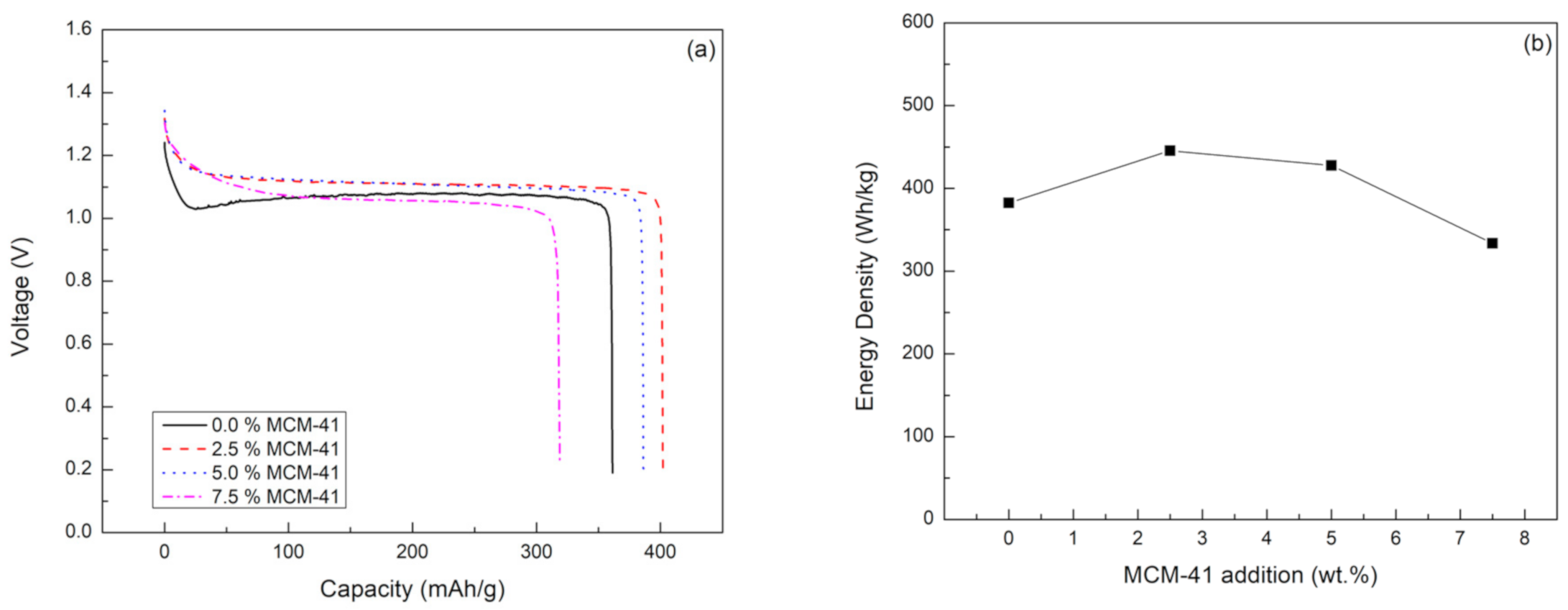
2.2. Primary Battery Performance
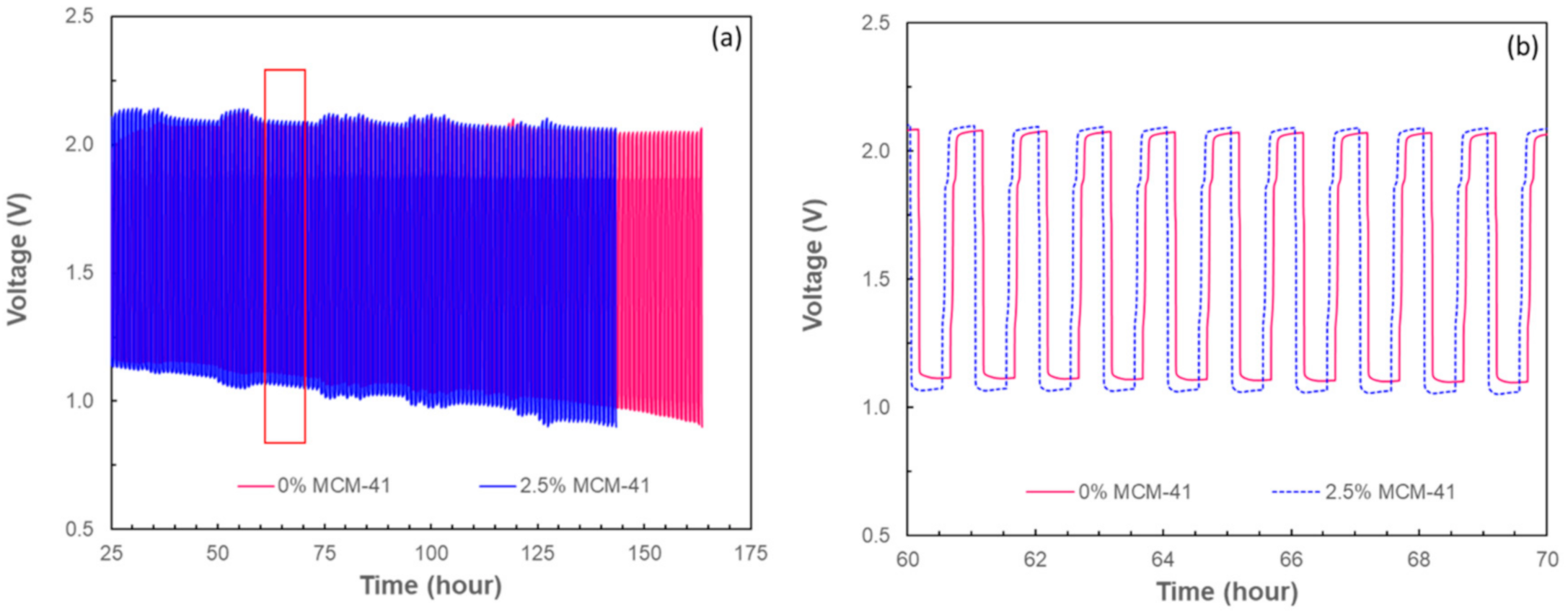
2.3. Rechargeability of ZABs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MCM-41
3.3. Preparation of the Membrane Separator
3.4. Material Characterization
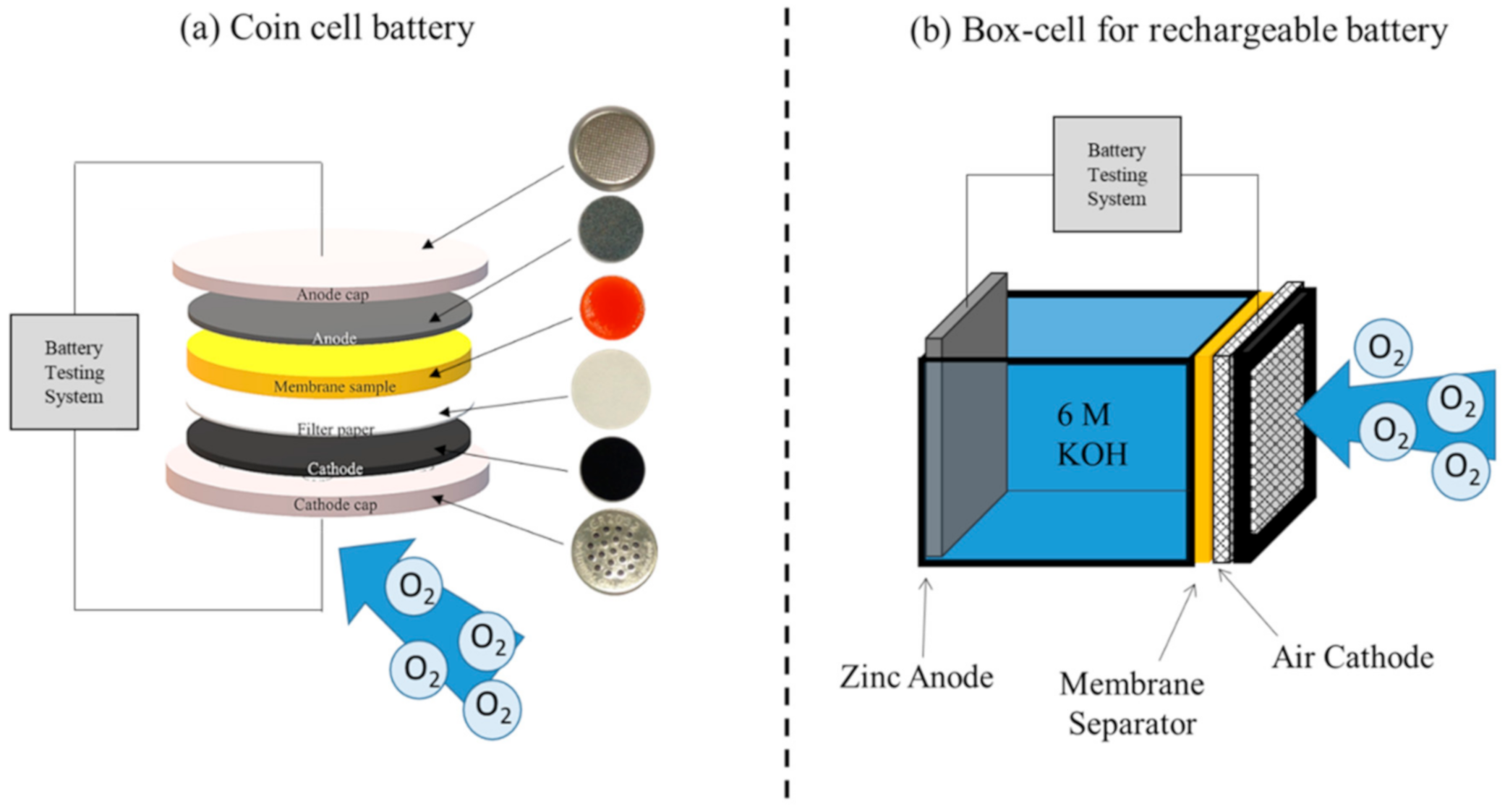
3.5. ZAB Fabrication and Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ZAB | zinc–air battery |
| PVA | polyvinyl alcohol |
| MCM-41 | Mobil Composition of Matter No. 41 |
| LIB | lithium-ion battery |
| PVAc | polyvinyl acetate |
| PEO | polyethylene oxide |
| PGE | polymer gel electrolyte |
| PVDF | polyvinylidene fluoride |
| PVDF-HFP | polyvinylidene fluoride-hexafluoropropylene |
| PTFE | polytetrafluoroethylene |
| TEOS | tetraethylorthosilicate |
| XRD | X-ray diffraction |
| SEM | scanning electron microscope |
| EIS | electrochemical impedance spectroscopy |
| EDS | energy-dispersive X-ray spectroscopy |
| ICP | inductively coupled plasma |
References
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc–Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc–air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Caramia, V.; Bozzini, B. Materials science aspects of zinc–air batteries: A review. Mater. Renew. Sustain. Energy 2014, 3, 28. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc–air batteries: Are they ready for prime time? Chem. Sci. 2019, 10, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Stock, D.; Dongmo, S.; Janek, J.; Schröder, D. Benchmarking Anode Concepts: The Future of Electrically Rechargeable Zinc–Air Batteries. Acs Energy Lett. 2019, 4, 1287–1300. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Vernardou, D.; Apostolopoulou, M.; Louloudakis, D.; Katsarakis, N.; Koudoumas, E. Hydrothermally grown β-V2O5 electrode at 95 °C. J. Colloid Interface Sci. 2014, 424, 1–6. [Google Scholar] [CrossRef]
- Hosseini, S.; Han, S.J.; Arponwichanop, A.; Yonezawa, T.; Kheawhom, S. Ethanol as an electrolyte additive for alkaline zinc-air flow batteries. Sci. Rep. 2018, 8, 11273. [Google Scholar] [CrossRef]
- Hosseini, S.; Abbasi, A.; Uginet, L.-O.; Haustraete, N.; Praserthdam, S.; Yonezawa, T.; Kheawhom, S. The Influence of Dimethyl Sulfoxide as Electrolyte Additive on Anodic Dissolution of Alkaline Zinc-Air Flow Battery. Sci. Rep. 2019, 9, 14958. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn–Air Batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tong, Y.; Yan, X.; Liang, J.; Dou, S.X. Recent Advances in Carbon-Based Bifunctional Oxygen Catalysts for Zinc-Air Batteries. Batter. Supercaps 2019, 2, 743–765. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. Int. J. Mol. Sci. 2019, 20, 3678. [Google Scholar] [CrossRef] [PubMed]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies 2019, 12, 4702. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Ding, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Investigation of the Environmental Stability of Poly(vinyl alcohol)–KOH Polymer Electrolytes for Flexible Zinc–Air Batteries. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F-127. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Qiu, H.; Yang, W.; Zhao, Z.; Zhao, J.; Cui, G. Pursuit of reversible Zn electrochemistry: A time-honored challenge towards low-cost and green energy storage. NPG Asia Mater. 2020, 12, 4. [Google Scholar] [CrossRef]
- Gan, W.; Zhou, D.; Zhou, L.; Zhang, Z.; Zhao, J. Zinc electrode with anion conducting polyvinyl alcohol/poly(diallyldimethylammonium chloride) film coated ZnO for secondary zinc air batteries. Electrochim. Acta 2015, 182, 430–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Cai, X.; Yao, J.; Li, M.; Zhang, X.; Liu, Q. High alkaline tolerant electrolyte membrane with improved conductivity and mechanical strength via lithium chloride/dimethylacetamide dissolved microcrystalline cellulose for Zn-Air batteries. Electrochim. Acta 2016, 220, 635–642. [Google Scholar] [CrossRef]
- Pan, Z.F.; An, L.; Zhao, T.S.; Tang, Z.K. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lim, J.-M.; Kim, H.-W.; Jeong, S.-H.; Eom, S.-W.; Hong Young, T.; Lee, S.-Y. Electrospun polyetherimide nanofiber mat-reinforced, permselective polyvinyl alcohol composite separator membranes: A membrane-driven step closer toward rechargeable zinc–air batteries. J. Membr. Sci. 2016, 499, 526–537. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lim, J.-M.; Lee, H.-J.; Eom, S.-W.; Hong, Y.T.; Lee, S.-Y. Artificially engineered, bicontinuous anion-conducting/-repelling polymeric phases as a selective ion transport channel for rechargeable zinc–air battery separator membranes. J. Mater. Chem. A 2016, 4, 3711–3720. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Cheng, M.-Y.; Xie, X.-L.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J. Alkali doped polyvinyl alcohol/graphene electrolyte for direct methanol alkaline fuel cells. J. Power Sources 2013, 239, 424–432. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.R.; Chang, C.-H.; Wu, K.C.-W.; Tung, K.-L.; Lue, S.J. Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef]
- Alabi, A.; AlHajaj, A.; Cseri, L.; Szekely, G.; Budd, P.; Zou, L. Review of nanomaterials-assisted ion exchange membranes for electromembrane desalination. npj Clean Water 2018, 1, 10. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zhang, B.; Liu, B.; Wang, J.; Song, Y. Novel sulfonated polyimide/polyvinyl alcohol blend membranes for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 2072–2081. [Google Scholar] [CrossRef]
- Pandey, J.; Tankal, B.R. Performance of the vanadium redox-flow battery (VRB) for Si-PWA/PVA nanocomposite membrane. J. Solid State Electrochem. 2016, 20, 2259–2265. [Google Scholar] [CrossRef]
- Klaysom, C.; Marschall, R.; Moon, S.-H.; Ladewig, B.P.; Lu, G.Q.M.; Wang, L. Preparation of porous composite ion-exchange membranes for desalination application. J. Mater. Chem. 2011, 21, 7401–7409. [Google Scholar] [CrossRef]
- Wang, X.-L.; Cai, Q.; Fan, L.-Z.; Hua, T.; Lin, Y.-H.; Nan, C.-W. Gel-based composite polymer electrolytes with novel hierarchical mesoporous silica network for lithium batteries. Electrochim. Acta 2008, 53, 8001–8007. [Google Scholar] [CrossRef]
- Yang, C.-C. Study of alkaline nanocomposite polymer electrolytes based on PVA–ZrO2–KOH. Mater. Sci. Eng. B 2006, 131, 256–262. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Saputra, H.; Othman, R.; Sutjipto, A.G.E.; Muhida, R. MCM-41 as a new separator material for electrochemical cell: Application in zinc–air system. J. Membr. Sci. 2011, 367, 152–157. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Qin, Y.; Ji, S.; Huo, Y.; Wang, Z.; Liu, Z.; Shen, J.; Liu, J. Recent Progress in Organic–Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Chem. A Eur. J. 2020, 26, 1720–1736. [Google Scholar] [CrossRef] [PubMed]
- Lue, S.J.; Mahesh, K.P.O.; Wang, W.-T.; Chen, J.-Y.; Yang, C.-C. Permeant transport properties and cell performance of potassium hydroxide doped poly(vinyl alcohol)/fumed silica nanocomposites. J. Membr. Sci. 2011, 367, 256–264. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Zhang, Y.; Zhao, B.; He, G. Enhanced hydroxide conductivity of imidazolium functionalized polysulfone anion exchange membrane by doping imidazolium surface-functionalized nanocomposites. RSC Adv. 2016, 6, 58380–58386. [Google Scholar] [CrossRef]
- Keane, D.A.; Hanrahan, J.P.; Copley, M.P.; Holmes, J.D.; Morris, M.A. A modified Stöber process for the production of mesoporous Sub 2 micron silica microspheres; applications in HPLC. J. Porous Mater. 2010, 17, 145–152. [Google Scholar] [CrossRef]
- Santos, F.; Tafur, J.P.; Abad, J.; Fernández Romero, A.J. Structural modifications and ionic transport of PVA-KOH hydrogels applied in Zn/Air batteries. J. Electroanal. Chem. 2019, 850, 113380. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Chung, H.-J.; Ivey, D.G. A study of alkaline gel polymer electrolytes for rechargeable zinc–air batteries. Electrochim. Acta 2019, 327, 135021. [Google Scholar] [CrossRef]
- Lao-atiman, W.; Bumroongsil, K.; Arpornwichanop, A.; Bumroongsakulsawat, P.; Olaru, S.; Kheawhom, S. Model-Based Analysis of an Integrated Zinc-Air Flow Battery/Zinc Electrolyzer System. Front. Energy Res. 2019, 7, 15. [Google Scholar] [CrossRef]



| Membrane Separator/Electrolyte | Electrochemical Properties | Battery Performance | Ref. | |||
|---|---|---|---|---|---|---|
| Electrolyte Uptake (%) | Ionic Conductivity (mS/cm) | Discharge Capacity (mAh/g) | Energy Density (mWh/g) | Rechargeable Cycle (Testing Conditions) | ||
| PVA | 118 | 13.5 | N/A | N/A | N/A | [22] |
| PVA on PEI nanomat support | 87 | 13.1 | 645 | N/A | 7 (N/A) | [22] |
| PVA/PAA nanomat | 31.2 | 6.6 | N/A | N/A | 250 (at 20 mAcm−2, 10 min/cycle) | [23] |
| PVA/PAA | 61.1 | 11.2 | N/A | N/A | 75 (at 20 mAcm−2, 10 min/cycle) | [23] |
| PVA-KOH | N/A | N/A | N/A | N/A | 37.5 (at 3 mA cm−2, 20 min/cycle) | [16] |
| PVA | 172 | 340 | 385 | 382 | 163 (at 5 mAcm−2, 1 h/cycle) | This work |
| 2.5 MCM-41/PVA | 169 | 380 | 400 | 446 | 145 (at 5 mAcm−2, 1 h/cycle) | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanthapong, S.; Kheawhom, S.; Klaysom, C. MCM-41/PVA Composite as a Separator for Zinc–Air Batteries. Int. J. Mol. Sci. 2020, 21, 7052. https://doi.org/10.3390/ijms21197052
Nanthapong S, Kheawhom S, Klaysom C. MCM-41/PVA Composite as a Separator for Zinc–Air Batteries. International Journal of Molecular Sciences. 2020; 21(19):7052. https://doi.org/10.3390/ijms21197052
Chicago/Turabian StyleNanthapong, Sirinuch, Soorathep Kheawhom, and Chalida Klaysom. 2020. "MCM-41/PVA Composite as a Separator for Zinc–Air Batteries" International Journal of Molecular Sciences 21, no. 19: 7052. https://doi.org/10.3390/ijms21197052
APA StyleNanthapong, S., Kheawhom, S., & Klaysom, C. (2020). MCM-41/PVA Composite as a Separator for Zinc–Air Batteries. International Journal of Molecular Sciences, 21(19), 7052. https://doi.org/10.3390/ijms21197052







