Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of pH on Anthocyanin Structure in External Solutions and Ion-Exchange Resins
2.1.1. In External Solutions
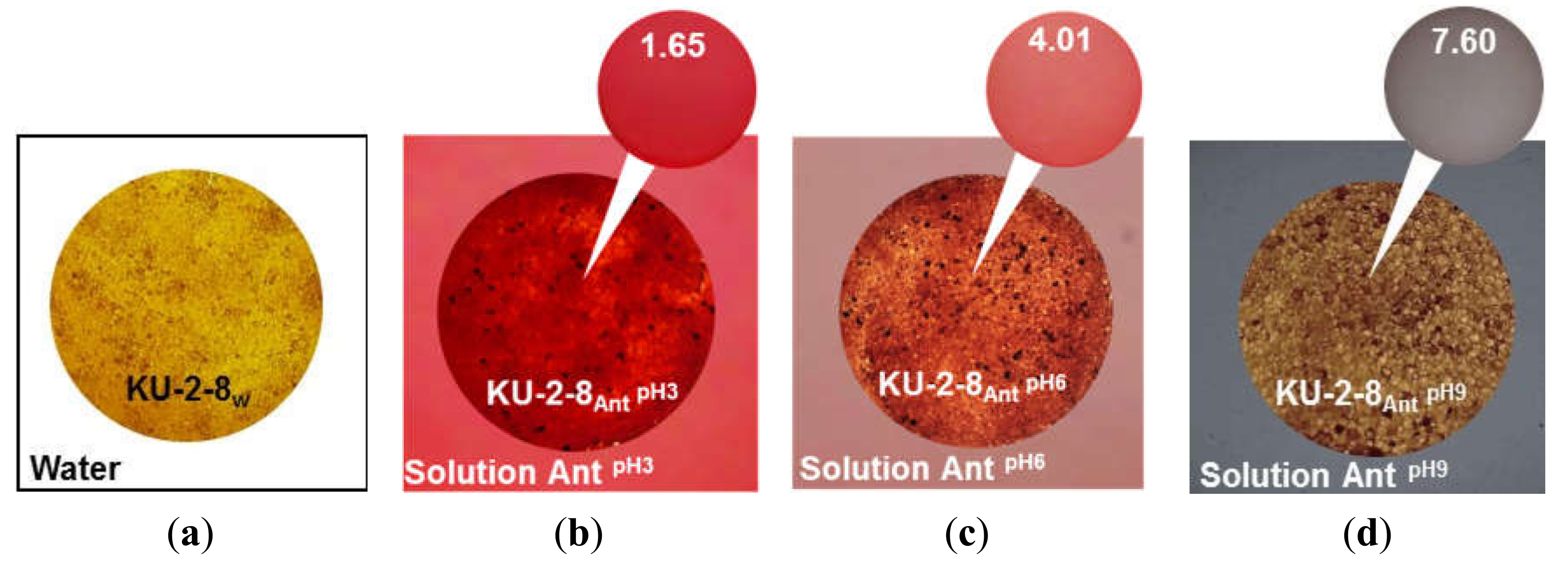
2.1.2. In Ion-Exchange Resins
2.2. Effect of the External Solution pH on Anthocyanin Adsorption by Ion-Exchange Resins
2.3. Effect of the Concentration of Anthocyanins in External Solutions on Their Adsorption by Resins
3. Materials and Methods
3.1. Ion-Exchange Resins
3.2. Solutions
3.3. Methods
3.3.1. The pH Differential Spectrophotometric Method
3.3.2. High-Performance Liquid Chromatography (HPLC) Analysis
3.3.3. Fourier Transform Infrared (FTIR) Spectroscopy and Optic Analysis
3.3.4. Kinetics and Equilibrium Isotherms of the Anthocyanins Adsorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A | Optical density |
| AER | Anion-exchange resins |
| Ant | Anthocyanin |
| BET | Brunauer–Emmett–Teller |
| ATR–FTIR | Attenuated total reflection–Fourier transform infrared spectroscopy |
| CER | Cation-exchange resins |
| Ce | Equilibrium concentration of anthocyanins in the solution, (mg dm−3) |
| Cs | Total concentration of anthocyanins in the solution, (mg dm−3) |
| CS0 | Total initial concentrations of anthocyanins in the external solution, (mg dm−3) |
| CSt | Total concentrations of anthocyanins at time t of contact of the external solution with the resin, (mg g−1) |
| CIER | Total concentration of anthocyanins in an ion-exchange resin, (mg g−1) |
| DVB | Divinylbenzene |
| ECH | Epichlorohydrin |
| IER | Ion-exchange resin |
| HPLC | High-performance liquid chromatography |
| Ka | Dissociation constant |
| KF | Equilibrium constant of adsorption in Freundlich isotherm, (mg dm−3)−1 |
| KL | Equilibrium constant of adsorption in Langmuir isotherm, (mg dm−3)−1 |
| l | Optical path length of a cuvette, (1 cm) |
| m | Mass of the swollen resin, (g) |
| M | Molecular weight of cyanidin-3-glucoside, (449.2 g mol−1) |
| n | Adsorption intensity |
| PEPAs | Polyethylene polyamines |
| PS | Polystyrene |
| qm | Amount of adsorbate corresponding to complete monolayer adsorption, (mg g−1) |
| qe | Amount of adsorbate adsorbed on the solid surface at equilibrium concentration, (mg g−1) |
| VIER | Volume of the swollen resin, (cm3) |
| V1 | Volume of the volumetric flask used to dilute the sample, (cm3) |
| Vs | Volume of solution in contact with the resin, (cm3) |
| ε | Molar extinction coefficient of cyanidin-3-glucoside, (26,900 [mol cm dm−3]−1) |
| ρ | Density of the swollen resin, (g cm−3) |
References
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine, Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2006; ISBN 978-0-470-01037-2. [Google Scholar]
- Coklar, H.; Akbulut, M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, I.L.F.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and Excretion of Black Currant Anthocyanins in Humans and Watanabe Heritable Hyperlipidemic Rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Kumar, A.V.; Raj, A.; Lakshmi, A.; Ojha, N.; Das, N. A green approach towards utilization of Floral wastes for the extraction of Natural Colorants. Res. J. Pharm. Technol. 2019, 12, 269. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Ponce, F.; Mirabal-Gallardo, Y.; Versari, A.; Laurie, F.; Versari, A. The use of cation exchange resins in wines: Effects on pH, tartrate stability, and metal content. Cienc. E Investig. Agrar. 2018, 45, 82–92. [Google Scholar] [CrossRef]
- Jordão, A.M.; Cosme, F. Recent advances in wine stabilization and conservation technologies; Nova Publishers: New York, NY, USA, 2016; ISBN 978-1-63484-883-1. [Google Scholar]
- Ibeas, V.; Correia, A.C.; Jordão, A.M. Wine tartrate stabilization by different levels of cation exchange resin treatments: Impact on chemical composition, phenolic profile and organoleptic properties of red wines. Food Res. Int. 2015, 69, 364–372. [Google Scholar] [CrossRef]
- Kelly, N.; Kelly, A.; O’Mahony, J. Strategies for enrichment and purification of polyphenols from fruit-based materials. Trends Food Sci. Technol. 2019, 83, 248–258. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Cassano, A.; Rastogi, N.K.; Basile, A. Membrane technologies for water treatment and reuse in the food and beverage industries. In Advances in Membrane Technologies for Water Treatment, 1st ed.; Basile, A., Cassano, A., Rastogi, N., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 551–580. ISBN 978-1-78242-121-4. [Google Scholar]
- Russo, C. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). J. Membr. Sci. 2007, 288, 239–246. [Google Scholar] [CrossRef]
- Bazinet, L.; Brianceau, S.; Dubé, P.; Desjardins, Y. Evolution of cranberry juice physico-chemical parameters during phenolic antioxidant enrichment by electrodialysis with filtration membrane. Sep. Purif. Technol. 2012, 87, 31–39. [Google Scholar] [CrossRef]
- Bazinet, L.; Doyen, A. Antioxidants, mechanisms, and recovery by membrane processes. Crit. Rev. Food Sci. Nutr. 2015, 57, 677–700. [Google Scholar] [CrossRef]
- Husson, E.; Araya-Farias, M.; Gagné, A.; Bazinet, L. Selective anthocyanins enrichment of cranberry juice by electrodialysis with filtration membrane: Influence of membranes characteristics. J. Membr. Sci. 2013, 448, 114–124. [Google Scholar] [CrossRef]
- Achaerandio, I.; Güell, C.; López, F. New approach to continuous vinegar decolourisation with exchange resins. J. Food Eng. 2007, 78, 991–994. [Google Scholar] [CrossRef]
- Coca, M.; Garcia, M.T.; Mato, S.; Cartón, A.; Gonzalez, G. Evolution of colorants in sugarbeet juices during decolorization using styrenic resins. J. Food Eng. 2008, 89, 429–434. [Google Scholar] [CrossRef]
- Luo, J.; Guo, S.; Qiang, X.; Hang, X.; Chen, X.; Wan, Y. Sustainable utilization of cane molasses by an integrated separation process: Interplay between adsorption and nanofiltration. Sep. Purif. Technol. 2019, 219, 16–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, X.; Tang, B. Environmentally friendly preparation of a strong basic anion exchange fibers and its application in sugar decolorization. React. Funct. Polym. 2014, 76, 41–48. [Google Scholar] [CrossRef]
- Li, J.; Liang, L.; Cheng, J.; Huang, Y.; Zhu, M.; Liang, S. Extraction of pigment from sugarcane juice alcohol wastewater and evaluation of its antioxidant and free radical scavenging activities. Food Sci. Biotechnol. 2012, 21, 1489–1496. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, R.; Quek, S.Y. Extraction of anthocyanins from natural sources—Methods and commercial considerations. In Food Chemistry, Function and Analysis; Brooks, M.S.-L., Celli, G.B., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 77–105. ISBN 978-1-78801-215-7. [Google Scholar]
- Dul, V.N.; Dargaeva, T.D.; Kir’janov, A.A.; Sajbel’, O.L.; Sokol’skaja, T.A.; Ferubko, E.V.; Shilina, N.L.; Gromakova, A.I. Method for producing therapeutic agent possessing antioxidant activity. R.U. Patent 2 568 908 C1, 20 November 2015. [Google Scholar]
- Milheiro, J.; Ferreira, L.C.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. A simple dispersive solid phase extraction clean-up/concentration method for selective and sensitive quantification of biogenic amines in wines using benzoyl chloride derivatisation. Food Chem. 2019, 274, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.R.; Kammerer, J.; Carle, R. Resin adsorption and ion exchange to recover and fractionate polyphenols. In Polyphenols in Plants; Watson, R.R., Ed.; Academic Press: New York, NY, USA, 2014; pp. 219–230. ISBN 978-0-12-397934-6. [Google Scholar]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Integrated extraction-adsorption process for selective recovery of antioxidant phenolics from food industry by-product. Chem. Eng. Process. Process. Intensif. 2018, 127, 83–92. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Díaz-Montaña, E.J.; Asuero, A.M.J.-M.A.A.G. Recovery of Anthocyanins Using Membrane Technologies: A Review. Crit. Rev. Anal. Chem. 2018, 48, 143–175. [Google Scholar] [CrossRef]
- Kammerer, J.; Carle, R.; Kammerer, D.R. Adsorption and Ion Exchange: Basic Principles and Their Application in Food Processing. J. Agric. Food Chem. 2011, 59, 22–42. [Google Scholar] [CrossRef]
- Buran, T.J.; Sandhu, A.K.; Li, Z.; Rock, C.R.; Yang, W.W.; Gu, L. Adsorption/desorption characteristics and separation of anthocyanins and polyphenols from blueberries using macroporous adsorbent resins. J. Food Eng. 2014, 128, 167–173. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, M.; Jiang, Q.; Zhang, Y.; Chang, X.; Zhan, K. Adsorption and Desorption Studies of Anthocyanins from Black Peanut Skins on Macroporous Resins. Int. J. Food Eng. 2015, 11, 841–849. [Google Scholar] [CrossRef]
- Wang, W.; Deng, J.; Shi, X.; Liu, Y. Isolation, purification and structure identification of anthocyanins from Vitis davidii Foex skin. Trans. Chin. Soc. Agric. Eng. 2016, 32, 296–301. [Google Scholar]
- Zhang, P.; Wang, L.; Fang, S. Modeling of the Adsorption/Desorption Characteristics and Properties of Anthocyanins from Extruded Red Cabbage Juice by Macroporous Adsorbent Resin. Int. J. Food Eng. 2019, 15, 15. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J. Batch and fixed bed column studies on phenolic adsorption from wine vinasses by polymeric resins. J. Food Eng. 2017, 209, 52–60. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, X.; Xu, Y.; Yu, Z. Purification of Anthocyanins from Extracts of Red Raspberry Using Macroporous Resin. Int. J. Food Prop. 2015, 18, 1046–1058. [Google Scholar] [CrossRef]
- Haddad, M.; Oie, C.; Duy, S.V.; Sauvé, S.; Barbeau, B. Adsorption of micropollutants present in surface waters onto polymeric resins: Impact of resin type and water matrix on performance. Sci. Total. Environ. 2019, 660, 1449–1458. [Google Scholar] [CrossRef]
- Ulbricht, M.; Ansorge, W.; Danielzik, I.; König, M.; Schuster, O. Fouling in microfiltration of wine: The influence of the membrane polymer on adsorption of polyphenols and polysaccharides. Sep. Purif. Technol. 2009, 68, 335–342. [Google Scholar] [CrossRef]
- Caetano, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Phenol removal from aqueous solution by adsorption and ion exchange mechanisms onto polymeric resins. J. Colloid Interface Sci. 2009, 338, 402–409. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]
- Rengaraj, S.; Moon, S.-H.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Agricultural solid waste for the removal of organics: Adsorption of phenol from water and wastewater by palm seed coat activated carbon. Waste Manag. 2002, 22, 543–548. [Google Scholar] [CrossRef]
- Radhika, M.; Palanivelu, K. Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent—Kinetics and isotherm analysis. J. Hazard. Mater. 2006, 138, 116–124. [Google Scholar] [CrossRef]
- Özkaya, B. Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. J. Hazard. Mater. 2006, 129, 158–163. [Google Scholar] [CrossRef]
- Taktak, F.; Ciğeroğlu, Z.; Öğen, Y.; Kirbaşlar, Ş.I. Resin-loaded cationic hydrogel: A new sorbent for recovering of grapefruit polyphenols. Chem. Eng. Commun. 2018, 205, 1442–1456. [Google Scholar] [CrossRef]
- Kaushik, A.; Basu, S.; Raturi, S.; Batra, V.; Balakrishnan, M. Recovery of antioxidants from sugarcane molasses distillery wastewater and its effect on biomethanation. J. Water Process. Eng. 2018, 25, 205–211. [Google Scholar] [CrossRef]
- Vidal, R.H.L.; Pereira, C.G. Ion exchange resin applied to obtain the clarified cashew juice. Sep. Sci. Technol. 2015, 150707113216002. [Google Scholar] [CrossRef]
- Sholokhova, A.Y.; Eliseeva, T.; Voronyuk, I.V. Sorption of Vanillin by Highly Basic Anion Exchangers under Dynamic Conditions. Russ. J. Phys. Chem. A 2018, 92, 2048–2052. [Google Scholar] [CrossRef]
- Robbins, R.D.; Johnson, D.K.; Collinz, T.M.; Ahmadiani, N.; Giusti, M.M. Method of Isolating Blue Anthocyanin Fractions. U.S. Patent 10119029 B2, 6 November 2018. [Google Scholar]
- Yang, L.; Tan, T. Enhancement of the isolation selectivity of isoflavonoid puerarin using oligo-β-cyclodextrin coupled polystyrene-based media. Biochem. Eng. J. 2008, 40, 189–198. [Google Scholar] [CrossRef]
- Geng, X.; Ren, P.; Pi, G.; Shi, R.; Yuan, Z.; Wang, C. High selective purification of flavonoids from natural plants based on polymeric adsorbent with hydrogen-bonding interaction. J. Chromatogr. A 2009, 1216, 8331–8338. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, M.; Cui, Y.; Alali, A.; Atkinson, J.D. Phenol adsorption and desorption with physically and chemically tailored porous polymers: Mechanistic variability associated with hyper-cross-linking and amination. J. Hazard. Mater. 2019, 361, 162–168. [Google Scholar] [CrossRef]
- Kammerer, J.; Boschet, J.; Kammerer, D.R.; Carle, R. Enrichment and fractionation of major apple flavonoids, phenolic acids and dihydrochalcones using anion exchange resins. LWT 2011, 44, 1079–1087. [Google Scholar] [CrossRef]
- Kammerer, J.; Schweizer, C.; Carle, R.; Kammerer, D.R. Recovery and fractionation of major apple and grape polyphenols from model solutions and crude plant extracts using ion exchange and adsorbent resins. Int. J. Food Sci. Technol. 2011, 46, 1755–1767. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, S.-T. Effect of pH on Fumaric Acid Adsorption onto IRA900 Ion Exchange Resin. Sep. Sci. Technol. 2014, 50, 56–63. [Google Scholar] [CrossRef]
- Hashim, H.; Wan Ahmad, W.Y.; Zubairi, S.I.; Maskat, M.Y. Effect of pH on adsorption of organic acids and phenolic compounds by amberlite ira 67 resin. J. Teknol. 2018, 81. [Google Scholar] [CrossRef] [Green Version]
- Lasanta, C.; Caro, I.; Perez, L. The influence of cation exchange treatment on the final characteristics of red wines. Food Chem. 2013, 138, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Chorfa, N.; Savard, S.; Belkacemi, K. An efficient method for high-purity anthocyanin isomers isolation from wild blueberries and their radical scavenging activity. Food Chem. 2016, 197, 1226–1234. [Google Scholar] [CrossRef]
- Meile, K.; Iljina, N.; Zhurinsh, A. Desorption of Phenols from Anion Exchange Resins after the Separation of Wood Pyrolysis Products. Key Eng. Mater. 2019, 800, 256–260. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, J.; Ma, Y.; Sun, X.; Li, D.; Li, L.; Bai, H.; Xin, G.; Meng, X. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa cultivated in Haicheng, Liaoning, China. Food Biosci. 2019, 30, 100413. [Google Scholar] [CrossRef]
- Shuang, C.; Wang, J.; Li, H.; Li, A.; Zhou, Q. Effect of the chemical structure of anion exchange resin on the adsorption of humic acid: Behavior and mechanism. J. Colloid Interface Sci. 2015, 437, 163–169. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Huang, W.; Tan, X.; Dong, H.; Goodman, B.A.; Du, H.; Lei, F.; Diao, K. Adsorption of phenolic compounds from water by a novel ethylenediamine rosin-based resin: Interaction models and adsorption mechanisms. Chemosphere 2019, 214, 821–829. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Traynor, M.; Ahmad, I. Impact of a commercially available ion-exchange resin used in red wines to reduce toxic compounds: Effect on pH, oxidation–reduction potential, color, and sensory attributes. J. Food Sci. Technol. 2018, 55, 4859–4866. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, W.; Yao, B.; Fang, L.; Li, W. Study on deacidification process of blackberry juice. Acta Hortic. 2016, 237–244. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Wang, D.; Zhou, X.; Tan, S. Methylation Formation to Improve Red Radish Anthocyanins Solubility in Cold Water. Int. J. Chem. Eng. Appl. 2013, 221–223. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Murugananthan, M.; Raju, G.B.; Prabhakar, S. Removal of tannins and polyhydroxy phenols by electro-chemical techniques. J. Chem. Technol. Biotechnol. 2005, 80, 1188–1197. [Google Scholar] [CrossRef]
- Mohammed-Ziegler, I.; Billes, F. Vibrational spectroscopic calculations on pyrogallol and gallic acid. J. Mol. Struct. Theochem. 2002, 618, 259–265. [Google Scholar] [CrossRef]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Holden-Day: Oakland, CA, US, 1977. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2010; ISBN 978-0-470-09307-8. [Google Scholar]
- Pappas, C.; Takidelli, C.; Tsantili, E.; Tarantilis, P.; Polissiou, M. Quantitative determination of anthocyanins in three sweet cherry varieties using diffuse reflectance infrared Fourier transform spectroscopy. J. Food Compos. Anal. 2011, 24, 17–21. [Google Scholar] [CrossRef]
- García-Tejeda, Y.V.; Salinas-Moreno, Y.; Hernandez-Martinez, A.R.; Martínez-Bustos, F. Encapsulation of Purple Maize Anthocyanins in Phosphorylated Starch by Spray Drying. Cereal Chem. J. 2016, 93, 130–137. [Google Scholar] [CrossRef]
- Baranović, G.; Šegota, S. Infrared spectroscopy of flavones and flavonols. Reexamination of the hydroxyl and carbonyl vibrations in relation to the interactions of flavonoids with membrane lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 473–486. [Google Scholar] [CrossRef]
- Laghi, L.; Versari, A.; Parpinello, G.P.; Nakaji, D.Y.; Boulton, R.B. FTIR Spectroscopy and Direct Orthogonal Signal Correction Preprocessing Applied to Selected Phenolic Compounds in Red Wines. Food Anal. Methods 2011, 4, 619–625. [Google Scholar] [CrossRef]
- Silva, S.D.; Feliciano, R.P.; Boas, L.V.; Bronze, M.R. Application of FTIR-ATR to Moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem. 2014, 150, 489–493. [Google Scholar] [CrossRef]
- Tinti, A.; Tugnoli, V.; Bonora, S.; Francioso, O. Recent applications of vibrational mid-Infrared (IR) spectroscopy for studying soil components: A review. J. Central Eur. Agric. 2015, 16, 1–22. [Google Scholar] [CrossRef]
- Günzler, H.; Gremlich, H.-U. IR Spectroscopy: An Introduction; Wiley-VCH: Weinheim, Germany, 2002; ISBN 978-3-527-28896-0. [Google Scholar]
- Vasil’Eva, V.I.; Goleva, E.A.; Selemenev, V.F. Features of the sorption of phenylalanine by profiled ion-exchange membranes. Russ. J. Phys. Chem. A 2016, 90, 2035–2043. [Google Scholar] [CrossRef]
- Grebeniyk, V.D.; Mazo, A.A. Water Demineralization with Ion Exchangers; Chemistry: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Pashkov, A.B.; Saldadze, K.M.; Lukyanova, N.L.; Polyakova, T.A. GOST 20301-74. Ion-Exchange Resins. Anionites. Specifications; Izdatel’stvo standartov: Moscow, Russia, 1992. [Google Scholar]
- Kravchenko, T.A.; Sakardina, E.A.; Kalinichev, A.I.; Zolotukhina, E.V. Stabilization of copper nanoparticles with volume- and surface-distribution inside ion-exchange matrices. Russ. J. Phys. Chem. A 2015, 89, 1648–1654. [Google Scholar] [CrossRef]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962; ISBN 0-486-68784-8. [Google Scholar]
- Moskvichev, V.; Yurchenko, V.S.; Genedi, A.S.; Chokina, B.C.; Samsonov, G.V. Synthesis, Structure, and Properties of Polymers; Nauka: Leningrad, Russia, 1970. (In Russian) [Google Scholar]
- Bdiri, M.; Dammak, L.; Chaabane, L.; Larchet, C.; Hellal, F.; Nikonenko, V.; Pismenskaya, N. Cleaning of cation-exchange membranes used in electrodialysis for food industry by chemical solutions. Sep. Purif. Technol. 2018, 199, 114–123. [Google Scholar] [CrossRef]
- Semushin, A.M.; Yakovlev, V.A.; Ivanova, E.V. Infrared Absorption Spectra of Ion-Exchange Materials. Reference Guide; Himiya: Leningrad, Russia, 1980. (In Russian) [Google Scholar]
- Uglyanskaya, V.A.; Chikin, G.A.; Selemenev, V.F.; Zav’yalova, V.A. Infrared Spectroscopy of Ion-Exchange Materials; VSU: Voronezh, Russia, 1989. (In Russian) [Google Scholar]
- Maes, A.M.; Pandey, T.P.; Vandiver, M.A.; Lundquist, L.K.; Yang, Y.; Horan, J.L.; Krosovsky, A.; Liberatore, M.; Seifert, S.; Herring, A.M. Preparation and characterization of an alkaline anion exchange membrane from chlorinated poly(propylene) aminated with branched poly(ethyleneimine). Electrochim. Acta 2013, 110, 260–266. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Kovaleva, T.A.; Artyukhov, V.G.; Karpov, S.I.; Seredin, P.V.; Bogachev, M.I. The research of interaction mechanism for inulinase molecule with the matrix of synthetic ionites. Fundam. Res. 2013, 4, 663–671. [Google Scholar]
- Lewis, J. The New Color Mixing Companion: Explore and Create Fresh and Vibrant Color Palettes with Paint, Collage, And Mixed Media; Quarry Books, an imprint of The Quarto Group: Beverly, MA, USA, 2018; ISBN 978-1-63159-549-3. [Google Scholar]
- Sarapulova, V.; Nevakshenova, E.; Nebavskaya, X.; Kozmai, A.; Aleshkina, D.; Pourcelly, G.; Nikonenko, V.V.; Pismenskaya, N. Characterization of bulk and surface properties of anion-exchange membranes in initial stages of fouling by red wine. J. Membr. Sci. 2018, 559, 170–182. [Google Scholar] [CrossRef]
- Koter, S.; Kultys, M. Modeling the electric transport of sulfuric and phosphoric acids through anion-exchange membranes. Sep. Purif. Technol. 2010, 73, 219–229. [Google Scholar] [CrossRef]
- Ramírez, P.; Alcaraz, A.; Mafe, S.; Pellicer, J. Donnan Equilibrium of Ionic Drugs in pH-Dependent Fixed Charge Membranes: Theoretical Modeling. J. Colloid Interface Sci. 2002, 253, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Franck-Lacaze, L.; Sistat, P.; Huguet, P. Determination of the pKa of poly (4-vinylpyridine)-based weak anion exchange membranes for the investigation of the side proton leakage. J. Membr. Sci. 2009, 326, 650–658. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Choi, W.J.; Park, J.M.; Yang, J.-W. Selective adsorption of flavonoid compounds from the leaf extract of Ginkgo bilobaL. Biotechnol. Tech. 1997, 11, 553–556. [Google Scholar] [CrossRef]
- Fu, B.; Liu, J.; Li, H.; Li, L.; Lee, F.S.; Wang, X. The application of macroporous resins in the separation of licorice flavonoids and glycyrrhizic acid. J. Chromatogr. A 2005, 1089, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Merle, G.G.; Wessling, M.M.; Nijmeijer, D.K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Kozmai, A.; Nikonenko, V.; Zyryanova, S.; Pismenskaya, N.; Dammak, L.; Baklouti, L. Modelling of anion-exchange membrane transport properties with taking into account the change in exchange capacity and swelling when varying bathing solution concentration and pH. J. Membr. Sci. 2019, 590, 117291. [Google Scholar] [CrossRef]
- Higa, M.; Tanaka, N.; Nagase, M.; Yutani, K.; Kameyama, T.; Takamura, K.; Kakihana, Y. Electrodialytic properties of aromatic and aliphatic type hydrocarbon-based anion-exchange membranes with various anion-exchange groups. Polymer 2014, 55, 3951–3960. [Google Scholar] [CrossRef]
- Harmon, K.M.; De Santis, N.J.; Brandt, D.O. Hydrogen bonding Part 39. Hydrogen bonding by α-CH in quaternary ammonium salts and the possible role of CH⋯B hydrogen bonds in acetylcholine—Receptor interactions. J. Mol. Struct. 1992, 265, 47–57. [Google Scholar] [CrossRef]
- Pan, B.; Xiong, Y.; Li, A.; Chen, J.; Zhang, Q.; Jin, X. Adsorption of aromatic acids on an aminated hypercrosslinked macroporous polymer. React. Funct. Polym. 2002, 53, 63–72. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Pawlak-Lemańska, K.; Szymusiak, H.; Tyrakowska, B.; Zieliński, R.; Soffers, A.E.; Rietjens, I.M.C.M. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free. Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- De Smedt, C.; Ferrer, F.; Leus, K.; Spanoghe, P. Removal of Pesticides from Aqueous Solutions by Adsorption on Zeolites as Solid Adsorbents. Adsorpt. Sci. Technol. 2015, 33, 457–485. [Google Scholar] [CrossRef] [Green Version]
- Ebadi, A.; Soltan, J.; Khudiev, A. What is the correct form of BET isotherm for modeling liquid phase adsorption? Adsorption 2009, 15, 65–73. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinet, L.; Dammak, L. Identification of phenolic compounds and their fouling mechanisms in ion-exchange membranes used at an industrial scale for wine tartaric stabilization by electrodialysis. Sep. Purif. Technol. 2020, 233, 115995. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. (Eds.) Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; ISBN 978-0-387-74116-1. [Google Scholar]
- Sholokhova, A.Y.; Eliseeva, T.; Voronyuk, I.V. Sorption of vanillin on highly basic anion exchanger under static conditions. Russ. J. Phys. Chem. A 2017, 91, 2237–2243. [Google Scholar] [CrossRef]
- Sholokhova, A.Y.; Eliseeva, T.V.; Buryak, A.K. The Influence of Experimental Factors on the Sorption of Vanillin by a Macroporous Highly Basic Anion Exchanger with a Low Degree of Cross-Linking. Colloid J. 2019, 81, 621–625. [Google Scholar] [CrossRef]
- Giles, C.H.; D’Silva, A.P.; A Easton, I. A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J. Colloid Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Anthocyanin Pigments-Bioactivity and Coloring Properties. J. Food Sci. 2006, 69, C419–C425. [Google Scholar] [CrossRef]











| Anthocyanin | Concentration, mg dm−3 |
|---|---|
| Cyanidin-3-galactoside | 105.6 |
| Cyanidin-3-glucoside | 20.8 |
| Cyanidin-3-arabinoside | 102.0 |
| Peonidine-3-galactoside | 51.8 |
| Peonidine-3-glucoside | 12.3 |
| Peonidine-3-arabinoside | 280.3 |
| Total concentration | 572.8 |
| IERs | Fixed Groups | Polymer Matrix | True Density, g cm−3 | Water Content, gH2O /gwet, % 1 | Total Ion-Exchange Capacity, mmol cm−3 |
|---|---|---|---|---|---|
| Anion-exchange resins | |||||
| EDE-10P | =NH+,-NH2+, ≡N | ECH+PEPA | 1.17 [82] | 46 | 2.34 [83] |
| AV-17-2P | -N+(CH3)3 | DVB2%+PS | 1.06 1 | 61 | 0.80 ± 0.02 [84] |
| AV-17-8 | -N+(CH3)3 | DVB8%+PS | 1.13 [82] | 45 | 1.12 ± 0.02 [84] |
| Cation-exchange resin | |||||
| KU-2-8 | -SO3− | DVB8%+PS | 1.25 [82] | 56 | 1.80 ± 0.01 [84] |
| External Solution | KU-2-8 | AV-17-8 | AV-17-2P | EDE-10P | |
|---|---|---|---|---|---|
| pH | 3.00 ± 0.05 | 2.5 ± 0.5 | 7.0 ± 0.6 | 6.6 ± 0.4 | 8 ± 1 |
| Anthocyanins’ electrical charge | 1+ | 1+ | 0 | 0 | 0, 1– (a small amount) |
| *CIER/CS0 (t = 180 min) 1 | - | 20 ± 1 | 13 ± 1 | 15 ± 1 | 29 |
| pH | 6.00 ± 0.05 | 4.0 ± 0.5 | 8 ± 1 | 8.0 ± 0.5 | 10.5 ± 0.5 |
| Anthocyanins’ electrical charge | 0 | 0, 1+ (a small amount) | 1– | 1– | 2– |
| *CIER/CS0 (t = 180 min) 1 | - | 10 ± 1 | 22 ± 1 | 22 ± 1 | 26 ± 1 |
| pH | 9.00 ± 0.05 | 7.6 ± 0.6 | 10.5 ± 1 | 11.0 ± 0.5 | 12 ± 1 |
| Anthocyanins’ electrical charge | 1– | 0, 1– (a small amount) | 2– | 2– | 2– |
| *CIER/CS0 (t = 180 min) 1 | - | 12 ± 1 | 22 ± 1 | 22 ± 1 | 14 ± 1 |
| KU-2-8 | АВ-17-8 | |||||||
|---|---|---|---|---|---|---|---|---|
| Langmuir (Section I) | Freundlich (Section I) | Freundlich (Section II) | BET | Langmuir (Section I) | Freundlich (Section I) | Freundlich (Section II) | BET | |
| R2 | 0.947 | 0.998 | 0.982 | 0.884 | 0.922 | 0.961 | 0.982 | 0.844 |
| qm (mg gdry–1) | −0.930 | - | 1.550 | −0.251 | - | 0.850 | ||
| KL (mg dm–3)−1 | −0.040 | - | - | −0.050 | - | - | ||
| KF (mg dm–3)−1 | - | 0.002 | 0.420 | - | - | 0.003 | 0.154 | - |
| Ks (mg dm–3)−1 | - | - | 0.110 | - | - | 0.067 | ||
| KB (mg dm–3)−1 | - | - | 0.008 | - | - | 0.008 | ||
| Buffer Solution | pH |
|---|---|
| Potassium chloride (0.05 mol kg−1) + hydrochloric acid (0.097 mol kg−1) | 1.01 |
| Potassium tetraoxalate dihydrate (0.05 mol kg−1) | 1.65 |
| Potassium hydrogen tartrate (0.05 mol kg−1) | 3.56 |
| Potassium hydrogen phthalate (0.05 mol kg−1) | 4.01 |
| Potassium dihydrogen phosphate (0.26 mol kg−1) + Disodium hydrogen phosphate (0.04 mol kg−1) | 7.00 |
| Sodium tetraborate decahydrate (0.01 mol kg−1) | 9.18 |
| Sodium carbonate (0.025 mol kg−1) + sodium hydrogen carbonate (0.025 mol kg−1) | 10.01 |
| Sodium carbonate (0.05 mol kg−1) + sodium tetraborate decahydrate (0.00135 mol kg−1) | 11.00 |
| Potassium chloride (0.05 mol kg−1) + sodium hydroxide (0.012 mol kg−1) | 12.00 |
| Model | Non-Linear Form | Linear Form |
|---|---|---|
| Langmuir | ||
| Freundlich | ||
| BET |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices. Int. J. Mol. Sci. 2020, 21, 7874. https://doi.org/10.3390/ijms21217874
Pismenskaya N, Sarapulova V, Klevtsova A, Mikhaylin S, Bazinet L. Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices. International Journal of Molecular Sciences. 2020; 21(21):7874. https://doi.org/10.3390/ijms21217874
Chicago/Turabian StylePismenskaya, Natalia, Veronika Sarapulova, Anastasia Klevtsova, Sergey Mikhaylin, and Laurent Bazinet. 2020. "Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices" International Journal of Molecular Sciences 21, no. 21: 7874. https://doi.org/10.3390/ijms21217874
APA StylePismenskaya, N., Sarapulova, V., Klevtsova, A., Mikhaylin, S., & Bazinet, L. (2020). Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices. International Journal of Molecular Sciences, 21(21), 7874. https://doi.org/10.3390/ijms21217874








