Mechanisms of Arrhythmias in the Brugada Syndrome
Abstract
1. Introduction
2. The Right Ventricular Outflow Tract
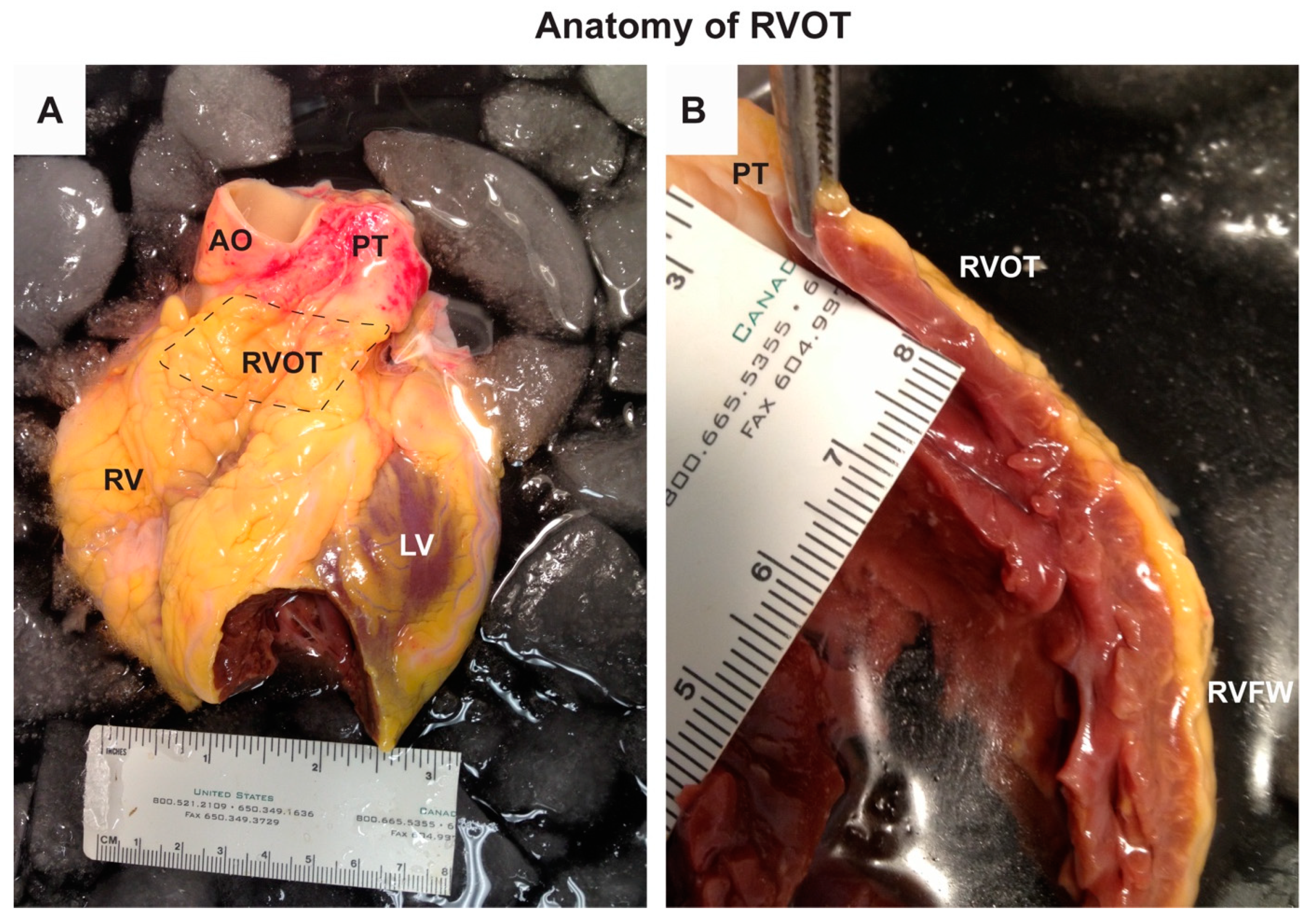
2.1. Anatomy of the RVOT
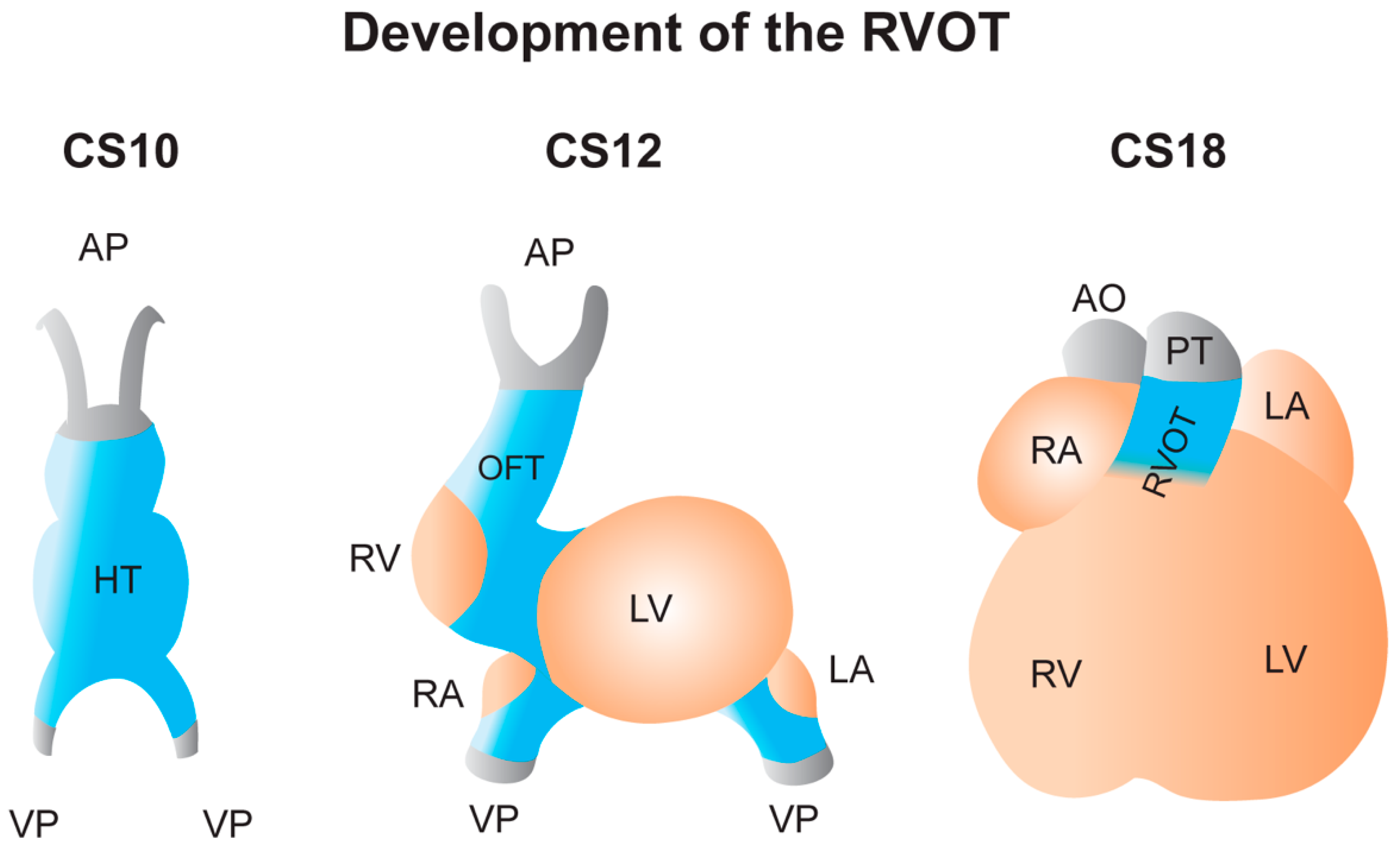
2.2. Development of the RVOT
2.3. Molecular and Electrophysiological Makeup of the RVOT
3. Mechanisms Underlying Arrhythmias in Brugada Syndrome
3.1. The Settlement of the Debate about the Depolarization and Repolarization Hypothesis
3.2. Structural Abnormalities Appear Crucial for Explaining Arrhythmias in Brugada Syndrome
4. Towards a Molecular Understanding of Brugada Syndrome
4.1. Known Genes Involved in the Brugada Syndrome Mechanism
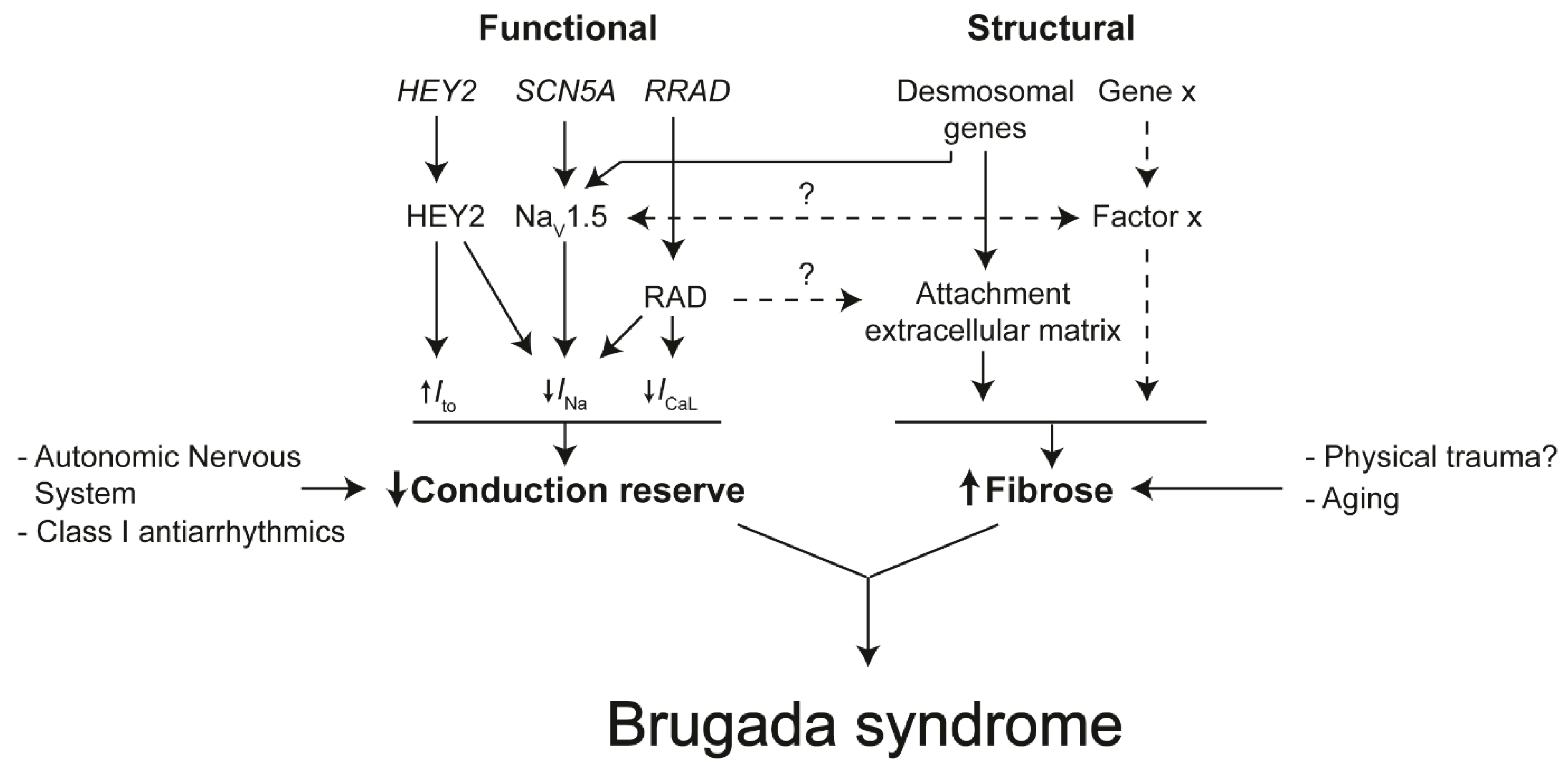
4.2. The Role of The Intercalated Disk in Reducing Conduction Reserve and Formation of Fibrosis
4.3. Insights From Human Induced Pluripotent Stem Cell Models
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Haïssaguerre, M.; Extramiana, F.; Hocini, M.; Cauchemez, B.; Jaïs, P.; Cabrera, J.A.; Farre, G.; Leenhardt, A.; Sanders, P.; Scavée, C.; et al. Mapping and ablation of ventricular fibrillation associated with long-QT and brugada syndromes. Circulation 2003, 108, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Nagase, S.; Miura, D.; Miura, A.; Hiramatsu, S.; Tada, T.; Murakami, M.; Nishii, N.; Nakamura, K.; Morita, S.T.; et al. Differential effects of cardiac sodium channel mutations on initiation of ventricular arrhythmias in patients with brugada syndrome. Heart Rhythm 2009, 6, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Viskin, S.; Rosso, R.; Rogowski, O.; Belhassen, B. The “short-coupled” variant of right ventricular outflow ventricular tachycardia: A not-so-benign form of benign ventricular tachycardia? J. Cardiovasc. Electrophysiol. 2005, 16, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Iwai, S.; Markowitz, S.M.; Shah, B.K.; Stein, K.M.; Lerman, B.B. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J. Am. Coll. Cardiol. 2007, 49, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Waxman, H.L.; Marchlinski, F.E.; Simson, M.B.; Cassidy, D.; Josephson, M.E. Right ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation 1983, 68, 917–927. [Google Scholar] [CrossRef]
- Sieira, J.; Dendramis, G.; Brugada, P. Pathogenesis and management of brugada syndrome. Nat. Rev. Cardiol. 2016, 13, 744–756. [Google Scholar] [CrossRef]
- Brugada, P.; Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. Multicent. Rep. J. Am. Coll Cardiol. 1992, 20, 1391–1396. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Antzelevitch, C.; Borggrefe, M.; Brugada, J.; Brugada, R.; Brugada, P.; Corrado, D.; Hauer, R.N.W.; Kass, R.S.; Nademanee, K.; et al. Proposed diagnostic criteria for the Brugada syndrome: Consensus report. Circulation 2002, 106, 2514–2519. [Google Scholar] [CrossRef]
- Brugada, J.; Brugada, P. Further characterization of the syndrome of right bundle branch block, ST segment elevation, and sudden cardiac death. J. Cardiovasc. Electrophysiol. 1997, 8, 325–331. [Google Scholar] [CrossRef]
- Rolf, S.; Bruns, H.J.; Wichter, T.; Kirchhof, P.; Ribbing, M.; Wasmer, K.; Paul, M.; Breithardt, G.; Haverkamp, W.; Eckardt, L. The ajmaline challenge in Brugada syndrome: Diagnostic impact, safety, and recommended protocol. Eur. Heart J. 2003, 24, 1104–1112. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Brugada, P.; Borggrefe, M.; Brugada, J.; Brugada, R.; Corrado, D.; Gussak, I.; LeMarec, H.; Nademanee, K.; Riera, A.R.P.; et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005, 111, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Kapplinger, J.D.; Tester, D.J.; Alders, M.; Benito, B.; Berthet, M.; Brugada, J.; Brugada, P.; Fressart, V.; Guerchicoff, A.; Harris-Kerr, C.; et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 2010, 7, 33–46. [Google Scholar] [CrossRef]
- Nielsen, M.W.; Holst, A.G.; Olesen, S.P.; Olesen, M.S. The genetic component of brugada syndrome. Front. Physiol. 2013, 4, 179. [Google Scholar] [CrossRef]
- Hu, D.; Barajas-Martínez, H.; Pfeiffer, R.; Dezi, F.; Pfeiffer, J.; Buch, T.; Betzenhauser, M.J.; Belardinelli, L.; Kahlig, K.M.; Rajamani, S.; et al. Mutations in SCN10A are responsible for a large fraction of cases of brugada syndrome. J. Am. Coll. Cardiol. 2014, 64, 66–79. [Google Scholar] [CrossRef]
- Minier, M.; Probst, V.; Berthome, P.; Tixier, R.; Briand, J.; Geoffroy, O.; Clementy, N.; Mansourati, J.; Jesel, L.; Dupuis, J.M.; et al. Age at diagnosis of brugada syndrome: Influence on clinical characteristics and risk of arrhythmia. Heart Rhythm 2020, 17, 743–749. [Google Scholar] [CrossRef]
- Sarquella-Brugada, G.; Campuzano, O.; Arbelo, E.; Brugada, J.; Brugada, R. Brugada syndrome: Clinical and genetic findings. Genet. Med. 2016, 18, 3–12. [Google Scholar] [CrossRef]
- Andorin, A.; Behr, E.R.; Denjoy, I.; Crotti, L.; Dagradi, F.; Jesel, L.; Sacher, F.; Petit, B.; Mabo, P.; Maltret, A.; et al. Impact of clinical and genetic findings on the management of young patients with brugada syndrome. Heart Rhythm 2016, 13, 1274–1282. [Google Scholar] [CrossRef]
- Oe, H.; Takagi, M.; Tanaka, A.; Namba, M.; Nishibori, Y.; Nishida, Y.; Kawarabayashi, T.; Yoshiyama, M.; Nishimoto, M.; Tanaka, K.; et al. Prevalence and clinical course of the juveniles with brugada-type ECG in Japanese population. PACE-Pacing Clin. Electrophysiol. 2005, 28, 549–554. [Google Scholar] [CrossRef]
- Sieira, J.; Brugada, P. The definition of the Brugada syndrome. Eur. Heart J. 2017, 38, 3029–3034. [Google Scholar] [CrossRef]
- Anderson, R.D.; Kumar, S.; Parameswaran, R.; Wong, G.; Voskoboinik, A.; Sugumar, H.; Watts, T.; Sparks, P.B.; Morton, J.B.; McLellan, A.; et al. Differentiating right-and left-sided outflow tract ventricular arrhythmias: Classical ECG signatures and prediction algorithms. Circ. Arrhythmia Electrophysiol. 2019, 12, e007392. [Google Scholar]
- Asirvatham, S.J. Correlative anatomy for the invasive electrophysiologist: Outflow tract and supravalvar arrhythmia. J. Cardiovasc. Electrophysiol. 2009, 20, 955–968. [Google Scholar]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92, i2–i13. [Google Scholar]
- Ghonim, S.; Voges, I.; Gatehouse, P.D.; Keegan, J.; Gatzoulis, M.A.; Kilner, P.J.; Babu-Narayan, S.V. Myocardial architecture, mechanics, and fibrosis in congenital heart disease. Front. Cardiovasc. Med. 2017, 4, 30. [Google Scholar] [CrossRef]
- Roney, C.H.; Bayer, J.D.; Cochet, H.; Meo, M.; Dubois, R.; Jaïs, P.; Vigmond, E.J. Variability in pulmonary vein electrophysiology and fibrosis determines arrhythmia susceptibility and dynamics. PLoS Comput. Biol. 2018, 14, e1006166. [Google Scholar]
- Roney, C.H.; Bayer, J.D.; Dubois, R.; Meo, M.; Cochet, H.; Jäis, P.; Vigmond, E.J. The combination of pulmonary vein electrophysiology and atrial fibrosis determines driver location. In Computing in Cardiology; IEEE: Rennes, France, 2017. [Google Scholar]
- Oken, D.E.; Boucek, R.J. Quantitation of collagen in human myocardium. Circ. Res. 1957, 5, 357–361. [Google Scholar]
- Miles, C.; Westaby, J.; Ster, I.C.; Asimaki, A.; Boardman, P.; Joshi, A.; Papadakis, M.; Sharma, S.; Behr, E.R.; Sheppard, M.N. Morphometric characterization of collagen and fat in normal ventricular myocardium. Cardiovasc. Pathol. 2020, 48, 107224. [Google Scholar] [CrossRef]
- Basso, C.; Thiene, G. Adipositas cordis, fatty infiltration of the right ventricle, and arrhythmogenic right ventricular cardiomyopathy. Just Matter Fat? Cardiovasc. Pathol. 2005, 14, 37–41. [Google Scholar] [CrossRef]
- Tansey, D.K.; Aly, Z.; Sheppard, M.N. Fat in the right ventricle of the normal heart. Histopathology 2005, 46, 98–104. [Google Scholar]
- Burke, A.P.; Farb, A.; Tashko, G.; Virmani, R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: Are they different diseases? Circulation 1998, 97, 1571–1580. [Google Scholar]
- Kelly, A.; Salerno, S.; Connolly, A.; Bishop, M.; Charpentier, F.; Stølen, T.; Smith, G.L. Normal interventricular differences in tissue architecture underlie right ventricular susceptibility to conduction abnormalities in a mouse model of brugada syndrome. Cardiovasc. Res. 2018, 114, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Boukens, B.J.; Remme, C.A. Intramural clefts and structural discontinuities in brugada syndrome: The missing gap? Cardiovasc. Res. 2018, 114, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Boukens, B.J.; Sulkin, M.S.; Gloschat, C.R.; Ng, F.S.; Vigmond, E.J.; Efimov, I.R. Transmural APD gradient synchronizes repolarization in the human left ventricular wall. Cardiovasc. Res. 2015, 108, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Felker, A.; Prummel, K.D.; Merks, A.M.; Mickoleit, M.; Brombacher, E.C.; Huisken, J.; Panáková, D.; Mosimann, C. Continuous addition of progenitors forms the cardiac ventricle in zebrafish. Nat. Commun. 2018, 9, 2001. [Google Scholar] [CrossRef] [PubMed]
- Desgrange, A.; Le Garrec, J.-F.; Meilhac, S.M. Left-right asymmetry in heart development and disease: Forming the right loop. Development 2018, 145, dev162776. [Google Scholar] [CrossRef]
- Boukens, B.J.; Christoffels, V.M.; Coronel, R.; Moorman, A.F.M. Developmental basis for electrophysiological heterogeneity in the ventricular and outflow tract myocardium as a substrate for life-threatening ventricular arrhythmias. Circ. Res. 2009, 104, 19–31. [Google Scholar] [CrossRef]
- van Eif, V.W.W.; Stefanovic, S.; van Duijvenboden, K.; Bakker, M.; Wakker, V.; de Gier-de Vries, C.; Zaffran, S.; Verkerk, A.O.; Boukens, B.J.; Christoffels, V.M. Transcriptome analysis of mouse and human sinoatrial node cells reveals a conserved genetic program. Development 2019, 146, dev173161. [Google Scholar] [CrossRef]
- Boukens, B.J.; Sylva, M.; de Gier-de Vries, C.; Remme, C.A.; Bezzina, C.R.; Christoffels, V.M.; Coronel, R. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ. Res. 2013, 113, 137–141. [Google Scholar] [CrossRef]
- Yelbuz, T.M.; Waldo, K.L.; Kumiski, D.H.; Stadt, H.A.; Wolfe, R.R.; Leatherbury, L.; Kirby, M.L. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation 2002, 106, 504–510. [Google Scholar] [CrossRef]
- Bajolle, F.; Zaffran, S.; Kelly, R.G.; Hadchouel, J.; Bonnet, D.; Brown, N.A.; Buckingham, M.E. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ. Res. 2006, 98, 421–428. [Google Scholar] [CrossRef]
- Inman, K.E.; Caiaffa, C.D.; Melton, K.R.; Sandell, L.L.; Achilleos, A.; Kume, T.; Trainor, P.A. Foxc2 is required for proper cardiac neural crest cell migration, outflow tract septation, and ventricle expansion. Dev. Dyn. 2018, 247, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Sicouri, S.; Litovsky, S.H.; Lukas, A.; Krishnan, S.C.; Di Diego, J.M.; Gintant, G.A.; Liu, D.W. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ. Res. 1991, 69, 1427–1449. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.C.; Shaw, R.M.; Rudy, Y. Effects of I(Kr) and I(Ks) heterogeneity on action potential duration and its rate dependence: A simulation study. Circulation 1999, 99, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Poelzing, S.; Akar, F.G.; Baron, E.; Rosenbaum, D.S. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2001–H2009. [Google Scholar] [CrossRef]
- Poelzing, S.; Rosenbaum, D.S. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1762–H1770. [Google Scholar] [CrossRef] [PubMed]
- Strom, M.; Wan, X.; Poelzing, S.; Ficker, E.; Rosenbaum, D.S. Gap junction heterogeneity as mechanism for electrophysiologically distinct properties across the ventricular wall. Am. J. Physiol Heart Circ. Physiol. 2010, 298, H787–H794. [Google Scholar] [CrossRef][Green Version]
- Gaborit, N.; Le Bouter, S.; Szuts, V.; Varro, A.; Escande, D.; Nattel, S.; Demolombe, S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007, 582, 675–693. [Google Scholar] [CrossRef]
- Boukens, B.J.; Walton, R.; Meijborg, V.M.; Coronel, R. Transmural electrophysiological heterogeneity, the T-wave and ventricular arrhythmias. Prog. Biophys. Mol. Biol. 2016, 122, 202–214. [Google Scholar] [CrossRef]
- Boukens, B.J.; Christoffels, V.M. Electrophysiological patterning of the heart. Pediatr. Cardiol. 2012, 25, 900–906. [Google Scholar] [CrossRef]
- Harvey, R.P. Patterning the vertebrate heart. Nat. Rev. Genet. 2002, 3, 544–556. [Google Scholar] [CrossRef]
- Black, B.L. Transcriptional pathways in second heart field development. Semin. Cell Dev. Biol. 2007, 18, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Boukens, B.J.; Coronel, R.; Christoffels, V.M. Embryonic development of the right ventricular outflow tract and arrhythmias. Heart Rhythm 2016, 13, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Aanhaanen, W.T.J.; Mommersteeg, M.T.M.; Norden, J.; Wakker, V.; de Gier-de Vries, C.; Anderson, R.H.; Kispert, A.; Moorman, A.F.M. Developmental origin, growth, and three-dimensional architecture of the atrioventricular conduction axis of the mouse heart. Circ. Res. 2010, 107, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Nakagawa, M.; Kajimoto, M.; Nobe, S.; Ooie, T.; Ichinose, M.; Yonemochi, H.; Ono, N.; Shimada, T.; Saikawa, T. Heterogeneous expression of connexin 43 in the myocardium of rabbit right ventricular outflow tract. Life Sci. 2005, 77, 52–59. [Google Scholar] [CrossRef]
- Park, D.S.; Cerrone, M.; Morley, G.; Vasquez, C.; Fowler, S.; Liu, N.; Bernstein, S.A.; Liu, F.Y.; Zhang, J.; Rogers, C.S.; et al. Genetically engineered SCN5A mutant pig hearts exhibit conduction defects and arrhythmias. J. Clin. Investig. 2015, 125, 403–412. [Google Scholar] [CrossRef]
- Remme, C.A.; Verkerk, A.O.; Nuyens, D.; van Ginneken, A.C.G.; van Brunschot, S.; Belterman, C.N.W.; Wilders, R.; van Roon, M.A.; Tan, H.L.; Wilde, A.A.M.; et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation 2006, 114, 2584–2594. [Google Scholar] [CrossRef]
- Zhang, Y.; Guzadhur, L.; Jeevaratnam, K.; Salvage, S.C.; Matthews, G.D.K.; Lammers, W.J.; Lei, M.; Huang, C.L.H.; Fraser, J.A. Arrhythmic substrate, slowed propagation and increased dispersion in conduction direction in the right ventricular outflow tract of murine Scn5a+/-hearts. Acta Physiol. 2014, 211, 559–573. [Google Scholar] [CrossRef]
- Heidecker, B. When high throughput meets mechanistic studies: A state-of-the-art approach in Brugada syndrome. Circ. Res. 2017, 121, 483–485. [Google Scholar] [CrossRef]
- Veerman, C.C.; Podliesna, S.; Tadros, R.; Lodder, E.M.; Mengarelli, I.; de Jonge, B.; Beekman, L.; Barc, J.; Wilders, R.; Wilde, A.A.M.; et al. The brugada syndrome susceptibility gene HEY2 modulates cardiac transmural ion channel patterning and electrical heterogeneity. Circ. Res. 2017, 121, 537–548. [Google Scholar] [CrossRef]
- Anderson, D.J.; Kaplan, D.I.; Bell, K.M.; Koutsis, K.; Haynes, J.M.; Mills, R.J.; Phelan, D.G.; Qian, E.L.; Leitoguinho, A.R.; Arasaratnam, D.; et al. NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nat. Commun. 2018, 9, 1373. [Google Scholar]
- Miao, L.; Li, J.; Jun, L.; Tian, X.; Lu, Y.; Hu, S.; Shieh, D.; Kanai, R.; Zhou, B.-Y.; Zhou, B.; et al. Notch signaling regulates Hey2 expression in a spatiotemporal dependent manner during cardiac morphogenesis and trabecular specification. Sci Rep. 2018, 8, 2678. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Barc, J.; Mizusawa, Y.; Remme, C.A.; Gourraud, J.-B.; Simonet, F.; Verkerk, A.O.; Schwartz, P.J.; Crotti, L.; Dagradi, F.; et al. Common variants at SCN5A-SCN10A and HEY2 are associated with brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 2013, 45, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Remme, C.A.; Verkerk, A.O.; Hoogaars, W.M.H.; Aanhaanen, W.T.J.; Scicluna, B.P.; Annink, C.; van den Hoff, M.J.B.; Wilde, A.A.M.; van Veen, T.A.B.; Veldkamp, M.W.; et al. The cardiac sodium channel displays differential distribution in the conduction system and transmural heterogeneity in the murine ventricular myocardium. Basic Res. Cardiol. 2009, 104, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, N.; Portero, V.; Al Sayed, Z.R.; Gourraud, J.-B.; Dilasser, F.; Jesel, L.; Guo, H.; Wu, H.; Gaborit, N.; Guilluy, C.; et al. RRAD mutation causes electrical and cytoskeletal defects in cardiomyocytes derived from a familial case of brugada syndrome. Eur. Heart J. 2019, 40, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Yada, H.; Murata, M.; Shimoda, K.; Yuasa, S.; Kawaguchi, H.; Ieda, M.; Adachi, T.; Murata, M.; Ogawa, S.; Fukuda, K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ. Res. 2007, 101, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Brugada, R.; Roberts, R. Brugada Syndrome: Why are there multiple answers to a simple question? Circulation 2001, 104, 3017–3019. [Google Scholar] [CrossRef]
- Meregalli, P.; Wilde, A.; Tan, H. Pathophysiological mechanisms of brugada syndrome: Depolarization disorder, repolarization disorder, or more? Cardiovasc. Res. 2005, 67, 367–378. [Google Scholar] [CrossRef]
- Nademanee, K.; Wilde, A.A.M. Repolarization versus depolarization defects in brugada syndrome: A tale of 2 different electrophysiologic settings? J. Am. Coll. Cardiol. Clin. Electrophysiol. 2017, 3, 364–366. [Google Scholar]
- Wilde, A.A.M.; Postema, P.G.; Di Diego, J.M.; Viskin, S.; Morita, H.; Fish, J.M.; Antzelevitch, C. The pathophysiological mechanism underlying brugada syndrome: Depolarization versus repolarization. J. Mol. Cell. Cardiol. 2010, 49, 543–553. [Google Scholar] [CrossRef]
- Yan, G.X.; Antzelevitch, C. Cellular basis for the brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 1999, 100, 1660–1666. [Google Scholar] [CrossRef]
- Lukas, A.; Antzelevitch, C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia: Role of the transient outward current. Circulation 1993, 88, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- Di Diego, J.M.; Sun, Z.Q.; Antzelevitch, C. I(to) and action potential notch are smaller in left vs. Right canine ventricular epicardium. Am. J. Physiol. 1996, 271, H548–H561. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Belardinelli, L. The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J. Cardiovasc. Electrophysiol. J. Cardiovasc. Electrophysiol. 2006, 17, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, L.; Kirchhof, P.; Johna, R.; Breithardt, G.; Borggrefe, M.; Haverkamp, W. Transient local changes in right ventricular monophasic action potentials due to ajmaline in a patient with Brugada syndrome. J. Cardiovasc. Electrophysiol. 1999, 10, 1010–1015. [Google Scholar] [CrossRef]
- Shimizu, W.; Matsuo, K.; Takagi, M.; Tanabe, Y.; Aiba, T.; Taguchi, A.; Suyama, K.; Kurita, T.; Aihara, N.; Kamakura, S. Body surface distribution and response to drugs of ST segment elevation in brugada syndrome: Clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J. Cardiovasc. Electrophysiol. 2000, 11, 396–404. [Google Scholar] [CrossRef]
- Takagi, M.; Aihara, N.; Kuribayashi, S.; Taguchi, A.; Shimizu, W.; Kurita, T.; Suyama, K.; Kamakura, S.; Hamada, S.; Takamiya, M. Localized right ventricular morphological abnormalities detected by electron-beam computed tomography represent arrhythmogenic substrates in patients with the brugada syndrome. Eur. Heart J. 2001, 22, 1032–1041. [Google Scholar] [CrossRef]
- Kurita, T.; Shimizu, W.; Inagaki, M.; Suyama, K.; Taguchi, A.; Satomi, K.; Aihara, N.; Kamakura, S.; Kobayashi, J.; Kosakai, Y. The electrophysiologic mechanism of ST-segment elevation in brugada syndrome. J. Am. Coll. Cardiol. 2002, 40, 330–334. [Google Scholar] [CrossRef]
- Veltmann, C.; Papavassiliu, T.; Konrad, T.; Doesch, C.; Kuschyk, J.; Streitner, F.; Haghi, D.; Michaely, H.J.; Schoenberg, S.O.; Borggrefe, M.; et al. Insights into the location of type I ECG in patients with brugada syndrome: Correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm 2012, 9, 414–421. [Google Scholar] [CrossRef]
- Coronel, R.; Casini, S.; Koopmann, T.T.; Wilms-Schopman, F.J.G.; Verkerk, A.O.; de Groot, J.R.; Bhuiyan, Z.; Bezzina, C.R.; Veldkamp, M.W.; Linnenbank, A.C.; et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of brugada syndrome. Circulation 2005, 112, 2769–2777. [Google Scholar] [CrossRef]
- Priori, S.G.; Napolitano, C.; Gasparini, M. Letters Regarding Article by Coronel et al. “Right ventricular fibrosis and conduction delay in a patient with clinical signs of brugada syndrome: A combined electrophysiological, genetic, histopathologic, and computational study”. Circulation 2005, 113, 2769–2777. [Google Scholar] [CrossRef]
- Nademanee, K.; Veerakul, G.; Chandanamattha, P.; Chaothawee, L.; Ariyachaipanich, A.; Jirasirirojanakorn, K.; Likittanasombat, K.; Bhuripanyo, K.; Ngarmukos, T. Prevention of ventricular fibrillation episodes in brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011, 123, 1270–1279. [Google Scholar] [PubMed]
- Sacher, F.; Jesel, L.; Jais, P.; Haïssaguerre, M. Insight into the mechanism of brugada syndrome: Epicardial substrate and modification during ajmaline testing. Heart Rhythm 2014, 11, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Szél, T.; Antzelevitch, C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of Brugada syndrome. J. Am. Coll. Cardiol. 2014, 63, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.M.W.; Ng, F.S.; Yao, C.; Roney, C.; Taraborrelli, P.; Linton, N.W.F.; Whinnett, Z.I.; Lefroy, D.C.; Davies, D.W.; Boon Lim, P.; et al. ST-elevation magnitude correlates with right ventricular outflow tract conduction delay in type I brugada ECG. Circ. Arrhythmia Electrophysiol. 2017, 10, e005107. [Google Scholar]
- Coronel, R.; de Bakker, J.M.T.; Wilms-Schopman, F.J.G.; Opthof, T.; Linnenbank, A.C.; Belterman, C.N.; Janse, M.J. Monophasic action potentials and activation recovery intervals as measures of ventricular action potential duration: Experimental evidence to resolve some controversies. Heart Rhythm 2006, 3, 1043–1050. [Google Scholar] [CrossRef]
- Nagase, S.; Kusano, K.F.; Morita, H.; Nishii, N.; Banba, K.; Watanabe, A.; Hiramatsu, S.; Nakamura, K.; Sakuragi, S.; Ohe, T. Longer repolarization in the epicardium at the right ventricular outflow tract causes type 1 electrocardiogram in patients with brugada syndrome. J. Am. Coll. Cardiol. 2008, 51, 1154–1161. [Google Scholar]
- Antzelevitch, C.; Patocskai, B. Ajmaline-induced slowing of conduction in the right ventricular outflow tract cannot account for ST elevation in patients with type I brugada ECG. Circ. Arrhythmia Electrophysiol. 2017, 10, e005775. [Google Scholar]
- Patocskai, B.; Yoon, N.; Antzelevitch, C. Mechanisms underlying epicardial radiofrequency ablation to suppress arrhythmogenesis in experimental models of brugada syndrome. JACC Clin. Electrophysiol. 2017, 3, 353–363. [Google Scholar]
- Janse, M.J.; Coronel, R.; Opthof, T.; Sosunov, E.A.; Anyukhovsky, E.P.; Rosen, M.R. Repolarization gradients in the intact heart: Transmural or apico-basal? Prog. Biophys. Mol. Biol. 2012, 109, 6–15. [Google Scholar]
- Boukens, B.J.; Meijborg, V.M.F.; Belterman, C.N.; Opthof, T.; Janse, M.J.; Schuessler, R.B.; Coronel, R.; Efimov, I.R. Local transmural action potential gradients are absent in the isolated, intact dog heart but present in the corresponding coronary-perfused wedge. Physiol. Rep. 2017, 5, e13251. [Google Scholar] [CrossRef]
- Morita, H.; Zipes, D.P.; Fukushima-Kusano, K.; Nagase, S.; Nakamura, K.; Morita, S.T.; Ohe, T.; Wu, J. Repolarization heterogeneity in the right ventricular outflow tract: Correlation with ventricular arrhythmias in brugada patients and in an in vitro canine brugada model. Heart Rhythm 2008, 5, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Watanabe, I.; Takagi, Y.; Okumura, Y.; Ashino, S.; Kofune, M.; Kofune, T.; Shindo, A.; Sugimura, H.; Nakai, T.; et al. Endocardial electrograms from the right ventricular outflow tract after induced ventricular fibrillation in patients with brugada syndrome. Circ. J. 2007, 71, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Kusano, K.F.; Miura, D.; Nagase, S.; Nakamura, K.; Morita, S.T.; Ohe, T.; Zipes, D.P.; Wu, J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008, 118, 1697–1704. [Google Scholar] [CrossRef]
- Postema, P.G.; van Dessel, P.F.H.M.; de Bakker, J.M.T.; Dekker, L.R.C.; Linnenbank, A.C.; Hoogendijk, M.G.; Coronel, R.; Tijssen, J.G.P.; Wilde, A.A.M.; Tan, H.L. Slow and discontinuous conduction conspire in Brugada syndrome: A right ventricular mapping and stimulation study. Circ. Arrhythmia Electrophysiol. 2008, 1, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Postema, P.G.; van Dessel, P.F.H.M.; Kors, J.A.; Linnenbank, A.C.; van Herpen, G.; Ritsema van Eck, H.J.; van Geloven, N.; de Bakker, J.M.T.; Wilde, A.A.M.; Tan, H.L. Local depolarization abnormalities are the dominant pathophysiologic mechanism for type 1 electrocardiogram in brugada syndrome. A study of electrocardiograms, vectorcardiograms, and body surface potential maps during ajmaline provocation. J. Am. Coll. Cardiol. 2010, 55, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Letsas, K.P.; Efremidis, M.; Vlachos, K.; Georgopoulos, S.; Karamichalakis, N.; Asvestas, D.; Valkanas, K.; Korantzopoulos, P.; Liu, T.; Sideris, A. Right ventricular outflow tract high-density endocardial unipolar voltage mapping in patients with Brugada syndrome: Evidence for electroanatomical abnormalities. Europace 2018, 20, f57–f63. [Google Scholar] [CrossRef]
- Pappone, C.; Mecarocci, V.; Manguso, F.; Ciconte, G.; Vicedomini, G.; Sturla, F.; Votta, E.; Mazza, B.; Pozzi, P.; Borrelli, V.; et al. New electromechanical substrate abnormalities in high-risk patients with Brugada syndrome. Heart Rhythm 2020, 17, 637–645. [Google Scholar] [CrossRef]
- Martini, B.; Nava, A.; Thiene, G.; Buja, G.F.; Canciani, B.; Scognamiglio, R.; Daliento, L.; Dalla Volta, S. Ventricular fibrillation without apparent heart disease: Description of six cases. Am. Heart J. 1989, 118, 1203–1209. [Google Scholar] [CrossRef]
- Martini, B.; Cannas, S.; Nava, A. Brugada by any other name? Eur. Heart J. 2001, 22, 1835–1836. [Google Scholar] [CrossRef][Green Version]
- Frustaci, A.; Priori, S.G.; Pieroni, M.; Chimenti, C.; Napolitano, C.; Rivolta, I.; Sanna, T.; Bellocci, F.; Russo, M.A. Cardiac histological substrate in patients with clinical phenotype of brugada syndrome. Circulation 2005, 112, 3680–3687. [Google Scholar] [CrossRef]
- Hoogendijk, M.G.; Opthof, T.; Postema, P.G.; Wilde, A.A.M.; de Bakker, J.M.T.; Coronel, R. The brugada ECG pattern: A marker of channelopathy, structural heart disease, or neither? Toward a unifying mechanism of the brugada syndrome. Circ. Arrhythmia Electrophysiol. 2010, 3, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, M.G.; Potse, M.; Vinet, A.; de Bakker, J.M.T.; Coronel, R. ST segment elevation by current-to-load mismatch: An experimental and computational study. Heart Rhythm 2011, 8, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, K.; Raju, H.; de Noronha, S.V.; Papadakis, M.; Robinson, L.; Rothery, S.; Makita, N.; Kowase, S.; Boonmee, N.; Vitayakritsirikul, V.; et al. Fibrosis, connexin-43, and conduction abnormalities in the brugada syndrome. J. Am. Coll. Cardiol. 2015, 66, 1976–1986. [Google Scholar] [CrossRef]
- Zumhagen, S.; Spieker, T.; Rolinck, J.; Baba, H.A.; Breithardt, G.; Böcker, W.; Eckardt, L.; Paul, M.; Wichter, T.; Schulze-Bahr, E. Absence of pathognomonic or inflammatory patterns in cardiac biopsies from patients with brugada syndrome. Circ. Arrhythmia Electrophysiol. 2009, 2, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Watanabe, I.; Okumura, Y.; Takagi, Y.; Ashino, S.; Kofune, M.; Sugimura, H.; Nakai, T.; Kasamaki, Y.; Hirayama, A.; et al. Right ventricular histological substrate and conduction delay in patients with brugada syndrome. Int. Heart J. 2010, 51, 17–23. [Google Scholar] [CrossRef]
- Tada, H.; Aihara, N.; Ohe, T.; Yutani, C.; Hamada, S.; Miyanuma, H.; Takamiya, M.; Kamakura, S. Arrhythmogenic right ventricular cardiomyopathy underlies syndrome of right bundle branch block, ST-segment elevation, and sudden death. Am. J. Cardiol. 1998, 81, 519–522. [Google Scholar] [CrossRef]
- Corrado, D.; Nava, A.; Buja, G.; Martini, B.; Fasoli, G.; Oselladore, L.; Turrini, P.; Thiene, G. Familial cardiomyopathy underlies syndrome of right bundle branch block, ST segment elevation and sudden death. J. Am. Coll. Cardiol. 1996, 27, 443–448. [Google Scholar] [CrossRef]
- van Rijen, H.V.M.; de Bakker, J.M.T. Penetrance of monogenetic cardiac conduction diseases. A matter of conduction reserve? Cardiovasc. Res. 2007, 76, 379–380. [Google Scholar] [CrossRef]
- Boyle, P.M.; Franceschi, W.H.; Constantin, M.; Hawks, C.; Desplantez, T.; Trayanova, N.A.; Vigmond, E.J. New insights on the cardiac safety factor: Unraveling the relationship between conduction velocity and robustness of propagation. J. Mol. Cell. Cardiol. 2019, 128, 117–128. [Google Scholar] [CrossRef]
- Hoogendijk, M.G.; Potse, M.; Linnenbank, A.C.; Verkerk, A.O.; den Ruijter, H.M.; van Amersfoorth, S.C.M.; Klaver, E.C.; Beekman, L.; Bezzina, C.R.; Postema, P.G.; et al. Mechanism of right precordial ST-segment elevation in structural heart disease: Excitation failure by current-to-load mismatch. Heart Rhythm 2010, 7, 238–248. [Google Scholar] [CrossRef]
- Joyner, R.W.; Kumar, R.; Wilders, R.; Jongsma, H.J.; Verheijck, E.E.; Golod, D.A.; van Ginneken, A.C.G.; Wagner, M.B.; Goolsby, W.N. Modulating L-type calcium current affects discontinuous cardiac action potential conduction. Biophys. J. 1996, 71, 237–245. [Google Scholar] [CrossRef]
- Rohr, S.; Kucera, J.P. Involvement of the calcium inward current in cardiac impulse propagation: Induction of unidirectional conduction block by nifedipine and reversal by bay K 8644. Biophys. J. 1997, 72, 754–766. [Google Scholar] [CrossRef]
- Huelsing, D.J.; Spitzer, K.W.; Cordeiro, J.M.; Pollard, A.E. Conduction between isolated rabbit purkinje and ventricular myocytes coupled by a variable resistance. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H1163–H1173. [Google Scholar] [CrossRef] [PubMed]
- Kléber, A.G.; Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef]
- Wellens, H.J.J.; Schwartz, P.J.; Lindemans, F.W.; Buxton, A.E.; Goldberger, J.J.; Hohnloser, S.H.; Huikuri, H.V.; Kääb, S.; La Rovere, M.T.; Malik, M. Risk stratification for sudden cardiac death: Current status and challenges for the future. Eur. Heart J. 2014, 35, 1642–1651. [Google Scholar] [CrossRef]
- Zhang, P.; Tung, R.; Zhang, Z.; Sheng, X.; Liu, Q.; Jiang, R.; Sun, Y.; Chen, S.; Yu, L.; Ye, Y.; et al. Characterization of the epicardial substrate for catheter ablation of brugada syndrome. Heart Rhythm 2016, 13, 2151–2158. [Google Scholar] [CrossRef]
- Veerakul, G.; Nademanee, K. Will we be able to cure brugada syndrome? Heart Rhythm 2016, 13, 2159–2160. [Google Scholar] [CrossRef]
- Ten Sande, J.N.; Coronel, R.; Conrath, C.E.; Driessen, A.H.G.; de Groot, J.R.; Tan, H.L.; Nademanee, K.; Wilde, A.A.M.; de Bakker, J.M.T.; van Dessel, P.F.H.M. ST-segment elevation and fractionated electrograms in brugada syndrome patients arise from the same structurally abnormal subepicardial RVOT area but have a different mechanism. Circ. Arrhythmia Electrophysiol. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Vigmond, E.J.; Efimov, I.R.; Rentschler, S.L.; Coronel, R.; Boukens, B.J. Fractionated electrograms with ST-segment elevation recorded from the human right ventricular outflow tract. Heart Case Rep. 2017, 3, 546–550. [Google Scholar] [CrossRef][Green Version]
- Kass, R.S.; Wiegers, S.E. The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac purkinje fibres. J. Physiol. 1982, 322, 541–558. [Google Scholar] [CrossRef]
- Marchal, G.A.; Verkerk, A.O.; Mohan, R.A.; Wolswinkel, R.; Boukens, B.J.D.; Remme, C.A. The sodium channel NaV1.5 impacts on early murine embryonic cardiac development, structure and function in a non-electrogenic manner. Acta Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rivaud, M.R.; Delmar, M.; Remme, C.A. Heritable arrhythmia syndromes associated with abnormal cardiac sodium channel function: Ionic and non-ionic mechanisms. Cardiovasc. Res. 2020, 116, 1557–1570. [Google Scholar] [PubMed]
- Cerrone, M.; Delmar, M. Desmosomes and the sodium channel complex: Implications for arrhythmogenic cardiomyopathy and brugada syndrome. Trends Cardiovasc. Med. 2014, 24, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, M.; Noorman, M.; Lin, X.; Chkourko, H.; Liang, F.X.; van der Nagel, R.; Hund, T.; Birchmeier, W.; Mohler, P.; van Veen, T.A.; et al. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc. Res. 2012, 95, 460–468. [Google Scholar] [PubMed]
- Corrado, D.; Zorzi, A.; Cerrone, M.; Rigato, I.; Mongillo, M.; Bauce, B.; Delmar, M. Relationship between arrhythmogenic right ventricular cardiomyopathy and brugada syndrome. Circ. Arrhythmia Electrophysiol. 2016, 9, e003631. [Google Scholar]
- Balse, E.; Steele, D.F.; Abriel, H.; Coulombe, A.; Fedida, D.; Hatem, S.N. Dynamic of ion channel expression at the plasma membrane of cardiomyocytes. Physiol. Rev. 2012, 92, 1317–1358. [Google Scholar]
- Van Tintelen, J.P.; Entius, M.M.; Bhuiyan, Z.A.; Jongbloed, R.; Wiesfeld, A.C.P.; Wilde, A.A.M.; van der Smagt, J.; Boven, L.G.; Mannens, M.M.A.M.; van Langen, I.M.; et al. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 2006, 113, 1650–1658. [Google Scholar] [CrossRef]
- Pieperhoff, S.; Schumacher, H.; Franke, W.W. The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur. J. Cell Biol. 2008, 87, 399–411. [Google Scholar] [CrossRef]
- Grossmann, K.S.; Grund, C.; Huelsken, J.; Behrend, M.; Erdmann, B.; Franke, W.W.; Birchmeier, W. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J. Cell Biol. 2004, 167, 149–160. [Google Scholar]
- Chen, S.N.; Gurha, P.; Lombardi, R.; Ruggiero, A.; Willerson, J.T.; Marian, A.J. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ. Res. 2014, 114, 454–468. [Google Scholar] [CrossRef]
- Dubash, A.D.; Kam, C.Y.; Aguado, B.A.; Patel, D.M.; Delmar, M.; Shea, L.D.; Green, K.J. Plakophilin-2 loss promotes TGF-β1/p38 MAPK-dependent fibrotic gene expression in cardiomyocytes. J. Cell Biol. 2016, 212, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, M.; Lin, X.; Zhang, M.; Agullo-Pascual, E.; Pfenniger, A.; Chkourko Gusky, H.; Novelli, V.; Kim, C.; Tirasawadichai, T.; Judge, D.P.; et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a brugada syndrome phenotype. Circulation 2014, 129, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Sato, P.Y.; Coombs, W.; Lin, X.; Nekrasova, O.; Green, K.J.; Isom, L.L.; Taffet, S.M.; Delmar, M. Interactions between ankyrin-G, plakophilin-2, and connexin43 at the cardiac intercalated disc. Circ. Res. 2011, 109, 193–201. [Google Scholar] [CrossRef]
- Jansen, J.A.; Noorman, M.; Musa, H.; Stein, M.; de Jong, S.; van der Nagel, R.; Hund, T.J.; Mohler, P.J.; Vos, M.A.; van Veen, T.A.; et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm 2012, 9, 600–607. [Google Scholar] [CrossRef]
- Lübkemeier, I.; Requardt, R.P.; Lin, X.; Sasse, P.; Andrié, R.; Schrickel, J.W.; Chkourko, H.; Bukauskas, F.F.; Kim, J.S.; Frank, M.; et al. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic Res. Cardiol. 2013, 108, 348. [Google Scholar] [CrossRef]
- Agullo-Pascual, E.; Lin, X.; Leo-Macias, A.; Zhang, M.; Liang, F.X.; Li, Z.; Pfenniger, A.; Lübkemeier, I.; Keegan, S.; Fenyo, D.; et al. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc. Res. 2014, 104, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Desplantez, T.; McCain, M.L.; Beauchamp, P.; Rigoli, G.; Rothen-Rutishauser, B.; Parker, K.K.; Kléber, A.G. Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current. Cardiovasc. Res. 2012, 94, 58–65. [Google Scholar] [CrossRef]
- Noorman, M.; Hakim, S.; Kessler, E.; Groeneweg, J.A.; Cox, M.G.P.J.; Asimaki, A.; van Rijen, H.V.M.; van Stuijvenberg, L.; Chkourko, H.; van der Heyden, M.A.G.; et al. Remodeling of the cardiac sodium channel, connexin43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm 2013, 10, 412–419. [Google Scholar] [CrossRef]
- Fidler, L.M.; Wilson, G.J.; Liu, F.; Cui, X.; Scherer, S.W.; Taylor, G.P.; Hamilton, R.M. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J. Cell Mol. Med. 2009, 13, 4219–4228. [Google Scholar] [CrossRef]
- Jansen, J.A.; van Veen, T.A.B.; de Jong, S.; van der Nagel, R.; van Stuijvenberg, L.; Driessen, H.; Labzowski, R.; Oefner, C.M.; Bosch, A.A.; Nguyen, T.Q.; et al. Reduced Cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ. Arrhythmia Electrophysiol. 2012, 5, 380–390. [Google Scholar] [CrossRef]
- Royer, A.; van Veen, T.A.B.; Le Bouter, S.; Marionneau, C.; Griol-Charhbili, V.; Léoni, A.L.; Steenman, M.; van Rijen, H.V.M.; Demolombe, S.; Goddard, C.A.; et al. Mouse model of SCN5A-linked hereditary Lenègre’s: Disease age-related conduction slowing and myocardial fibrosis. Circulation 2005, 111, 1738–1746. [Google Scholar]
- Sato, P.Y.; Musa, H.; Coombs, W.; Guerrero-Serna, G.; Patiño, G.A.; Taffet, S.M.; Isom, L.L.; Delmar, M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ. Res. 2009, 105, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, Z.; Loh, L.J.; Zhao, Y.; Li, G.; Liew, R.; Islam, O.; Wu, J.; Chung, Y.Y.; Teo, W.S.; et al. Identification of an I Na-dependent and I to-mediated proarrhythmic mechanism in cardiomyocytes derived from pluripotent stem cells of a Brugada syndrome patient. Sci. Rep. 2018, 8, 11246. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Sallam, K.; Wu, H.; Li, Y.; Itzhaki, I.; Garg, P.; Zhang, Y.; Vermglinchan, V.; Lan, F.; Gu, M.; et al. Patient-specific and genome-edited induced pluripotent stem cell–derived cardiomyocytes elucidate single-cell phenotype of brugada syndrome. J. Am. Coll. Cardiol. 2016, 68, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A brief review of current maturation methods for human induced pluripotent stem cells-derived cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.C.; Mengarelli, I.; Guan, K.; Stauske, M.; Barc, J.; Tan, H.L.; Wilde, A.A.M.; Verkerk, A.O.; Bezzina, C.R. HiPSC-derived cardiomyocytes from Brugada Syndrome patients without identified mutations do not exhibit clear cellular electrophysiological abnormalities. Sci. Rep. 2016, 6, 30967. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.C.; Harmer, S.C.; Poliandri, A.; Nobles, M.; Edwards, E.C.; Ware, J.S.; Sharp, T.V.; McKay, T.R.; Dunkel, L.; Lambiase, P.D.; et al. Ajmaline blocks INa and IKr without eliciting differences between brugada syndrome patient and control human pluripotent stem cell-derived cardiac clusters. Stem Cell Res. 2017, 25, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Selga, E.; Sendfeld, F.; Martinez-Moreno, R.; Medine, C.N.; Tura-Ceide, O.; Wilmut, S.I.; Pérez, G.J.; Scornik, F.S.; Brugada, R.; Mills, N.L. Sodium channel current loss of function in induced pluripotent stem cell-derived cardiomyocytes from a Brugada syndrome patient. J. Mol. Cell. Cardiol. 2018, 114, 10–19. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Müller, J.; Zhao, Z.; Cyganek, L.; Zhong, R.; Zhang, F.; Kleinsorge, M.; Lan, H.; Li, X.; Xu, Q.; et al. Studying brugada syndrome with an scn1b variants in human-induced pluripotent stem cell-derived cardiomyocytes. Front. Cell Dev. Biol. 2019, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Albers, S.; Cyganek, L.; Zhao, Z.; Lan, H.; Li, X.; Xu, Q.; Kleinsorge, M.; Huang, M.; Liao, Z.; et al. A cellular model of Brugada syndrome with SCN10A variants using human-induced pluripotent stem cell-derived cardiomyocytes. Europace 2019, 21, 1410–1421. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blok, M.; Boukens, B.J. Mechanisms of Arrhythmias in the Brugada Syndrome. Int. J. Mol. Sci. 2020, 21, 7051. https://doi.org/10.3390/ijms21197051
Blok M, Boukens BJ. Mechanisms of Arrhythmias in the Brugada Syndrome. International Journal of Molecular Sciences. 2020; 21(19):7051. https://doi.org/10.3390/ijms21197051
Chicago/Turabian StyleBlok, Michiel, and Bastiaan J. Boukens. 2020. "Mechanisms of Arrhythmias in the Brugada Syndrome" International Journal of Molecular Sciences 21, no. 19: 7051. https://doi.org/10.3390/ijms21197051
APA StyleBlok, M., & Boukens, B. J. (2020). Mechanisms of Arrhythmias in the Brugada Syndrome. International Journal of Molecular Sciences, 21(19), 7051. https://doi.org/10.3390/ijms21197051




