Selective Elimination of NG2-Expressing Hair Follicle Stem Cells Exacerbates the Sensitization Phase of Contact Dermatitis in a Transgenic Rat Model
Abstract
1. Introduction
2. Results
2.1. Expression of HSVtk in NG2+ HFSCs of Tg Rats
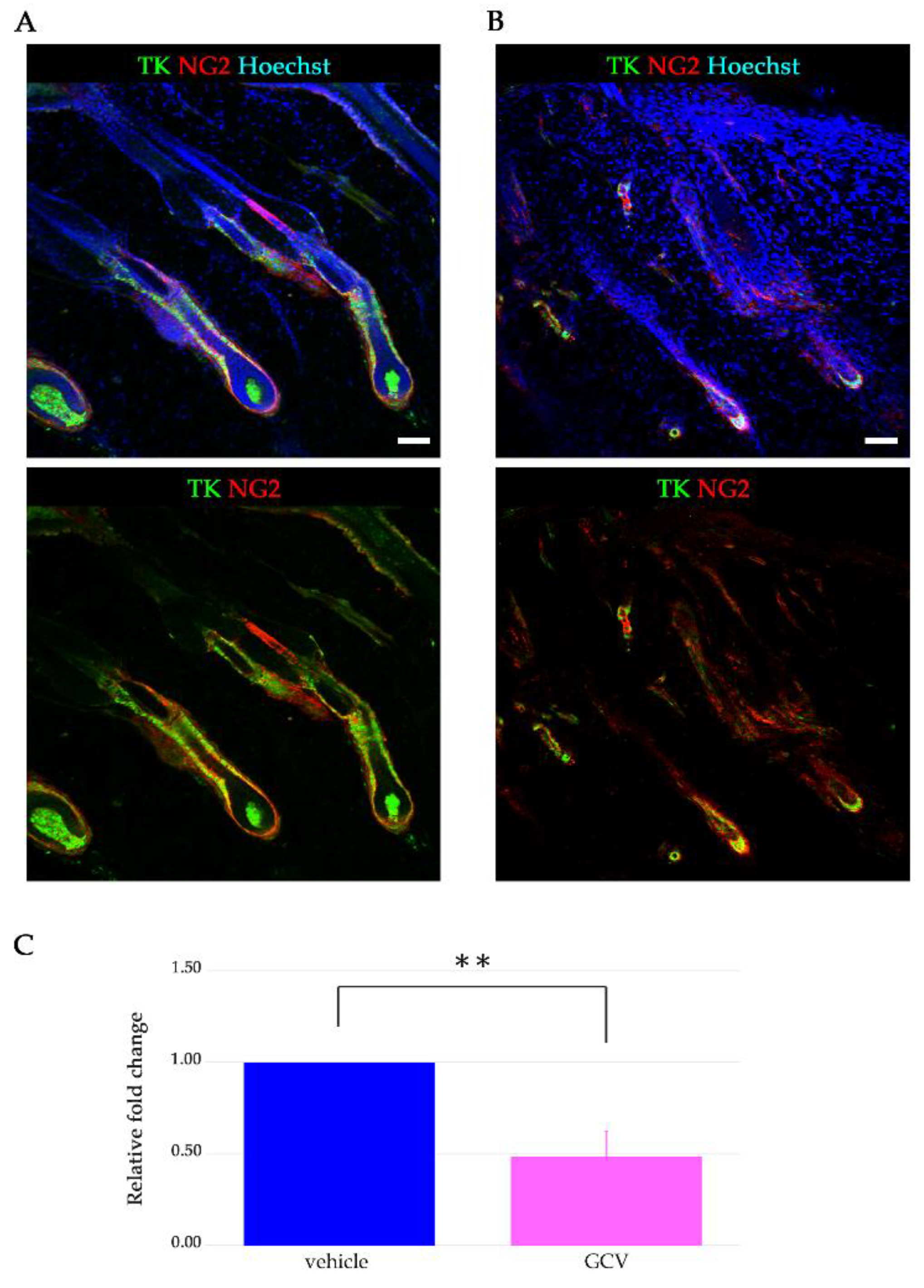
2.2. GCV Induced Programmed Cell Death in the Dividing NG2+ Cells of the Hair Follicles during Anagen
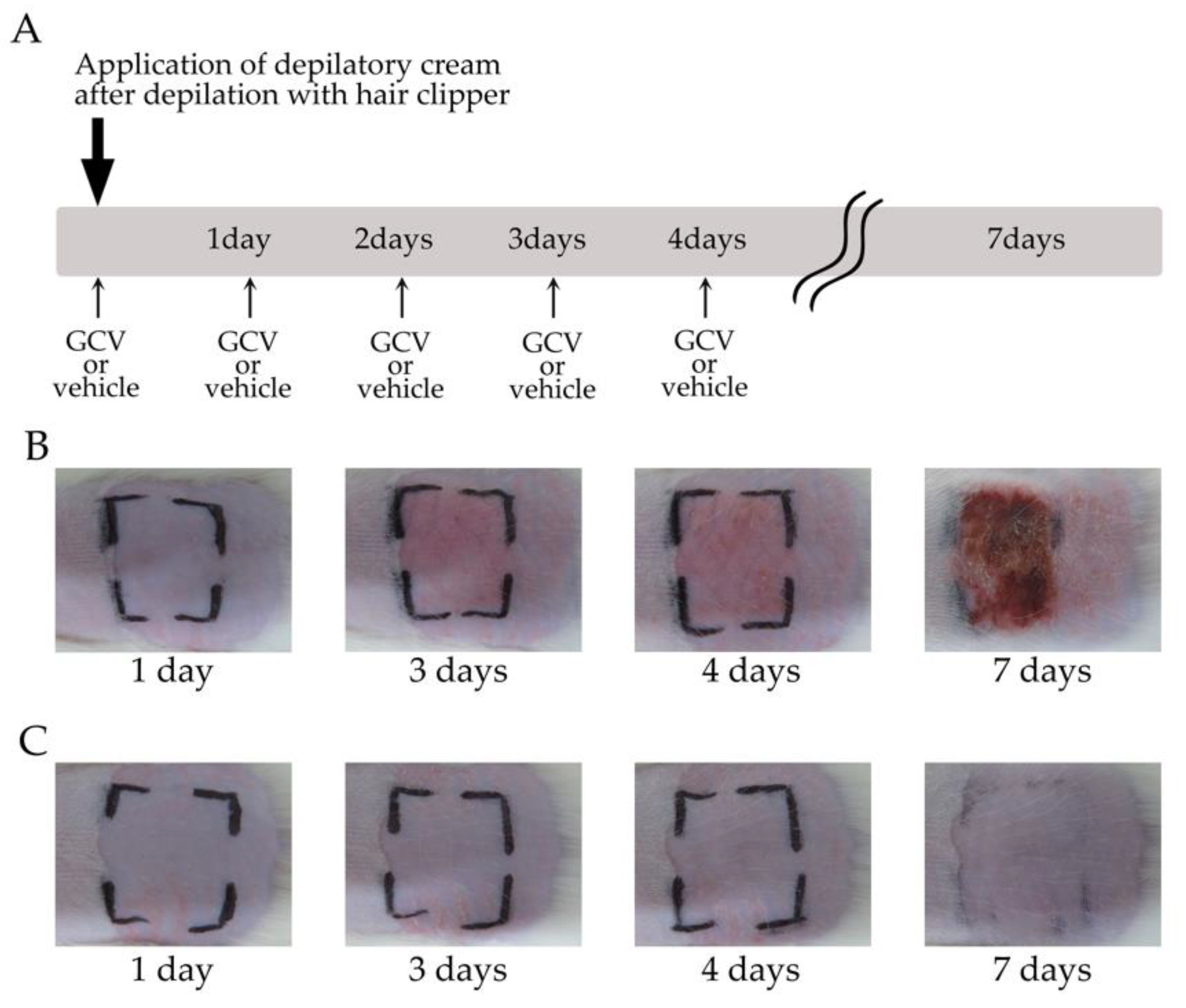
2.3. Selective Elimination of NG2+ HFSCs during Anagen-Induced Contact Dermatitis with a Depilatory Cream
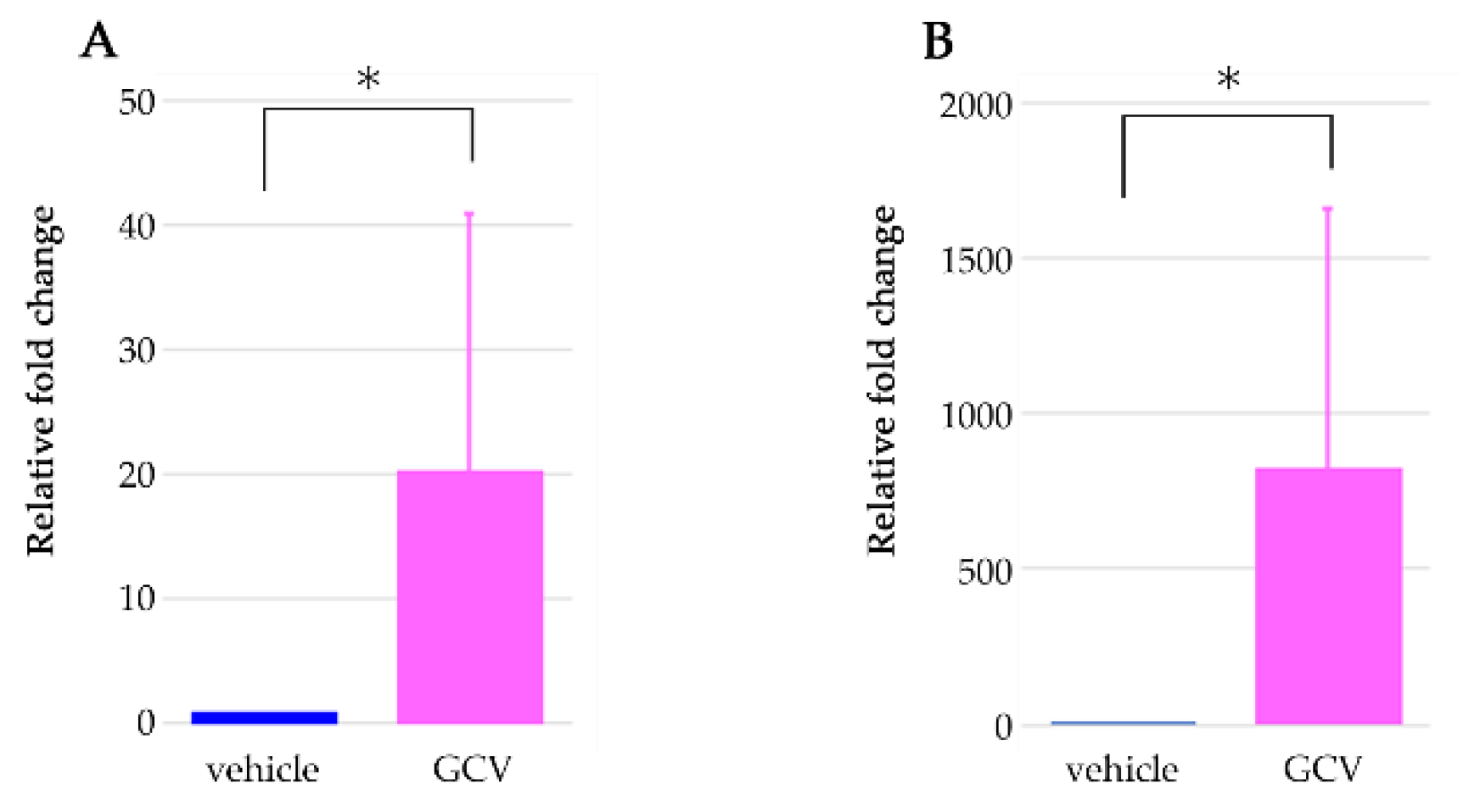
2.4. Selective Ablation of NG2+ HFSCs during Anagen Exacerbated the Sensitization Phase of DNFB-Induced Contact Dermatitis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Selective Elimination of NG2+ HFSCs during Depilation-Induced Anagen
4.3. DNFB-Induced Contact Dermatitis
4.4. Immunohistochemistry
4.5. Real-Time Polymerase Chain Reaction Assay
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HFSCs | hair follicle stem cells |
| HSVtk | herpes simplex virus thymidine kinase |
| GCV | ganciclovir |
| Tg | transgenic |
| sHG | secondary hair germ |
| CSPG4 | chondroitin sulfate proteoglycan 4 |
| DNFB | dinitrofluorobenzene |
| TUNEL | terminal deoxynucleotidyl transferase dUDP nick-end labeling |
| WT | Wild type |
References
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Mesa, K.R.; Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013, 502, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Trempus, C.S.; Morris, R.J.; Bortner, C.D.; Cotsarelis, G.; Faircloth, R.S.; Reece, J.M.; Tennant, R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Investig. Dermatol. 2003, 120, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Jaks, V.; Barker, N.; Kasper, M.; van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgård, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008, 40, 1291–1299. [Google Scholar] [CrossRef]
- Tamura, Y.; Takata, K.; Eguchi, A.; Kataoka, Y. In vivo monitoring of hair cycle stages via bioluminescence imaging of hair follicle NG2 cells. Sci. Rep. 2018, 8, 393. [Google Scholar] [CrossRef]
- Paus, R.; van der Veen, C.; Eichmüller, S.; Kopp, T.; Hagen, E.; Müller-Röver, S.; Hofmann, U. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Investig. Dermatol. 1998, 111, 7–18. [Google Scholar] [CrossRef]
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014, 12, e1002002. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X.; et al. Macrophages induce AKT/β-catenin-dependent Lgr5(+) stem cell activation and hair follicle regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C.; et al. Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Kobayashi, T.; Sugihara, E.; Yamada, T.; Ikuta, K.; Pittaluga, S.; Saya, H.; Amagai, M.; Nagao, K. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015, 21, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, U.; Tokura, Y.; Nishijima, T.; Takigawa, M.; Paus, R. Hair cycle-dependent changes in skin immune functions: Anagen-associated depression of sensitization for contact hypersensitivity in mice. J. Investig. Dermatol. 1996, 106, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Tokura, Y.; Rückert, R.; Paus, R. The anagen hair cycle induces systemic immunosuppression of contact hypersensitivity in mice. Cell Immunol. 1998, 184, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Tamura, Y.; Yamato, M.; Kume, S.; Eguchi, A.; Takata, K.; Watanabe, Y.; Kataoka, Y. NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci. Rep. 2017, 7, 42041. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009, 4, 155–169. [Google Scholar] [CrossRef]
- Peszkowski, M.J.; Warfvinge, G.; Larsson, A. Allergic and irritant contact responses to DNFB in BN and LEW rat strains with different TH1/TH2 profiles. Acta Derm. Venereol. 1994, 74, 371–374. [Google Scholar] [CrossRef]
- Saint-Mezard, P.; Krasteva, M.; Chavagnac, C.; Bosset, S.; Akiba, H.; Kehren, J.; Kanitakis, J.; Kaiserlian, D.; Nicolas, J.F.; Berard, F. Afferent and efferent phases of allergic contact dermatitis (ACD) can be induced after a single skin contact with haptens: Evidence using a mouse model of primary ACD. J. Investig. Dermatol. 2003, 120, 641–647. [Google Scholar] [CrossRef]
- Elliott, J.C.; Picker, M.J.; Nelson, C.J.; Carrigan, K.A.; Lysle, D.T. Sex differences in opioid-induced enhancement of contact hypersensitivity. J. Investig. Dermatol. 2003, 121, 1053–1059. [Google Scholar] [CrossRef][Green Version]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Nosbaum, A.; Vocanson, M.; Rozieres, A.; Hennino, A.; Nicolas, J.F. Allergic and irritant contact dermatitis. Eur. J. Dermatol. 2009, 19, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Brys, A.K.; Rodriguez-Homs, L.G.; Suwanpradid, J.; Atwater, A.R.; MacLeod, A.S. Shifting Paradigms in Allergic Contact Dermatitis: The Role of Innate Immunity. J. Investig. Dermatol. 2020, 140, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; Chavagnac, C.; Vocanson, M.; Rozieres, A.; Benetiere, J.; Pernet, I.; Denis, A.; Nicolas, J.F.; Hennino, A. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J. Investig. Dermatol. 2007, 127, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Paus, R. Melanogenesis is coupled to murine anagen: Toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J. Investig. Dermatol. 1993, 101, 90S–97S. [Google Scholar] [CrossRef]
- Welker, P.; Foitzik, K.; Bulfone-Paus, S.; Henz, B.M.; Paus, R. Hair cycle-dependent changes in the gene expression and protein content of transforming factor beta 1 and beta 3 in murine skin. Arch Dermatol. Res. 1997, 289, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Foitzik, K.; Welker, P.; Bulfone-Paus, S.; Eichmüller, S. Transforming growth factor-beta receptor type I and type II expression during murine hair follicle development and cycling. J. Investig. Dermatol. 1997, 109, 518–526. [Google Scholar] [CrossRef]
- Botchkareva, N.V.; Botchkarev, V.A.; Chen, L.H.; Lindner, G.; Paus, R. A role for p75 neurotrophin receptor in the control of hair follicle morphogenesis. Dev. Biol. 1999, 216, 135–153. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef]
- Paus, R.; Ito, N.; Takigawa, M.; Ito, T. The hair follicle and immune privilege. J. Investig. Dermatol. Symp. Proc. 2003, 8, 188–194. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Myung, P.; Ito, M. Dissecting the bulge in hair regeneration. J. Clin. Investig. 2012, 122, 448–454. [Google Scholar] [CrossRef] [PubMed]




| Depilatory Cream | Redness (Erythema) | More Severe (Dry, Cracked, Scaly, Skin) | |
|---|---|---|---|
| GCV group | + | 15/15 | 13/15 |
| Vehi group | + | 0/10 | 0/10 |
| GCV group | - | 1/8 | 0/8 |
| Vehi group | - | 0/5 | 0/5 |
| DNFB | Redness (Erythema) | More Severe (Dry, Cracked, Scaly, Skin) | |
|---|---|---|---|
| GCV group | + | 4/4 | 4/4 |
| Vehi group | + | 4/4 | 0/4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, Y.; Takata, K.; Eguchi, A.; Kataoka, Y. Selective Elimination of NG2-Expressing Hair Follicle Stem Cells Exacerbates the Sensitization Phase of Contact Dermatitis in a Transgenic Rat Model. Int. J. Mol. Sci. 2020, 21, 6922. https://doi.org/10.3390/ijms21186922
Tamura Y, Takata K, Eguchi A, Kataoka Y. Selective Elimination of NG2-Expressing Hair Follicle Stem Cells Exacerbates the Sensitization Phase of Contact Dermatitis in a Transgenic Rat Model. International Journal of Molecular Sciences. 2020; 21(18):6922. https://doi.org/10.3390/ijms21186922
Chicago/Turabian StyleTamura, Yasuhisa, Kumi Takata, Asami Eguchi, and Yosky Kataoka. 2020. "Selective Elimination of NG2-Expressing Hair Follicle Stem Cells Exacerbates the Sensitization Phase of Contact Dermatitis in a Transgenic Rat Model" International Journal of Molecular Sciences 21, no. 18: 6922. https://doi.org/10.3390/ijms21186922
APA StyleTamura, Y., Takata, K., Eguchi, A., & Kataoka, Y. (2020). Selective Elimination of NG2-Expressing Hair Follicle Stem Cells Exacerbates the Sensitization Phase of Contact Dermatitis in a Transgenic Rat Model. International Journal of Molecular Sciences, 21(18), 6922. https://doi.org/10.3390/ijms21186922




