Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata
Abstract
1. Introduction
2. Results
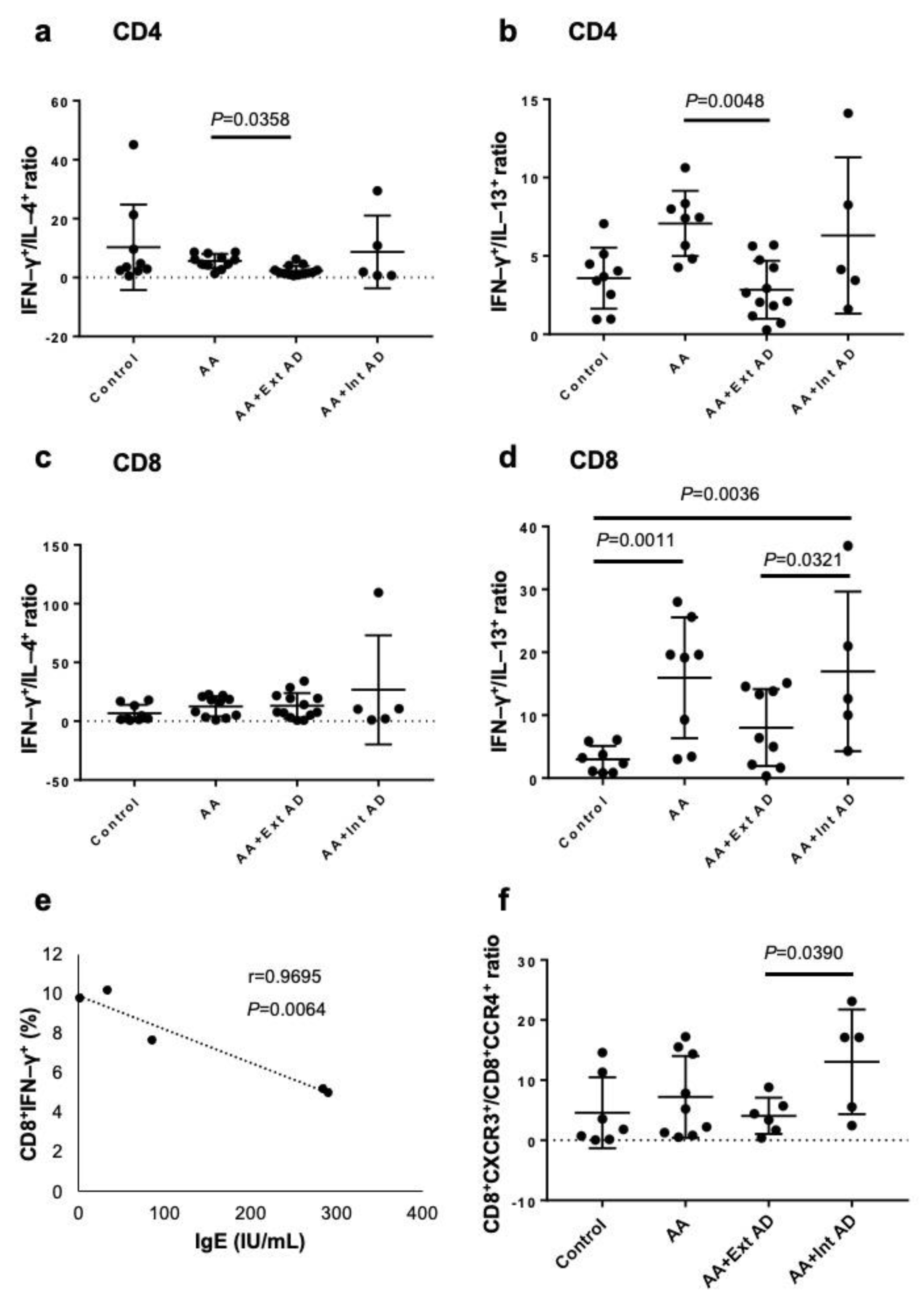
2.1. Type 2 Polarization in Circulating T Cells from AA Patients with Extrinsic AD
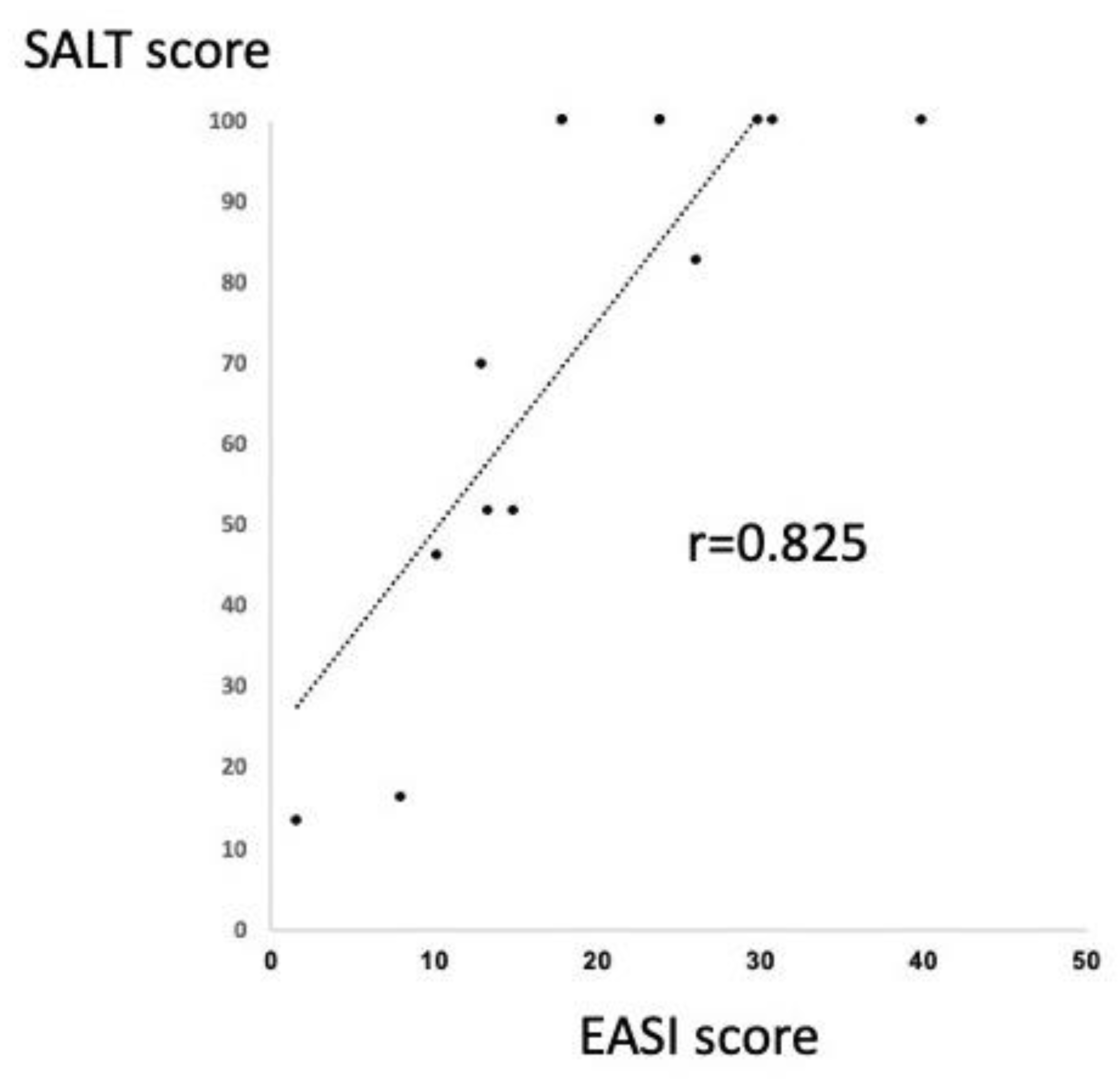
2.2. Significant Correlation between SALT and EASI Score in the Patients with AA and Extrinsic AD
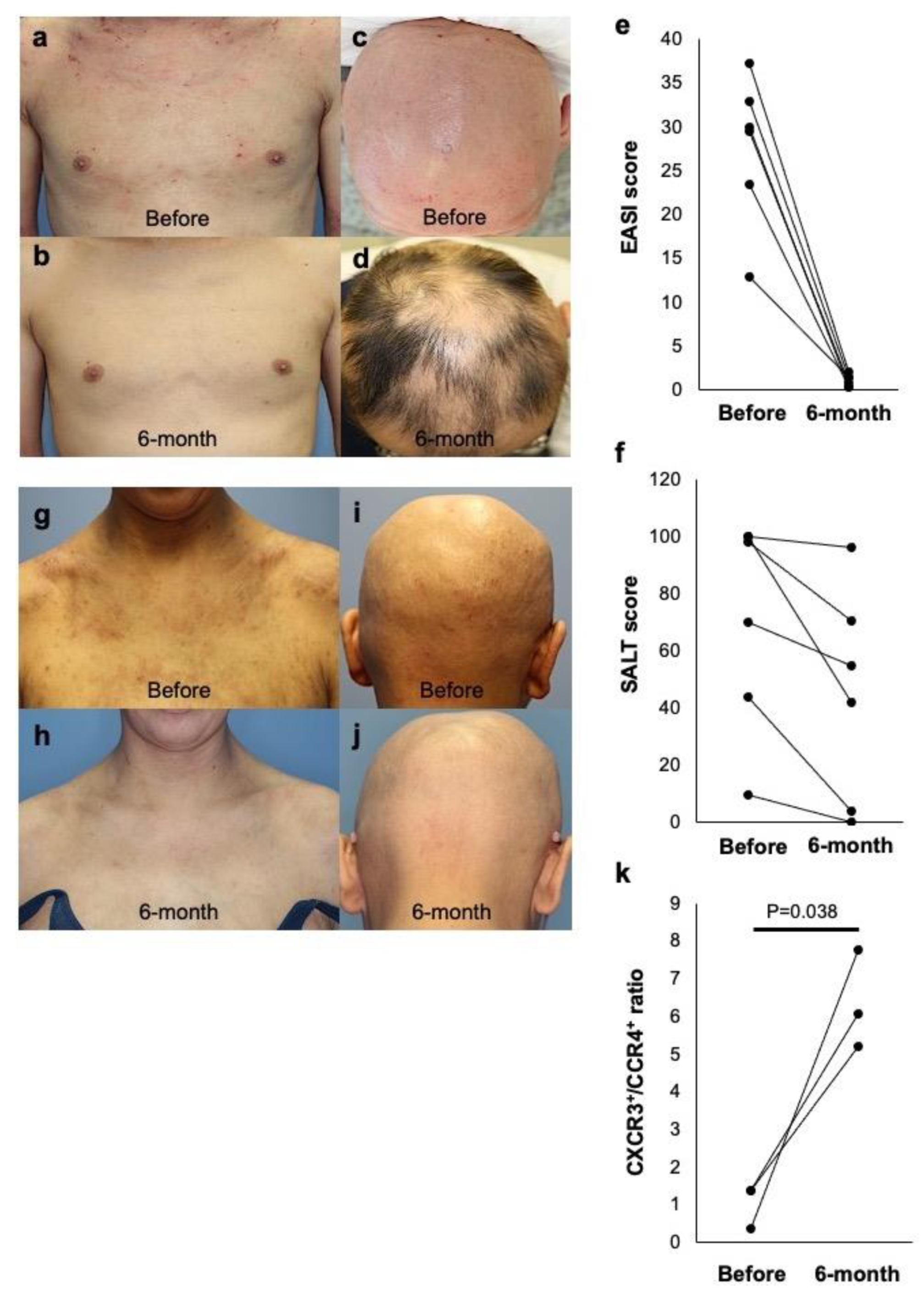
2.3. Dual Efficacy of Dupilumab for AD and AA in Extrinsic AD Patients
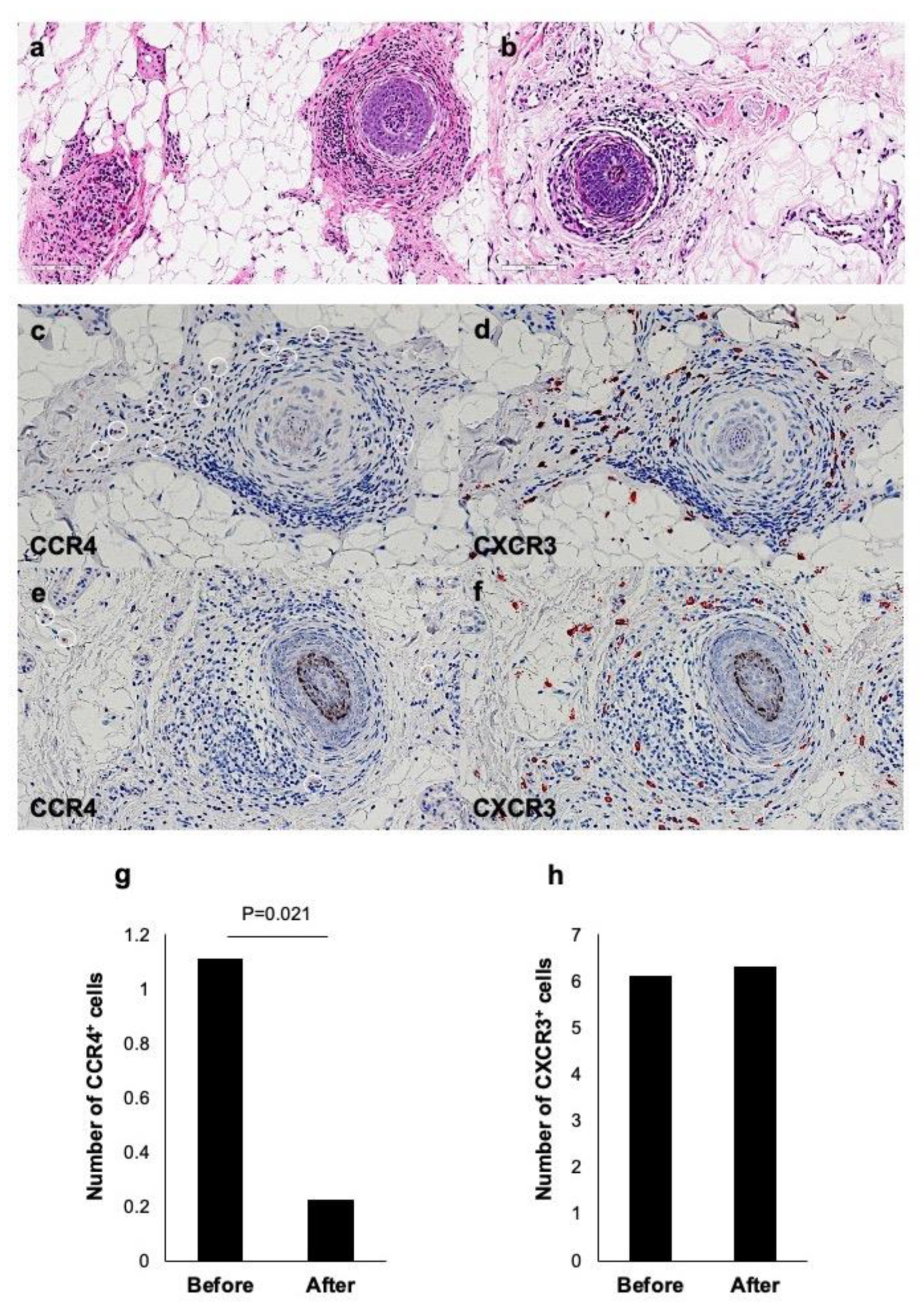
2.4. Improvement of Histopathological Changes by Dupilumab in AA with Extrinsic AD
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Dupilumab Treatment
4.3. Eczema Area and Severity Index (EASI) Score and Severity of Alopecia Tool (SALT) Score
4.4. Flow Cytometric Analysis
4.5. Intracytoplasmic Cytokines
4.6. Histopathology and Immunohistochemical Staining
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ito, T.; Ito, N.; Bettermann, A.; Tokura, Y.; Takigawa, M.; Paus, R. Collapse and resto-ration of MHC class-I-dependent immune privilege: Exploiting the human hair follicle as a model. Am. J. Pathol. 2004, 164, 623–634. [Google Scholar] [CrossRef]
- Paus, R.; Ito, N.; Takigawa, M.; Ito, T. The hair follicle and immune privilege. J. Investig. Dermatol. Symp. Proc. 2003, 8, 188–194. [Google Scholar] [CrossRef]
- Żeberkiewicz, M.; Rudnicka, L.; Malejczyk, J. Immunology of alopecia areata. Cent. Eur. J. Immunol. 2020, 45, 325–333. [Google Scholar] [CrossRef] [PubMed]
- McElwe, K.J.; Gilhar, A.; Tobin, D.J.; Ramot, Y.; Sundberg, J.P.; Nakamura, M.; Bertolini, M.; Inui, S.; Tokura, Y.; King, L.E., Jr.; et al. What causes alopecia areata? Exp. Dermatol. 2013, 22, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Kam, Y.; Assy, B.; Kalish, R.S. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: Evidence for loss of immune privilege. J. Invest. Dermatol. 2005, 124, 288–289. [Google Scholar] [CrossRef]
- Nakamura, M.; Jo, J.; Tabata, Y.; Ishikawa, O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (Alopecia Areata) in a C3H/HeJ mouse model. Am. J. Pathol. 2008, 172, 650–658. [Google Scholar] [CrossRef]
- Freyschmidt-Paul, P.; Zoller, M.; McElwee, K.J.; Sundberg, J.; Happle, R.; Hoffmann, R. The functional relevance of the type 1 cytokines IFN-gamma and IL-2 in alopecia areata of C3H/HeJ mice. J. Investig. Dermatol. Symp. Proc. 2005, 10, 282–283. [Google Scholar] [CrossRef]
- Freyschmidt-Paul, P.; McElwee, K.J.; Hoffmann, R.; Sundberg, J.P.; Vitacolonna, M.; Kissling, S.; Zöller, M. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br. J. Dermatol. 2006, 155, 515–521. [Google Scholar] [CrossRef]
- Sato-Kawamura, M.; Aiba, S.; Tagami, H. Strong expression of CD40, CD54 and HLA-DR antigen and lack of evidence for direct cellular cytotoxicity are unique immunohistopathological features in alopecia areata. Arch. Dermatol. Res. 2003, 294, 536–543. [Google Scholar] [CrossRef]
- Ito, T.; Tokura, Y. The role of cytokines and chemokines in the T-cell-mediated autoimmune process in alopecia areata. Exp. Dermatol. 2014, 23, 787–791. [Google Scholar] [CrossRef]
- Smogorzewski, J.; Sierro, T.; Compoginis, G.; Kim, G. Remission of alopecia universalis in a patient with atopic dermatitis treated with dupilumab. JAAD. Case. Rep. 2019, 5, 116–117. [Google Scholar] [CrossRef]
- Barroso-Garcia, B.; Rial, M.J.; Molina, A.; Sastre, J. Alopecia Areata in Severe Atopic Dermatitis Treated with Dupilumab. J. Investig. Allergol. Clin. Immunol. 2018, 28, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.; Sperling, L.; Lin, J. Drug-induced alopecia after dupilumab therapy. JAAD. Case. Rep. 2019, 5, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Koto, M.; Hoashi, T.; Saeki, H. Case of alopecia areata during dupilumab treatment for atopic dermatitis. J. Dermatol. 2019, 46, e332–e333. [Google Scholar] [CrossRef]
- Salguero-Fernandez, I.; Gonzalez de Domingo, M.A.; Suarez, D.; Roustan-Gullon, G. Dermatitis and alopecia in a patient treated with dupilumab: A new adverse effect? Clin. Exp. Dermatol. 2019, 44, e41–e43. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Kamata, M.; Watanabe, A.; Agematsu, A.; Nagata, M.; Fukaya, S.; Hayashi, K.; Fukuyasu, A.; Tanaka, T.; Ishikawa, T.; et al. Dupilumab Improved Alopecia Areata in a Patient with Atopic Dermatitis: A Case Report. Acta. Derm. Venereol. 2019, 99, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.Y.; Wen, H.C.; Hashim, P.W.; Nia, J.K.; Malik, K.; Estrada, Y.; Kimmel, G.W.; Taliercio, M.; Krueger, J.G.; et al. Alopecia areata is characterized by expansion of circulating Th2/Tc2/Th22, within the skin-homing and systemic T-cell populations. Allergy 2018, 73, 713–723. [Google Scholar] [CrossRef]
- Nomura, T.; Honda, T.; Kabashima, K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int. Immunol. 2018, 30, 419–428. [Google Scholar] [CrossRef]
- Honda, T.; Nomura, T.; Kabashima, K. Advances in atopic dermatitis and urticarial in 2016. J. Allergy. Clin. Immunol. 2017, 140, 369–376. [Google Scholar] [CrossRef]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A multicentric study on prevalence of clinical patterns and clinical phenotypes in adult atopic dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef]
- Dattola, A.; Bennardo, L.; Silvestri, M.; Nisticò, S.P. What’s new in the treatment of atopic dermatitis? Dermatol. Ther. 2019, 32, e12787. [Google Scholar] [CrossRef]
- Seegraber, M.; Srour, J.; Walter, A.; Knop, M.; Wollenberg, A. Dupilumab for treatment of atopic dermatitis. Expert. Rev. Clin. Pharmacol. 2018, 11, 467–474. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Hong, H.C.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, s28–s36. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Katoh, N.; Fujieda, S.; Izuhara, K.; Oishi, K. Dupilumab: Basic aspects and applications to allergic diseases. Allergol. Int. 2020, 69, 187–196. [Google Scholar] [CrossRef]
- Darrigade, A.S.; Legrand, A.; Andreu, N.; Jacquemin, C.; Boniface, K.; Taieb, A.; Seneschal, J. Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br. J. Dermatol. 2018, 179, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Patruno, C.; Napolitano, M.; Ferrillo, M.; Fabbrocini, G. Dupilumab and alopecia: A Janus effect. Dermatol. Ther. 2019, 32, e13023. [Google Scholar] [CrossRef]
- Penzi, L.R.; Yasuda, M.; Manatis-Lornell, A.; Hagigeorges, D.; Senna, M.M. Hair Regrowth in a Patient With Long-standing Alopecia Totalis and Atopic Dermatitis Treated With Dupilumab. JAMA Dermatol. 2018, 154, 1358–1360. [Google Scholar] [CrossRef]
- Alniemi, D.T.; McGevna, L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019, 5, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, H.; Mullen, R.; Zirwas, M. Alopecia Universalis and Atopic Dermatitis Improvement with Dupilumab: Demonstration of a Shared Pathophysiology and Clinical Efficacy. Skinmed 2019, 17, 139–140. [Google Scholar] [PubMed]
- Whiting, D.A. Histopathologic features of alopecia areata: A new look. Arch. Dermatol. 2003, 139, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.S.; El Shabrawi-Caelen, L. ‘Follicular Swiss cheese’ pattern--another histopathologic clue to alopecia areata. J. Cutan. Pathol. 2011, 38, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Levitt, J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018, 4, 143–144. [Google Scholar] [CrossRef]
- Chung, J.; Slaught, C.L.; Simpson, E.L. Alopecia areata in 2 patients treated with dupilumab: New onset and worsening. JAAD Case Rep. 2019, 5, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Yazdanyar, S.; Jemec, G.B.E. Alopecia Areata after Treatment with Dupilumab. Dermatitis 2019, 30, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Carnicle, J.M.; Hendricks, A.J.; Shi, V.Y. Reactivation of Alopecia Areata after Dupilumab Therapy for Atopic Dermatitis. Dermatitis 2019. [Google Scholar] [CrossRef]
- Ito, T.; Hashizume, H.; Shimauchi, T.; Funakoshi, A.; Ito, N.; Fukamizu, H.; Takigawa, M.; Tokura, Y. CXCL10 produced from hair follicles induces Th1 and Tc1 cell infiltration in the acute phase of alopecia areata followed by sustained Tc1 accumulation in the chronic phase. J. Dermatol. Sci. 2013, 69, 140–147. [Google Scholar] [CrossRef]
- Hendricks, A.; Yosipovitch, G.; Shi, V. Dupilumab use in dermatologic conditions beyond atopic dermatitis—A systemic review. J. Dermatol. Treat. 2021, 32, 19–28. [Google Scholar] [CrossRef]
- Pourang, A.; Mesinkovska, N.A. New and Emerging Therapies for Alopecia Areata. Drugs 2020, 80, 635–646. [Google Scholar] [CrossRef]
- Andersen, Y.M.; Egeberg, A.; Gislason, G.H.; Skov, L.; Thyssen, J.P. Autoimmune diseases in adults with atopic dermatitis. J. Am. Acad. Dermatol. 2017, 76, 274–280. [Google Scholar] [CrossRef]
- Hendricks, A.J.; Lio, P.A.; Shi, V.Y. Dupilumab and Alopecia: Causative or Therapeutic? Dermatology 2019, 235, 306–307. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, P.L.; Castelo-Soccio, L. Dupilumab Therapy for Alopecia Areata in Pediatric Patients with Concomitant Atopic Dermatitis. J. Am. Acad. Dermatol. 2021, 20. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.J.; Lee, Y.B.; Lee, W.S. Hair regrowth outcomes of contact immunotherapy for patients with alopecia areata: A systematic review and meta-analysis. JAMA Dermatol. 2018, 154, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Kitagaki, H.; Ono, N.; Hayakawa, K.; Kitazawa, T.; Watanabe, K.; Shiohara, T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J. Immunol. 1997, 159, 2484–2491. [Google Scholar] [PubMed]
- Weise, K.; Kretzschmar, L.; John, S.M.; Hamm, H. Topical immunotherapy in alopecia areata: Anamnestic and clinical criteria of prognostic significance. Dermatology 1996, 192, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhao, Y.; Zhang, X.; Qi, S.; Li, S.; Ye, Y.; Yang, J.; Caulloo, S.; McElwee, K.J.; Zhang, X. Serum level of IL-4 predicts response to topical immunotherapy with diphenylcyclopropenone in alopecia areata. Exp. Dermatol. 2020, 29, 231–238. [Google Scholar] [CrossRef]
- Yoshino, T.; Asada, H.; Ando, Y.; Fujii, H.; Yamaguchi, Y.; Yoshikawa, K.; Itami, S. Impaired responses of peripheral blood mononuclear cells to T-cell stimulants in alopecia areata patients with a poor response to topical immunotherapy. Br. J. Dermatol. 2001, 145, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M. The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp. Dermatol. 2001, 10, 11–18. [Google Scholar] [CrossRef]
- Barbier, N.; Paul, C.; Luger, T.; Allen, R.; De Prost, Y.; Papp, K.; Eichenfield, L.F.; Cherill, R.; Hanifin, J. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br. J. Dermatol. 2004, 150, 96–102. [Google Scholar] [CrossRef]
- Olsen, E.A.; Hordinsky, M.K.; Price, V.H.; Roberts, J.L.; Shapiro, J.; Canfield, D.; Duvic, M.; King Jr, L.E.; McMichael, A.J.; Randall, V.A.; et al. Alopecia areata investigational assessment guidelines--Part II. National Alopecia Areata Foundation. J. Am. Acad. Dermatol. 2004, 51, 440–447. [Google Scholar] [CrossRef] [PubMed]
| Cases | Age | Sex | Tx Period (Week) | Type of AD | IgE Level before Tx (IU/mL) | IgE Level at 20 Weeks (IU/mL) | EASI Score before Tx | EASI Score after Tx | SALT Score before Tx | SALT Score after Tx | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | M | 70 | Extrinsic | 58737 | 8577 | 32.9 | 1.5 | 9.4 | 0 | Hair regrowth at 12 weeks. |
| 2 | 35 | M | 70 | Extrinsic | 5869 | 1207 | 29.9 | 0.4 | 100 | 42.0 | Hair regrowth at 8 weeks. |
| 3 | 47 | M | 66 | Extrinsic | 10522 | 4634 | 37.3 | 2.1 | 100 | 96.0 | Slight hair growth at 26 weeks. |
| 4 | 48 | F | 64 | Extrinsic | 14665 | 6496 | 29.4 | 0.8 | 98.0 | 70.4 | Hair regrowth at 24 weeks. |
| 5 | 48 | F | 58 | Extrinsic | 3152 | 1442 | 23.4 | 0.4 | 44.0 | 4.0 | Hair regrowth at 12 weeks. |
| 6 | 30 | M | 40 | Extrinsic | 7372 | 4800 | 13.0 | 1.5 | 70.0 | 54.8 | Hair regrowth at 16 weeks. |
| 7 | 38 | F | 70 | Intrinsic | 122 | 22 | 35.0 | 1.0 | 100 | 100 | No hair regrowth. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kageyama, R.; Ito, T.; Hanai, S.; Morishita, N.; Nakazawa, S.; Fujiyama, T.; Honda, T.; Tokura, Y. Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata. Int. J. Mol. Sci. 2021, 22, 2618. https://doi.org/10.3390/ijms22052618
Kageyama R, Ito T, Hanai S, Morishita N, Nakazawa S, Fujiyama T, Honda T, Tokura Y. Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata. International Journal of Molecular Sciences. 2021; 22(5):2618. https://doi.org/10.3390/ijms22052618
Chicago/Turabian StyleKageyama, Reiko, Taisuke Ito, Shiho Hanai, Naomi Morishita, Shinsuke Nakazawa, Toshiharu Fujiyama, Tetsuya Honda, and Yoshiki Tokura. 2021. "Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata" International Journal of Molecular Sciences 22, no. 5: 2618. https://doi.org/10.3390/ijms22052618
APA StyleKageyama, R., Ito, T., Hanai, S., Morishita, N., Nakazawa, S., Fujiyama, T., Honda, T., & Tokura, Y. (2021). Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata. International Journal of Molecular Sciences, 22(5), 2618. https://doi.org/10.3390/ijms22052618






