Potential of Extracellular Vesicle-Associated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury
Abstract
1. Introduction
2. Traumatic Brain Injury: A Representative Potentially Mesenchymal Stem Cell-Treated Disease
3. Immunomodulatory Effects of Mesenchymal Stem Cells
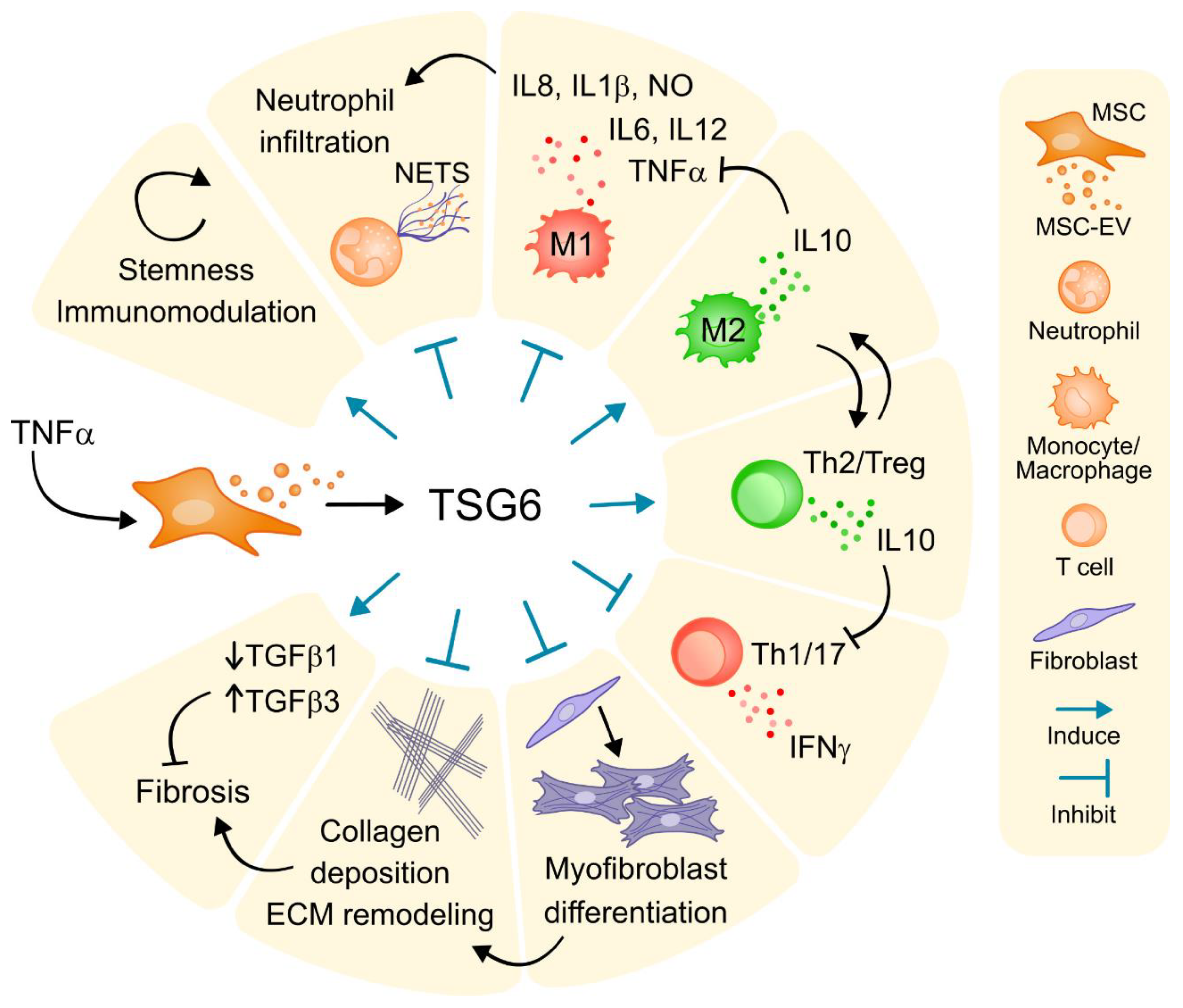
4. Tumor Necrosis Factor (TNF)-Stimulated Protein 6 (TSG-6): Central Role in Therapeutic Benefit of Mesenchymal Stem Cells
5. Extracellular Vesicles (EV): Novel Paracrine Multifunctional Agents by Mesenchymal Stem Cells
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASC | Adipose-derived mesenchymal stromal cells |
| CCM | Concentrated conditioned media |
| EV | Extracellular vesicles |
| mTBI | Mild traumatic brain injury |
| MSC | Mesenchymal stromal cells |
| MI | Myocardial infarction |
| SEC | Size-exclusion chromatography |
| siRNA | Small interfering RNA |
| TNF | Tumor necrosis factor |
| TSG-6 | Tumor necrosis factor-stimulated gene-6 |
References
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Franquesa, M.; Bayes-Genis, A.; Borràs, F.E. Mesenchymal Stem Cells Induce Expression of CD73 in Human Monocytes In Vitro and in a Swine Model of Myocardial Infarction In Vivo. Front. Immunol. 2017, 8, 1577. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Nardelli, A.; Lucarelli, E.; Zannetti, A.; Brunetti, A. Autocrine signals increase ovine mesenchymal stem cells migration through Aquaporin-1 and CXCR4 overexpression. J. Cell Physiol. 2018, 233, 6241–6249. [Google Scholar] [CrossRef]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Sussman, E.S.; Pendharkar, A.V.; Ho, A.L.; Ghajar, J. Mild traumatic brain injury and concussion: Terminology and classification. Handb. Clin. Neurol. 2018, 158, 21–24. [Google Scholar]
- Najem, D.; Rennie, K.; Ribecco-Lutkiewicz, M.; Ly, D.; Haukenfrers, J.; Liu, Q.; Nzau, M.; Fraser, D.D.; Bani-Yaghoub, M. Traumatic brain injury: Classification, models, and markers. Biochem. Cell Biol. 2018, 96, 391–406. [Google Scholar] [CrossRef]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; von Steinbuechel, N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Algattas, H.; Huang, J.H. Traumatic Brain Injury pathophysiology and treatments: Early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 2013, 15, 309–341. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Gonzales-Portillo, G.S.; Acosta, S.; de la Pena, I.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis. Treat 2015, 11, 97–106. [Google Scholar] [PubMed]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef]
- De Witte, S.F.; Franquesa, M.; Baan, C.C.; Hoogduijn, M.J. Toward Development of iMesenchymal Stem Cells for Immunomodulatory Therapy. Front. Immunol. 2016, 6, 648. [Google Scholar] [CrossRef]
- Lee, H.J.; Ko, J.H.; Jeong, H.J.; Ko, A.Y.; Kim, M.K.; Wee, W.R.; Yoon, S.; Oh, J.Y. Mesenchymal stem/stromal Cells Protect Against Autoimmunity via CCL2-dependent Recruitment of Myeloid-Derived Suppressor Cells. J. Immunol. 2015, 194, 3634–3645. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Foley, J.E.; Farthing, D.E.; Gress, R.E.; Laurence, A.; Eckhaus, M.A.; Métais, J.; Rose, J.J.; Hakim, F.T.; Felizardo, T.C.; et al. Bone Marrow-Derived Mesenchymal Stromal Cells Harness Purinergenic Signaling to Tolerize Human Th1 Cells in Vivo. Stem Cells 2015, 33, 1200–1212. [Google Scholar] [CrossRef]
- Kerkelä, E.; Laitinen, A.; Räbinä, J.; Valkonen, S.; Takatalo, M.; Larjo, A.; Veijola, J.; Lampinen, M.; Siljander, P.; Lehenkari, P.; et al. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation With T Cells. Stem Cells 2016, 34, 781–790. [Google Scholar]
- Moll, G.; Jitschin, R.; von Bahr, L.; Rasmusson-Duprez, I.; Sundberg, B.; Lönnies, L.; Elgue, G.; Nilsson-Ekdahl, K.; Mougiakakos, D.; Lambris, J.D.; et al. Mesenchymal Stromal Cells Engage Complement and Complement Receptor Bearing Innate Effector Cells to Modulate Immune Responses. PLoS ONE 2011, 6, e21703. [Google Scholar] [CrossRef]
- English, K.; Barry, F.P.; Field-Corbett, C.P.; Mahon, B.P. IFN-gamma and TNF-alpha Differentially Regulate Immunomodulation by Murine Mesenchymal Stem Cells. Immunol. Lett. 2007, 110, 91–100. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, W.; Li, C.; You, S.; Liao, L.; Han, Q.; Deng, W.; Zhao, R.C.H. Effects of Mesenchymal Stem Cells on Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells. Stem Cells Dev. 2004, 13, 263–271. [Google Scholar] [CrossRef]
- Djouad, F.; Charbonnier, L.; Bouffi, C.; Louis-Plence, P.; Bony, C.; Apparailly, F.; Cantos, C.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cells Inhibit the Differentiation of Dendritic Cells Through an interleukin-6-dependent Mechanism. Stem Cells 2007, 25, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Ye, L.; Zhang, T.; Cheng, J.; Chen, G.; Zhang, Q.; Yang, Y. Umbilical Cord-derived Mesenchymal Stem Cells Instruct Monocytes Towards an IL10-producing Phenotype by Secreting IL6 and HGF. Sci. Rep. 2016, 6, 37566. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Geutskens, S.B.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica 2013, 98, 888–895. [Google Scholar] [CrossRef]
- Luu, N.T.; McGettrick, H.M.; Buckley, C.D.; Newsome, P.N.; Rainger, G.E.; Frampton, J.; Nash, G.B. Crosstalk between Mesenchymal Stem Cells and Endothelial Cells Leads to Downregulation of Cytokine-Induced Leukocyte Recruitment. Stem Cells 2013, 31, 2690–2702. [Google Scholar] [PubMed]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Deng, W.; Chen, W.; Zhang, Z.; Huang, S.; Kong, W.; Sun, Y.; Tang, X.; Yao, G.; Feng, X.; Chen, W.; et al. Mesenchymal Stem Cells Promote CD206 Expression and Phagocytic Activity of Macrophages through IL-6 in Systemic Lupus Erythematosus. Clin. Immunol. 2015, 161, 209–216. [Google Scholar] [CrossRef]
- Chen, P.-M.; Liu, K.-J.; Hsu, P.-J.; Wei, C.-F.; Bai, C.-H.; Ho, L.-J.; Sytwu, H.-K.; Yen, B.L. Induction of Immunomodulatory Monocytes by Human Mesenchymal Stem Cell-Derived Hepatocyte Growth Factor Through ERK1/2. J. Leukoc Biol. 2014, 96, 295–303. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC Suppression Correlates With Cytokine Induction of Indoleamine 2,3-dioxygenase and Bystander M2 Macrophage Differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human Bone Marrow Stromal Cells Inhibit Allogeneic T-cell Responses by Indoleamine 2,3-dioxygenase-mediated Tryptophan Degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, Q.; Bu, H.; Lin, F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010, 19, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, C.; Shi, B.; Zhang, Z.; Feng, R.; Guo, M.; Lu, L.; Shi, S.; Gao, X.; Chen, W.; et al. Mesenchymal Stem Cells Control Complement C5 Activation by Factor H in Lupus Nephritis. EBioMedicine 2018, 32, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rafei, M.; Hsieh, J.; Fortier, S.; Li, M.; Shala Yuan, S.; Birman, E.; Forner, K.; Boivin, M.-N.; Doody, K.; Tremblay, M.; et al. Mesenchymal Stromal Cell-Derived CCL2 Suppresses Plasma Cell Immunoglobulin Production via STAT3 Inactivation and PAX5 Induction. Blood 2008, 112, 4991–4998. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef]
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone Marrow Mesenchymal Progenitor Cells Inhibit Lymphocyte Proliferation by Activation of the Programmed Death 1 Pathway. Eur. J. Immunol. 2005, 35, 1482–1490. [Google Scholar] [CrossRef]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, S.; Wu, S.; Qi, J.; Li, W.; Liu, S.; Cong, Y.; Chen, H.; Lu, L.; Shi, S.; et al. Clearance of Apoptotic Cells by Mesenchymal Stem Cells Contributes to Immunosuppression via PGE2. EBioMedicine 2019, 45, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Burand, A.J., Jr.; Di, L.; Boland, L.K.; Boyt, D.T.; Schrodt, M.V.; Santillan, D.A.; Ankrum, J.A. Aggregation of Human Mesenchymal Stromal Cells Eliminates Their Ability to Suppress Human T Cells. Front. Immunol. 2020, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Meyer, J.R.; Greco, S.J.; Corcoran, K.E.; Bryan, M.; Rameshwar, P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: Role of mesenchymal stem cell-derived TGF-beta. J. Immunol. 2010, 184, 5885–5894. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176.e20. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Zhang, L.; Lin, H.; Hu, J.; Li, D.; Shi, S.; Cui, S.; Zhou, J.; Ji, J.; et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS ONE 2012, 7, e43768. [Google Scholar] [CrossRef]
- Magaña-Guerrero, F.S.; Domínguez-López, A.; Martínez-Aboytes, P.; Buentello-Volante, B.; Garfias, Y. Human Amniotic Membrane Mesenchymal Stem Cells inhibit Neutrophil Extracellular Traps through TSG-6. Sci. Rep. 2017, 7, 12426. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef]
- Kato, T.; Okumi, M.; Tanemura, M.; Yazawa, K.; Kakuta, Y.; Yamanaka, K.; Tsutahara, K.; Doki, Y.; Mori, M.; Takahara, S.; et al. Adipose tissue-derived stem cells suppress acute cellular rejection by TSG-6 and CD44 interaction in rat kidney transplantation. Transplantation 2014, 98, 277–284. [Google Scholar] [CrossRef]
- Jiang, D.; Muschhammer, J.; Qi, Y.; Kügler, A.; de Vries, J.C.; Saffarzadeh, M.; Sindrilaru, A.; Beken, S.V.; Wlaschek, M.; Kluth, M.A.; et al. Suppression of Neutrophil-Mediated Tissue Damage-A Novel Skill of Mesenchymal Stem Cells. Stem Cells 2016, 34, 2393–2406. [Google Scholar] [CrossRef]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.; Willemze, R.; Fibbe, W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Morbelli, S.; Morando, S.; Massollo, M.; Marini, C.; Bertoni, A.; Frassoni, F.; Bartolomé, S.T.; Sambuceti, G.; Traggiai, E.; et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17384–17389. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Barry, F.P.; Mahon, B.P. Murine Mesenchymal Stem Cells Suppress Dendritic Cell Migration, Maturation and Antigen Presentation. Immunol. Lett. 2008, 115, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Shahir, M.; Hashemi, S.M.; Asadirad, A.; Varahram, M.; Kazempour-Dizaji, M.; Folkerts, G.; Garssen, J.; Adcock, I.; Mortaz, E. Effect of Mesenchymal Stem Cell-Derived Exosomes on the Induction of Mouse Tolerogenic Dendritic Cells. J. Cell Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Engela, A.U.; Baan, C.C.; Litjens, N.H.R.; Franquesa, M.; Betjes, M.G.H.; Weimar, W.; Hoogduijn, M.J. Mesenchymal Stem Cells Control Alloreactive CD8(+) CD28(-) T Cells. Clin. Exp. Immunol. 2013, 174, 449–458. [Google Scholar] [CrossRef]
- Liu, X.; Feng, T.; Gong, T.; Shen, C.; Zhu, T.; Wu, Q.; Li, Q.; Li, H. Human Umbilical Cord Mesenchymal Stem Cells Inhibit the Function of Allogeneic Activated Vγ9Vδ2 T Lymphocytes In Vitro. Biomed. Res. Int. 2015, 2015, 317801. [Google Scholar] [CrossRef]
- Li, H.; Guo, Z.; Jiang, X.; Zhu, H.; Li, X.; Mao, N. Mesenchymal Stem Cells Alter Migratory Property of T and Dendritic Cells to Delay the Development of Murine Lethal Acute Graft-Versus-Host Disease. Stem Cells 2008, 26, 2531–2541. [Google Scholar] [CrossRef]
- Benvenuto, F.; Voci, A.; Carminati, E.; Gualandi, F.; Mancardi, G.; Antonio Uccelli, A.; Vergani, L. Human Mesenchymal Stem Cells Target Adhesion Molecules and Receptors Involved in T Cell Extravasation. Stem Cell Res. Ther. 2015, 6, 245. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Noël, D.; Fernandez, X.; Khoury, M.; Figueroa, F.; Carrión, F.; Jorgensen, C.; Djouad, F. Mesenchymal Stem Cells Repress Th17 Molecular Program Through the PD-1 Pathway. PLoS ONE 2012, 7, e45272. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal Stem Cells Generate a CD4+CD25+Foxp3+ Regulatory T Cell Population During the Differentiation Process of Th1 and Th17 Cells. Stem Cell Res. Ther. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.; Pène, J.; Moquet-Torcy, G.; Jorgensen, C.; Yssel, H. Mesenchymal Stem Cells Inhibit Human Th17 Cell Differentiation and Function and Induce a T Regulatory Cell Phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef]
- Engela, A.U.; Hoogduijn, M.J.; Boer, K.; Litjens, N.H.R.; Betjes, M.G.H.; Weimar, W.; Baan, C.C. Human Adipose-Tissue Derived Mesenchymal Stem Cells Induce Functional De-Novo Regulatory T Cells With Methylated FOXP3 Gene DNA. Clin. Exp. Immunol. 2013, 173, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced Immunoregulation Involves FAS-ligand-/FAS-mediated T Cell Apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, J.S.; Yoon, I.H.; Shin, J.S.; Nam, H.Y.; Yang, S.H.; Kim, S.J.; Park, C.G. Immunomodulation of Delayed-Type Hypersensitivity Responses by Mesenchymal Stem Cells Is Associated With Bystander T Cell Apoptosis in the Draining Lymph Node. J. Immunol. 2010, 185, 4022–4029. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T Cell Apoptosis by Tryptophan Catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Plumas, J.; Chaperot, L.; Richard, M.-J.; Molens, J.-P.; Bensa, J.-C.; Favrot, M.-C. Mesenchymal Stem Cells Induce Apoptosis of Activated T Cells. Leukemia 2005, 19, 1597–1604. [Google Scholar] [CrossRef]
- Benvenuto, F.; Ferrari, S.; Gerdoni, E.; Gualandi, F.; Frassoni, F.; Pistoia, V.; Mancardi, G.; Uccelli, A. Human Mesenchymal Stem Cells Promote Survival of T Cells in a Quiescent State. Stem Cells 2007, 25, 1753–1760. [Google Scholar] [CrossRef]
- Crop, M.J.; Baan, C.C.; Korevaar, S.S.; Ijzermans, J.N.M.; Weimar, W.; Hoogduijn, M.J. Human Adipose Tissue-Derived Mesenchymal Stem Cells Induce Explosive T-cell Proliferation. Stem Cells Dev. 2010, 19, 1843–1853. [Google Scholar] [CrossRef]
- Franquesa, M.; Mensah, F.K.; Huizinga, R.; Strini, T.; Boon, L.; Lombardo, E.; DelaRosa, O.; Laman, J.D.; Grinyó, J.M.; Weimar, W.; et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells 2015, 33, 880–891. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef]
- Comoli, P.; Ginevri, F.; Maccario, R.; Avanzini, M.A.; Marconi, M.; Groff, A.; Cometa, A.; Cioni, M.; Porretti, L.; Barberi, W.; et al. Human Mesenchymal Stem Cells Inhibit Antibody Production Induced in Vitro by Allostimulation. Nephrol. Dial Transplant 2008, 23, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, I.; Le Blanc, K.; Sundberg, B.; Ringdén, O. Mesenchymal Stem Cells Stimulate Antibody Secretion in Human B Cells. Scand J. Immunol. 2007, 65, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chan, K.-H.; Lai, W.-H.; Siu, C.-W.; Kwan, S.-C.; Tse, H.-F.; Wing-Lok Ho, P.; Wing-Man Ho, J. Human Mesenchymal Stem Cells Upregulate CD1dCD5(+) Regulatory B Cells in Experimental Autoimmune Encephalomyelitis. Neuroimmunomodulation 2013, 20, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Luk, F.; Carreras-Planella, L.; Korevaar, S.S.; de Witte, S.F.H.; Borràs, F.E.; Betjes, M.G.H.; Baan, C.C.; Hoogduijn, M.J.; Franquesa, M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front. Immunol. 2017, 8, 1042. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, X.; Liu, Q.; Zhang, X.; Huang, K.; Liu, L.; Li, H.; Zhou, M.; Huang, F.; Fan, Z.; et al. Mesenchymal Stromal Cells Infusions Improve Refractory Chronic Graft Versus Host Disease Through an Increase of CD5+ Regulatory B Cells Producing Interleukin 10. Leukemia 2015, 29, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Planella, L.; Monguió-Tortajada, M.; Borràs, F.E.; Franquesa, M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front. Immunol. 2019, 10, 1288. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef]
- Krampera, M.; Pasini, A.; Pizzolo, G.; Cosmi, L.; Romagnani, S.; Annunziato, F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr. Opin. Pharmacol. 2006, 6, 435–441. [Google Scholar] [CrossRef]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Cutler, A.J.; Limbani, V.; Girdlestone, J.; Navarrete, C.V. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J. Immunol. 2010, 185, 6617–6623. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Cronstein, B. Regulation of inflammation by adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Sitkovsky, M. Extracellular adenosine-mediated modulation of regulatory T cells. Front. Immunol. 2014, 5, 304. [Google Scholar] [CrossRef]
- Regateiro, F.S.; Cobbold, S.P.; Waldmann, H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin. Exp. Immunol. 2013, 171, 1–7. [Google Scholar] [CrossRef]
- Chatterjee, D.; Tufa, D.M.; Baehre, H.; Hass, R.; Schmidt, R.E.; Jacobs, R. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood 2014, 123, 594–595. [Google Scholar] [CrossRef]
- Beavis, P.A.; Stagg, J.; Darcy, P.K.; Smyth, M.J. CD73: A potent suppressor of antitumor immune responses. Trends Immunol. 2012, 33, 231–237. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Pujal, J.M.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.R.; Bayes-Genis, A.; Borràs, F.E. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: Implications for nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory properties of mesenchymal stem cells: Cytokines and factors. Am. J. Reprod. Immunol. 2012, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G.; Samburski, S.S.; Jalkanen, S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 2003, 17, 1328–1330. [Google Scholar] [CrossRef]
- Maksimow, M.; Kyhälä, L.; Nieminen, A.; Kylänpää, L.; Aalto, K.; Elima, K.; Mentula, P.; Lehti, M.; Puolakkainen, P.; Yegutkin, G.G.; et al. Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis. Crit. Care Med. 2014, 42, 2556–2564. [Google Scholar] [CrossRef]
- Airas, L.; Niemelä, J.; Salmi, M.; Puurunen, T.; Smith, D.J.; Jalkanen, S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kD adhesion molecule, on lymphocytes and endothelial cells. J. Cell Biol. 1997, 136, 421–431. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Bönner, F.; Borg, N.; Burghoff, S.; Schrader, J. Resident cardiac immune cells and expression of the ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PLoS ONE 2012, 7, e34730. [Google Scholar] [CrossRef]
- Gálvez-Montón, C.; Bragós, R.; Soler-Botija, C.; Díaz-Güemes, I.; Prat-Vidal, C.; Crisóstomo, V.; Sánchez-Margallo, F.M.; Llucià-Valldeperas, A.; Bogónez-Franco, P.; Perea-Gil, I.; et al. Noninvasive Assessment of an Engineered Bioactive Graft in Myocardial Infarction: Impact on Cardiac Function and Scar Healing. Stem Cells Transl. Med. 2017, 6, 647–655. [Google Scholar] [CrossRef]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koç, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef]
- Lu, W.; Xie, Z.; Tang, Y.; Bai, L.; Yao, Y.; Fu, C.; Ma, G. Photoluminescent Mesoporous Silicon Nanoparticles with siCCR2 Improve the Effects of Mesenchymal Stromal Cell Transplantation after Acute Myocardial Infarction. Theranostics 2015, 5, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, R.; Sánchez-Margallo, F.M.; Álvarez, V.; Usón, A.; Casado, J.G. Surgical meshes coated with mesenchymal stem cells provide an anti-inflammatory environment by a M2 macrophage polarization. Acta Biomater. 2016, 31, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Dayan, V.; Yannarelli, G.; Billia, F.; Filomeno, P.; Wang, X.H.; Davies, J.E.; Keating, A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 2011, 106, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Ben-Mordechai, T.; Holbova, R.; Landa-Rouben, N.; Harel-Adar, T.; Feinberg, M.S.; Abd Elrahman, I.; Blum, G.; Epstein, F.H.; Silman, Z.; Cohen, S.; et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 2013, 62, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78–79, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, R.; Wang, Y.; Huang, W.; Hu, B.; Zhu, G.; Zhang, R.; Li, F.; Han, J.; Li, Y. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res. 2019, 1724, 146422. [Google Scholar] [CrossRef]
- Prockop, D.J. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016, 51, 7–13. [Google Scholar] [CrossRef]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.; Day, A.J.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.; Duan, H.; Chai, J.; Yang, J.; Li, X.; Yu, Y.; Zhang, X.; Hu, X.; Xiao, M.; et al. TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci. Rep. 2016, 6, 30121. [Google Scholar] [CrossRef]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Gentry, J.; Klaic, L.; Del Mar, N.; Reiner, A.; Yang, C.H.; Pfeffer, L.M.; Sohl, N.; et al. TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation. Stem Cell Res Ther. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Yu, J.M.; Foskett, A.M.; Peltier, G.; Reneau, J.C.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 16766–16771. [Google Scholar] [CrossRef]
- Romano, B.; Elangovan, S.; Erreni, M.; Sala, E.; Petti, L.; Kunderfranco, P.; Massimino, L.; Restelli, S.; Sinha, S.; Lucchetti, D.; et al. TNF-Stimulated Gene-6 Is a Key Regulator in Switching Stemness and Biological Properties of Mesenchymal Stem Cells. Stem Cells 2019, 37, 973–987. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Katsuda, T.; Ochiya, T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res. Ther. 2015, 6, 212. [Google Scholar] [CrossRef]
- Ng, S.Y.; Wah Lee, A.Y. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front Cell Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.J. MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Verweij, F.J.; Revenu, C.; Arras, G.; Dingli, F.; Loew, D.; Pegtel, D.M.; Follain, G.; Allio, G.; Goetz, J.G.; Zimmermann, P.; et al. Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Dev. Cell 2019, 48, 573–589.e4. [Google Scholar] [CrossRef] [PubMed]
- Nizamudeen, Z.; Markus, R.; Lodge, R.; Parmenter, C.; Platt, M.; Chakrabarti, L.; Sottile, V. Rapid and accurate analysis of stem cell-derived extracellular vesicles with super resolution microscopy and live imaging. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef] [PubMed]
- Budoni, M.; Fierabracci, A.; Luciano, R.; Petrini, S.; Di Ciommo, V.; Muraca, M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013, 22, 369–379. [Google Scholar] [CrossRef]
- Park, K.S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef]
- Krämer-Albers, E.V. Ticket to Ride: Targeting Proteins to Exosomes for Brain Delivery. Mol. Ther. 2017, 25, 1264–1266. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef]
- Williams, A.M.; Bhatti, U.F.; Brown, J.F.; Biesterveld, B.E.; Kathawate, R.G.; Graham, N.J.; Chtraklin, K.; Siddiqui, A.Z.; Dekker, S.E.; Andjelkovic, A.; et al. Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain barrier integrity in swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2020, 88, 207–218. [Google Scholar] [CrossRef]
- Gézsi, A.; Kovács, Á.; Visnovitz, T.; Buzás, E.I. Systems biology approaches to investigating the roles of extracellular vesicles in human diseases. Exp. Mol. Med. 2019, 51, 33. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rufino, J.D.; Espinosa-Lara, N.; Osugui, L.; Sanchez-Guijo, F. Targeting the Immune System with Mesenchymal Stromal Cell-Derived Extracellular Vesicles: What Is the Cargo’s Mechanism of Action? Front. Bioeng. Biotechnol. 2019, 7, 308. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, S.; Thueson, S.; Ponnalagu, D.; Alam, M.A.; Gheorghe, C.P.; Aghai, Z.; Singh, H.; Bhandari, V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. 2018, 9, 173. [Google Scholar] [CrossRef]
- An, J.H.; Li, Q.; Ryu, M.O.; Nam, A.R.; Bhang, D.H.; Jung, Y.C.; Song, W.J.; Youn, H.Y. TSG-6 in extracellular vesicles from canine mesenchymal stem/stromal is a major factor in relieving DSS-induced colitis. PLoS ONE 2020, 15, e0220756. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef]
- Chou, K.J.; Hsu, C.Y.; Huang, C.W.; Chen, H.Y.; Ou, S.H.; Chen, C.L.; Lee, P.T.; Fang, H.C. Secretome of Hypoxic Endothelial Cells Stimulates Bone Marrow-Derived Mesenchymal Stem Cells to Enhance Alternative Activation of Macrophages. Int. J. Mol. Sci. 2020, 21, 4409. [Google Scholar] [CrossRef]
- Lin, Y.H.; Kang, L.; Feng, W.H.; Cheng, T.L.; Tsai, W.C.; Huang, H.T.; Lee, H.C.; Chen, C.H. Effects of Lipids and Lipoproteins on Mesenchymal Stem Cells Used in Cardiac Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 4770. [Google Scholar] [CrossRef]
- Yang, J.W.; Shin, Y.Y.; Seo, Y.; Kim, H.S. Therapeutic Functions of Stem Cells from Oral Cavity: An Update. Int. J. Mol. Sci. 2020, 21, 4389. [Google Scholar] [CrossRef]
| Molecule | Target | Effect | Expression | Reference |
|---|---|---|---|---|
| CCL2/MCP-1 | Monocytes | Recruitment | Constitutive (mouse and human) | [16] |
| CD39, CD73 | Monocytes, DC, B, T cells | Adenosine production for immune suppression | Constitutive (mouse and human) | [17,18] |
| CD59 | MAC | Inhibition of MAC formation, final step of complement system-mediated cell lysis | Human | [19] |
| COX2 | Production of PGE2, see PGE2 | [20,21] | ||
| IL6 | Monocytes | Impaired differentiation to dendritic cells | Mouse and human | [22,23] |
| M2 skewing | Human | [24] | ||
| T cells | Inhibition of mitogenic or allogeneic T cell proliferation | Mouse | [22] | |
| Endothelial cells | Reduced leukocyte recruitment and transendothelial migration | Human | [25] | |
| IL10 | Not expressed by murine or human MSC | [20,26,27] | ||
| Anti-inflammatory environment, induction of Treg | Induced to monocytes (human) | [23,28,32] | ||
| Neutrophils | Reduced neutrophil infiltrate by inducing IL10 expression to resident macrophages | Induced to resident macrophages (mouse) | [26] | |
| HGF | Monocytes | M2 skewing | Mouse and human | [23,28] |
| T cells | Inhibition of allogeneic proliferation | Constitutive (mouse) | [20] | |
| Constitutive (human) | [29] | |||
| HLA-G | NK cells | Reduced NK cytolytic activity | Human | [30] |
| T cells | Inhibition of allogeneic proliferation and induction of Treg | |||
| IDO | NK cells | Inhibition of IL2-induced proliferation | Human | [31] |
| Monocytes | M2 skewing: increased CD206, decreased CD80; increased IL10 and decreased TNFα production | Human | [32] | |
| T cells | Suppression of T cell proliferation by depletion of the essential amino acid tryptophan and kynurenine accumulation | Inducible by IFNγ (mouse) | [20] | |
| Inducible by IFNγ (human) | [33] | |||
| Factor H | C3b | Inhibition of complement activation: blockage of C3b activation, cofactor for C3b elimination, deployment of C3 convertase (C3bBb) | Constitutive and inducible by IFNγ (human) | [34,35] |
| M-CSF | monocytes | M2 skewing, impaired differentiation and maturation of dendritic cells | Constitutive (human) | [24] |
| MMP | B cells | Reduced IgG/IgM production by MMP processing of CCL2 for reduced STAT3 and induced PAX5 | Constitutive (mouse) | [36] |
| iNOS | T cells | Nitric oxide production for T cell suppression through inhibition of Stat5 phosphorylation | Inducible by allogeneic T cell contact (mouse) | [37] |
| PD-L1/2 | T cells | Inhibition of proliferation and cytokine production (IL2), T cell death | Constitutive (mouse) | [38] |
| Inducible by IFNγ and TNFα (human) | [20] | |||
| PGE2 | NK | Inhibition of cytotoxic activity | Human | [31] |
| Monocytes | M2 skewing: increased IL10 and decreased TNFα and IL6 production | Mouse | [26] | |
| M2 skewing | Human | [39] | ||
| Impaired differentiation to dendritic cells and maturation | [40] | |||
| T cells | Inhibition of allogeneic proliferation | Constitutive and increased by IFNγ and TNFα (mouse, human) | [20,41] | |
| T cells | Inhibition of T cell response and proliferation | Mouse in vitro and in vivo Human in vitro | [21,42,43] | |
| TGFβ | T cells | Inhibition of allogeneic proliferation | Constitutive (mouse, human) | [20,29] |
| Induction of Treg | Human | [24,44] | ||
| TSG6 | Neutrophils | Reduced neutrophil infiltration and activation | Mouse in vitro and Rat in vivo | [45,46] |
| Diminish ROS and NETs release | Human | [47] | ||
| Monocytes | M2 skewing, limit inflammation and fibrosis | Mouse in vitro and in vivo | [48] | |
| Decreased NF-κB-mediated inflammatory cytokines production through CD44 receptor signaling | Human | [49] | ||
| T cells | Suppress alloreactive T cells, attenuate acute kidney rejection | Rat in vivo | [50] |
| Target | Mechanism | Effect | Model | Reference |
|---|---|---|---|---|
| Neutrophils | IL10, TSG6 SOD3 | Reduction of infiltration by inducing IL10 expression to resident macrophages, blockage of CXCL8 by TSG6 production. Prevention of neutrophil death, ROS, NETs, and matrix degrading neutrophil elastase, gelatinase, and myeloperoxidase release | Mouse in vitro and in vivo | [26,45,51] |
| TSG6, SOD3, HLA-G | Reduction of infiltration | Rat in vivo | [46] | |
| Decreased oxidase-1, HO-1; reduced VEGF; reduced IL8, IFNγ and increased COX2 for a dampened oxidative; vascular; and inflammatory activity. Reduced neutrophil death, ROS and NETs release | Human in vitro | [30,47,51] | ||
| Natural Killer (NK) cells | Contact-dep; contact-indep: IDO, PGE2, HLA-G, TGFβ1 | Downregulation of NK activating receptors, inhibition of IL2-induced proliferation (through IDO), cytotoxic activity (through PGE2) and IFNγ production | Human in vitro | [31,41] |
| Monocytes/macrophages | Support survival in in vitro culture | Human in vitro | [24,39] | |
| Contact-dep; contact-indep: HGF, PGE2, TSG6 | M2 skewing: increased CD206, decreased CD80; increased IL10 and decreased TNFα production | Mouse in vitro | [26,28,48] | |
| Contact-dep; contact-indep: M-CSF, HGF, PGE2 | Human in vitro | [24,28,32,39] | ||
| IL6 | Impaired differentiation to dendritic cells and maturation: reduced CD1a, HLA-II and costimulatory molecules expression and less T cell priming | Mouse in vitro | [22] | |
| Contact-dep; contact-indep: PGE2, IL6 | Human in vitro | [21,40] | ||
| Partly by IL6 and M-CSF | [24,52] | |||
| Dendritic cells | Reduced CCR7 expression to inhibit migration to lymph nodes | Mouse in vitro and in vivo | [53,54] | |
| Reduced cross-presentation to CD8+ T cells | Mouse in vivo | [53] | ||
| Decreased MHC-II and costimulatory molecules expression, impaired cytokine production | Mouse in vitro and in vivo | [41,53,54] | ||
| TGF-β, IL-10, IL-6 | Expression of DC costimulatory markers and ability of DCs to modulate lymphocyte proliferation | Mouse in vitro | [55] | |
| T cells | NO, PGE2, IL6 | Inhibition of mitogenic or allogeneic T cell proliferation | Mouse in vitro | [22,37,38] |
| TSG6 | Rat in vitro | [50] | ||
| Baboon in vitro | [56] | |||
| Contact-dep: PD-L1; contact-indep: PGE2, IDO, HGF, TGFβ, adenosine, HLA-G | Human in vitro | [18,29,30,33] | ||
| Impaired cytotoxic activity of CD8+ T cells | Human in vitro | [44,57] | ||
| Impaired cytotoxic activity of γΔ T cells | Mouse in vitro | [58] | ||
| Upregulation of CCR7 and CD62L for retention in secondary lymphoid organs | Mouse in vitro | [59] | ||
| Reduced CXCR3 (CXCL10-R) and adhesion molecules expression for reduced transendothelial migration | Human in vitro | [60] | ||
| M2/MDSC induction | Shift to Th2 from Th1 or Th17 polarization | Mouse in vitro | [58,61] | |
| Human in vitro | [41,44] | |||
| IDO | Induction of Tregs | Mouse in vitro | [62] | |
| Contact-dep | Human in vitro | [63] | ||
| Contact-indep: TGFβ, HLA-G, PGE2 | Induction of Tregs | [30,44,64] | ||
| Need M2 skewing (CCL18 and IL10 production) | [24,39] | |||
| IDO | Apoptosis of activated T cells | Mouse in vitro | [65,66,67] | |
| Inhibition of T cell proliferation | Human in vitro | [33,38,68] | ||
| Promote survival and expansion of quiescent T cells | Mouse and human in vitro | [52,69,70] | ||
| B cells | Contact-dep: PD-1 | Inhibition of mitogenic proliferation | Mouse and human in vitro | [38,71] |
| IL1RA | Impaired B cell maturation and plasmablast differentiation | Mouse and human in vitro | [71,72] | |
| MMP processing of CCL2 for reduced STAT3 activation and induced PAX5 transcription | Reduced production of IgG and IgM under strong stimulation | Mouse in vitro | [36] | |
| Human in vitro | [73,74] | |||
| Contact-dep; contact-indep: IDO | Induction of Bregs | Mouse and human in vitro | [71,75,76,77,78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roura, S.; Monguió-Tortajada, M.; Munizaga-Larroudé, M.; Clos-Sansalvador, M.; Franquesa, M.; Rosell, A.; Borràs, F.E. Potential of Extracellular Vesicle-Associated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 6761. https://doi.org/10.3390/ijms21186761
Roura S, Monguió-Tortajada M, Munizaga-Larroudé M, Clos-Sansalvador M, Franquesa M, Rosell A, Borràs FE. Potential of Extracellular Vesicle-Associated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury. International Journal of Molecular Sciences. 2020; 21(18):6761. https://doi.org/10.3390/ijms21186761
Chicago/Turabian StyleRoura, Santiago, Marta Monguió-Tortajada, Micaela Munizaga-Larroudé, Marta Clos-Sansalvador, Marcella Franquesa, Anna Rosell, and Francesc E. Borràs. 2020. "Potential of Extracellular Vesicle-Associated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury" International Journal of Molecular Sciences 21, no. 18: 6761. https://doi.org/10.3390/ijms21186761
APA StyleRoura, S., Monguió-Tortajada, M., Munizaga-Larroudé, M., Clos-Sansalvador, M., Franquesa, M., Rosell, A., & Borràs, F. E. (2020). Potential of Extracellular Vesicle-Associated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury. International Journal of Molecular Sciences, 21(18), 6761. https://doi.org/10.3390/ijms21186761









