Dietary Supplementation with Spray-Dried Porcine Plasma Attenuates Colon Inflammation in a Genetic Mouse Model of Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
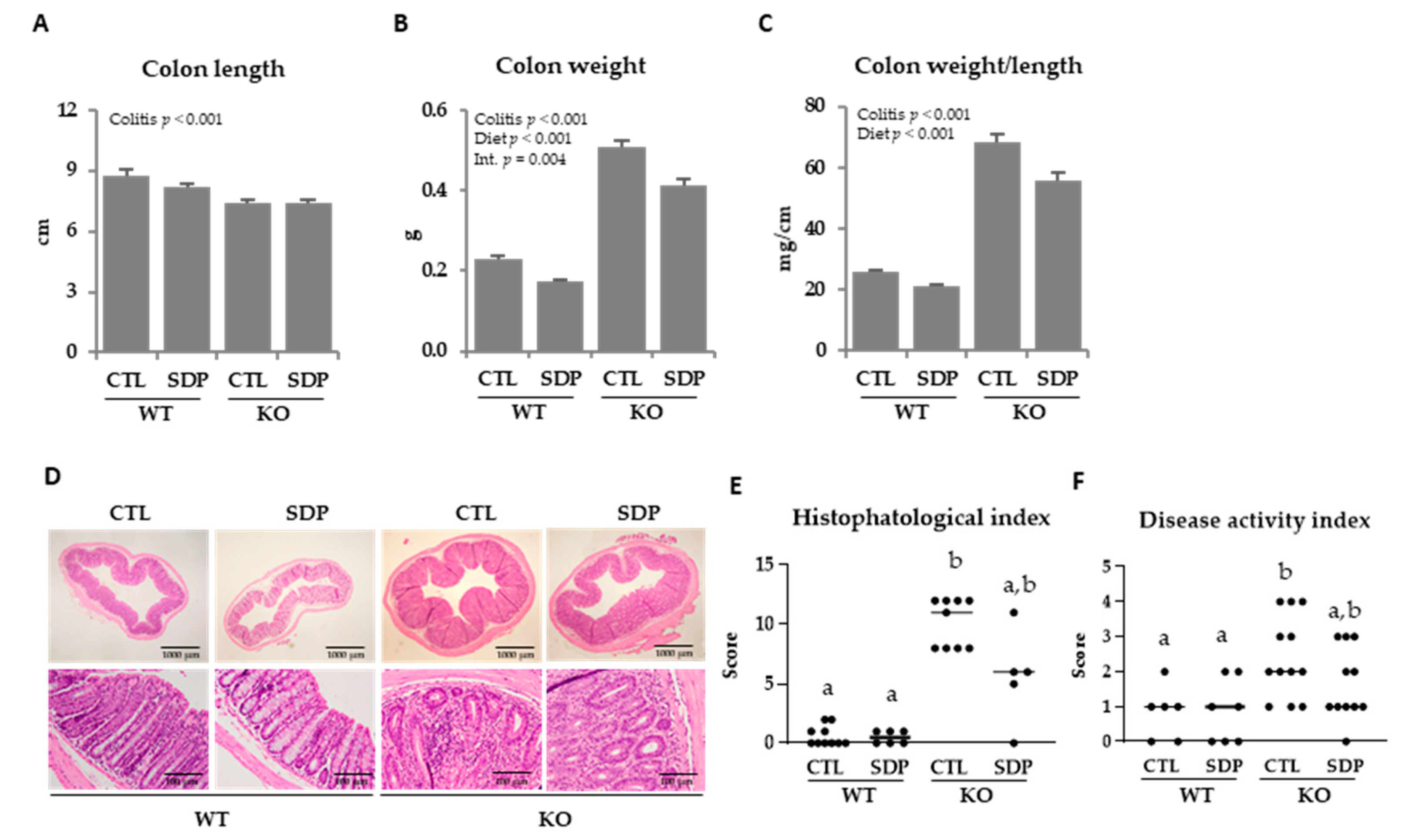
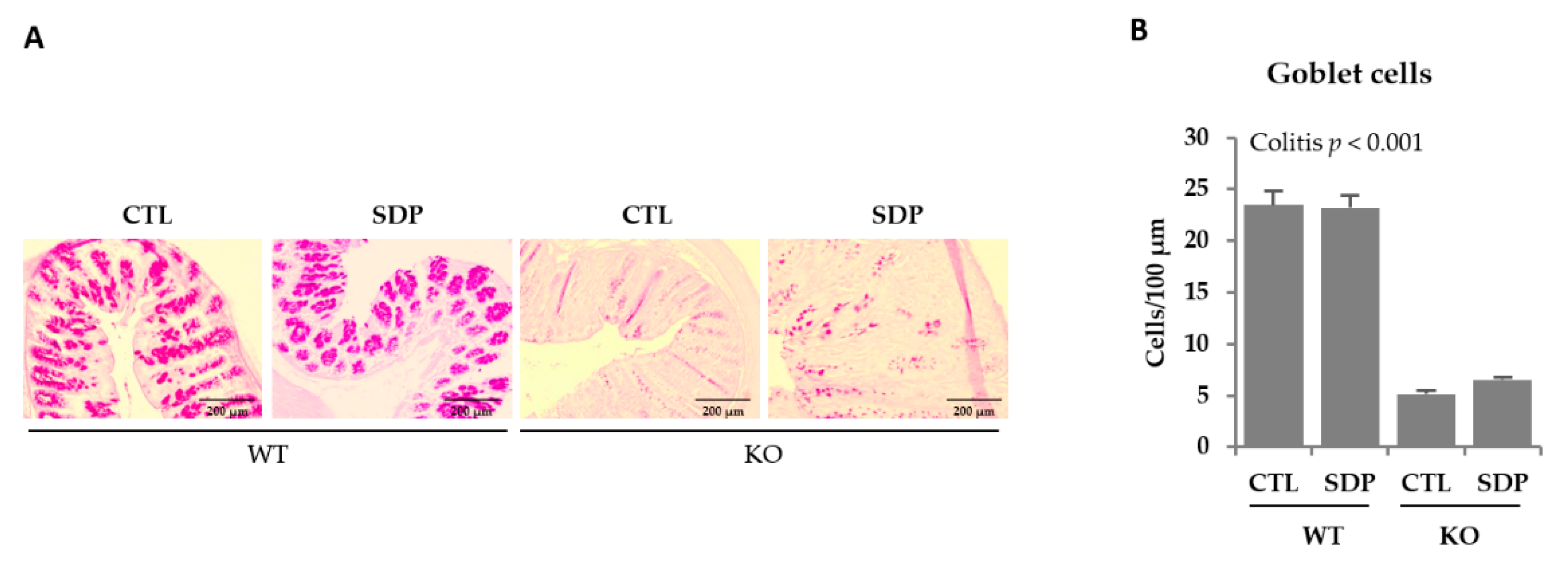
2.1. Morphological Effects of SDP in Mdr1a KO Mice
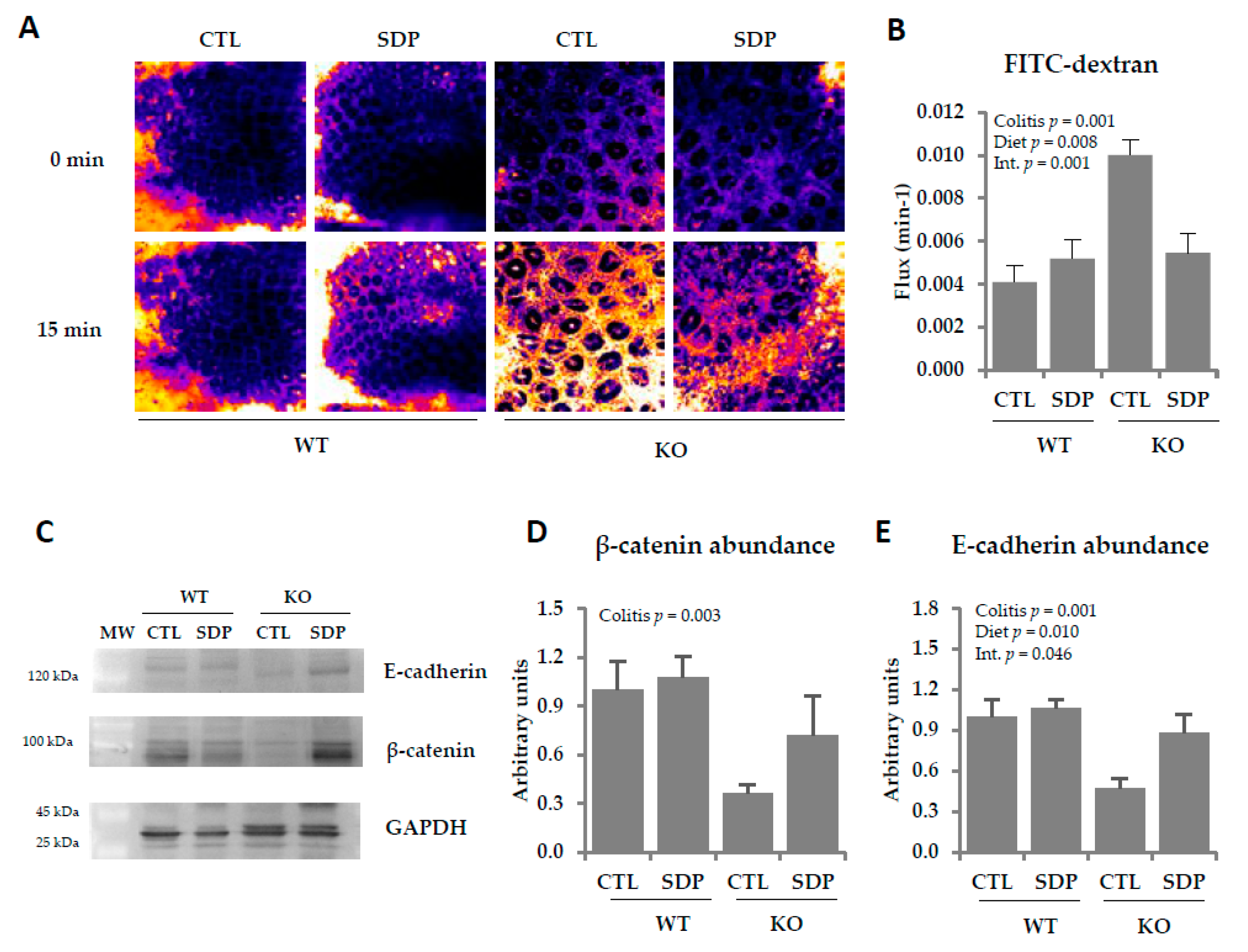
2.2. Effects of SDP on Colon Permeability in Mdr1a Knockout Mice
2.3. Effects of SDP on the Immune System in the Lamina Propria and Intraepithelial Lymphocytes in Mdr1a KO Mice
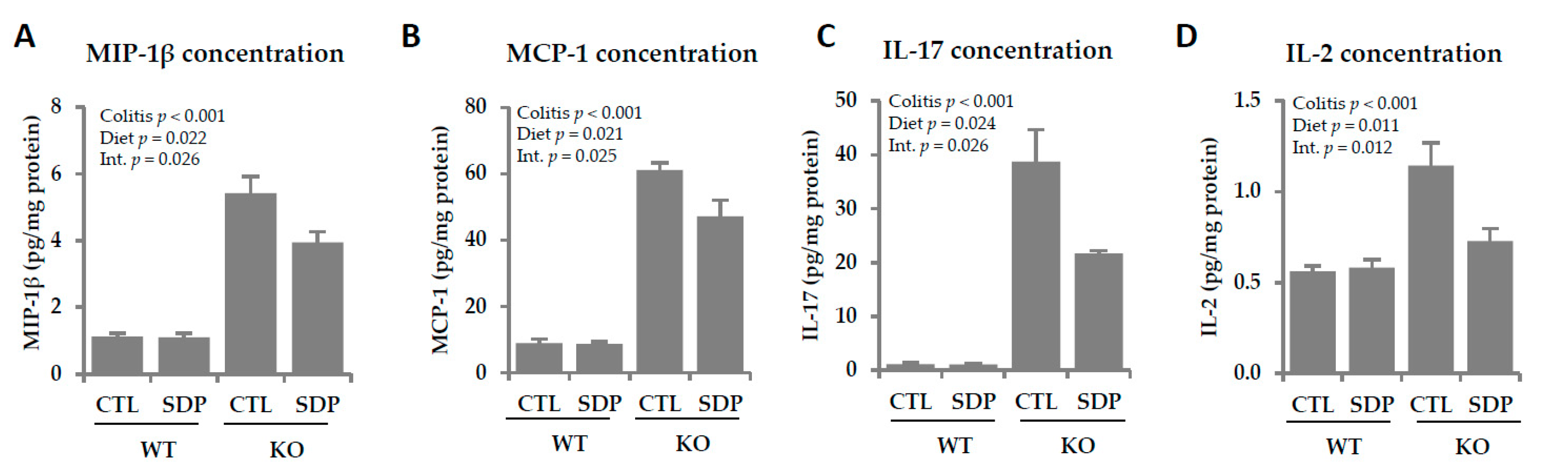
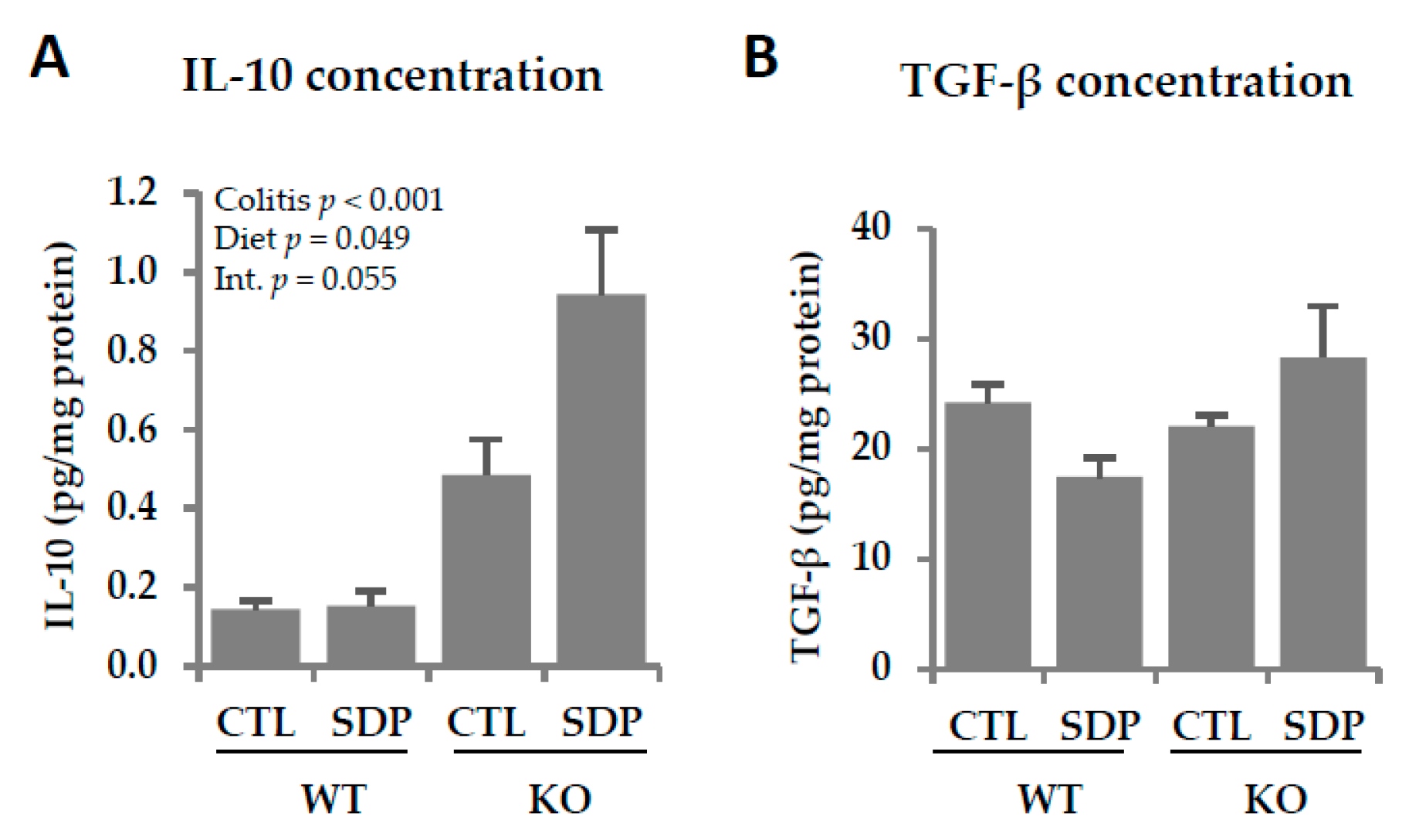
2.4. Effects of SDP Suplementation on Inflammation Markers in Mdr1a KO Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Morphological Study
4.3. Intraepithelial Lymphocytes and Lamina Propria Lymphocytes Isolation
4.4. Cell Staining
4.5. Epithelial Fluorescent Dextran Permeability
4.6. Western Blot
4.7. Real-Time PCR
4.8. Cytokine Concentration
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CTL | Control |
| FITC | Fluorescein isothiocyanate |
| GALT | Gut-associated lymphoid tissue |
| IBD | Inflammatory bowel disease |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| Inos | Inducible nitric oxide synthase |
| KO | Knockout |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MIP-1β | Macrophage inflammatory protein 1 beta |
| Muc1 | Mucin 1 |
| Muc2 | Mucin 2 |
| Muc4 | Mucin 4 |
| Muc6 | Mucin 6 |
| MW | Molecular weight |
| SDP | Spray-dried porcine plasma |
| Tff3 | Trefoil factor 3 |
| TGF-β | Transforming growth factor-beta |
| Th | T helper |
| Treg | Regulatory Th lymphocytes |
| TNF-α | Tumor necrosis factor-alpha |
| WT | Wild type |
References
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases. Gastroenterology 2014, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.Y.; Kim, S.; Son, M.J.; Son, C.Y.; Kim, J.Y.; Kronbichler, A.; Lee, K.H.; Shin, J.I. Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun. Rev. 2019, 18, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am. J. Transl. Res. 2016, 8, 2490–2497. [Google Scholar] [PubMed]
- Goto, Y.; Ivanov, I.I. Intestinal epithelial cells as mediators of commensal-host immune crosstalk. Immumol. Cell Biol. 2013, 91, 204–214. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Pedicord, A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, A.V. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells 2019, 8, 193. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Smitham, J.; Barrett, K.E. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/-mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G153–G162. [Google Scholar] [CrossRef]
- Ho, G.T.; Aird, R.E.; Liu, B.; Boyapati, R.K.; Kennedy, N.A.; Dorward, D.A.; Noble, C.L.; Shimizu, T.; Carter, R.N.; Chew, E.T.S.; et al. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 2018, 11, 120–130. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Miró, L.; Maijó, M.; Polo, J.; Campbell, J.; Russell, L.; Crenshaw, J.; Weaver, E.; Moretó, M. Dietary intervention with serum-derived bovine immunoglobulins protects barrier function in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Miró, L.; Maijó, M.; Polo, J.; Campbell, J.; Russell, L.; Crenshaw, J.; Weaver, E.; Moretó, M. Oral serum-derived bovine immunoglobulin/protein isolate has immunomodulatory effects on the colon of mice that spontaneously develop colitis. PLoS ONE 2016, 11, e0154823. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.; Weaver, E.; Klein, G.; Wignall, A.; Wozniak, B.; Plews, E.; Mayo, B.; White, I.; Keefe, D. Serum-derived bovine immunoglobulin/protein isolate in the alleviation of chemotherapy-induced mucositis. Support. Care Cancer 2016, 24, 377–385. [Google Scholar] [CrossRef][Green Version]
- Lallès, J.P.; Bosi, P.; Janczyk, P.; Koopmans, J.S.; Torrallardona, D. Impact of bioactive substances on the gastrointestinal tract and performance of weaned piglets: A review. Animals 2009, 3, 1625–1643. [Google Scholar] [CrossRef]
- Weaver, A.C.; Campbell, J.M.; Crenshaw, J.D.; Polo, J.; Kim, D.W. Efficacy of dietary spray dried plasma protein to mitigate the negative effects on performance of pigs fed diets with corn naturally contaminated with multiple mycotoxins. J. Anim. Sci. 2014, 92, 3878–3886. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Amat, C.; Polo, J.; Campbell, J.M.; Crenshaw, J.; Russell, L.; Moretó, M. Spray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned rats. J. Nutr. 2006, 136, 2838–2843. [Google Scholar] [CrossRef]
- Miró, L.; Garcia-Just, A.; Amat, C.; Polo, J.; Moretó, M.; Pérez-Bosque, A. Dietary animal plasma proteins improve the intestinal immune response in senescent mice. Nutrients 2017, 9, 1346. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Miró, L.; Polo, J.; Russell, L.; Campbell, J.; Weaver, E.; Crenshaw, J.; Moretó, M. Dietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in rats. J. Nutr. 2010, 140, 25–30. [Google Scholar] [CrossRef]
- Garcia-Just, A.; Miró, L.; Pérez-Bosque, A.; Amat, C.; Polo, J.; Pallàs, M.; Griñán-Ferré, C.; Moretó, M. Dietary spray-dried porcine plasma prevents cognitive decline in senescent mice and reduces neuroinflammation and oxidative stress. J. Nutr. 2020, 150, 303–311. [Google Scholar] [CrossRef]
- Sanchez-Muñoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.I.; Cho, S.; Choi, N.J. Effect of dietary probiotics on colon length in an inflammatory bowel disease-induced murine model: A meta-analysis. J. Dairy Sci. 2020, 103, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Yoseph, B.P.; Klingensmith, N.J.; Liang, Z.; Breed, E.R.; Burd, E.M.; Mittal, R.; Dominguez, J.A.; Petrie, B.; Ford, M.L.; Coopersmith, C.M. Mechanisms of intestinal barrier dysfunction in sepsis. Shock 2016, 46, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Boltin, D.; Perets, T.T.; Vilkin, A.; Niv, Y. Mucin function in inflammatory bowel disease: An update. J. Clin. Gastroenterol. 2013, 47, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.H.; Hasnain, S.Z.; Florin, T.H.J.; McGuckin, M.A. Mucins in inflammatory bowel diseases and colorectal cancer. J. Gastroenterol. Hepatol. 2012, 27, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil factor peptides and gastrointestinal function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef]
- Kjellev, S. The trefoil factor family-small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar] [CrossRef]
- Feng, L.; Chen, S.; Zhang, L.; Chen, Z. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice. Environ. Pollut. 2019, 254, 112960. [Google Scholar] [CrossRef]
- Sorribas, M.; de Gottardi, A.; Moghadamrad, S.; Hassan, M.; Spadoni, I.; Rescigno, M.; Wiest, R. Isoproterenol disrupts intestinal barriers activating gut-liver-axis: Effects on intestinal mucus and vascular barrier as entry sites. Digestion 2019, 24, 1–13. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vanuytsel, T.; Farré, R.; Verstockt, S.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Schuit, F.; Vermeire, S.; Arijs, I.; et al. Genetic and transcriptomic bases of intestinal epithelial barrier dysfunction in inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 1718–1729. [Google Scholar] [CrossRef]
- Breugelmans, T.; Van Spaendonk, H.; De Man, J.G.; De Schepper, H.U.; Jauregui-Amezaga, A.; Macken, E.; Lindén, S.K.; Pintelon, I.; Timmermans, J.P.; De Winter, B.Y.; et al. In-depth study of transmembrane mucins in association with intestinal barrier dysfunction during the course of T cell transfer and DSS-induced colitis. J. Crohns Colitis 2020, 31, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Cuellar, J.G.; Diehl, M.L.; deFreytas, A.M.; Zhang, J.; Carraway, K.L.; Voynow, J.A. Neutrophil elastase increases MUC4 expression in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L671–L679. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.E.; Cho, M.L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Muthas, D.; Reznichenko, A.; Balendran, C.A.; Böttcher, G.; Clausen, I.G.; Kärrman Mårdh, C.; Ottosson, T.; Uddin, M.; MacDonald, T.T.; Danese, S.; et al. Neutrophils in ulcerative colitis: A review of selected biomarkers and their potential therapeutic implications. Scand. J. Gastroenterol. 2017, 52, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M. New therapeutic strategies for treatment of inflammatory bowel disease. Mucosal Immunol. 2008, 1, 175–182. [Google Scholar] [CrossRef]
- Ajuebor, M.N.; Kunkel, S.L.; Hogaboam, C.M. The role of CCL3/macrophage inflammatory protein-1α in experimental colitis. Eur. J. Pharmacol. 2004, 497, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Park, S.; Kaplan, M.H.; Olson, M.R. Effector T helper cell subsets in inflammatory bowel diseases. Front. Immunol. 2018, 9, 1212. [Google Scholar] [CrossRef]
- Wallace, K.L.; Zheng, L.B.; Kanazawa, Y.; Shih, D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6–21. [Google Scholar] [CrossRef]
- Műzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Miró, L.; Polo, J.; Russell, L.; Campbell, J.; Weaver, E.; Crenshaw, J.; Moretó, M. Dietary plasma proteins modulate the immune response of diffuse gut-associated lymphoid tissue in rats challenged with Staphylococcus aureus enterotoxin B. J. Nutr. 2008, 138, 533–537. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Miró, L.; Amat, C.; Polo, J.; Moretó, M. The anti-inflammatory effect of spray-dried plasma is mediated by a reduction in mucosal lymphocyte activation and infiltration in a mouse model of intestinal inflammation. Nutrients 2016, 8, 657. [Google Scholar] [CrossRef]
- Maijó, M.; Miró, L.; Polo, J.; Campbell, J.; Russell, L.; Crenshaw, J.; Weaver, E.; Moretó, M.; Pérez-Bosque, A. Dietary plasma proteins modulate the adaptive immune response in mice with acute lung inflammation. J. Nutr. 2012, 142, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Yousaf, W.; Giannella, R.; Shata, M.T. Th17 cells: Interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease. Expert Rev. Clin. Immunol. 2012, 8, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Laan, M.; Cui, Z.H.; Hoshino, H.; Lötvall, J.; Sjöstrand, M.; Gruenert, D.C.; Skoogh, B.E.; Lindén, A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999, 162, 2347–2352. [Google Scholar] [PubMed]
- Maijó, M.; Miró, L.; Polo, J.; Campbell, J.; Russell, L.; Crenshaw, J.; Weaver, E.; Moretó, M.; Pérez-Bosque, A. Dietary plasma proteins attenuate the innate immunity response in a mouse model of acute lung injury. Br. J. Nutr. 2012, 107, 867–875. [Google Scholar] [CrossRef]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef]
- Izcue, A.; Coombes, J.L.; Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 2009, 27, 313–338. [Google Scholar] [CrossRef]
- Coombes, J.L.; Maloy, K.J. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin. Immunol. 2007, 19, 116–126. [Google Scholar] [CrossRef]
- Eastaff-Leung, N.; Mabarrack, N.; Barbour, A.; Cummins, A.; Barry, S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 2010, 30, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. TGF-β and regulatory T cell in immunity and autoimmunity. J. Clin. Immunol. 2008, 28, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Lee, J.J.; Che, T.M.; Soares-Almeida, J.A.; Chun, J.L.; Campbell, J.M.; Polo, J.; Crenshaw, J.D.; Seo, S.W.; et al. Spray-dried plasma attenuates inflammation and improves pregnancy rate of mated female mice. J. Anim. Sci. 2015, 93, 298–305. [Google Scholar] [CrossRef]
- Maynard, C.L.; Weaver, C.T. Diversity i the contribution of IL-10 to T-cell-mediated immune regulation. Immunol. Rev. 2008, 226, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Angulo, S.; Llopis, M.; Antolín, M.; Gironella, M.; Sans, M.; Malagelada, J.R.; Piqué, J.M.; Guarner, F.; Panés, J. Lactobacillus casei prevents the upregulation of ICAM-1 expression and leukocyte recruitment in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1155–G1162. [Google Scholar] [CrossRef]
- Moretó, M.; Miró, L.; Amat, C.; Polo, J.; Manichanh, C.; Pérez-Bosque, A. Dietary supplementation with spray-dried porcine plasma has prebiotic effects on gut microbiota in mice. Sci. Rep. 2020, 10, 2926. [Google Scholar] [CrossRef]
- Weigmann, B.; Tubbe, I.; Seidel, D.; Nicolaev, A.; Becker, C.; Neurath, M.F. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2007, 2, 2307–2311. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Curran-Everett, D.; Benos, J.D. Guidelines for reporting statistics in journals published by the American Physiological Society. Am. J. Physiol. Endocrinol. Metab. 2004, 287, 189–191. [Google Scholar] [CrossRef]

| WT | KO | p Value | |||||
|---|---|---|---|---|---|---|---|
| CTL | SDP | CTL | SDP | Colitis | Diet | Int. | |
| Muc1 | 1.00 ± 0.04 | 1.98 ± 0.03 | 5.90 ± 0.50 | 5.00 ± 0.36 | <0.001 | ns | 0.016 |
| Muc2 | 1.00 ± 0.09 | 0.84 ± 0.04 | 0.21 ± 0.03 | 0.35 ± 0.03 | <0.001 | ns | 0.023 |
| Muc4 | 1.00 ± 0.17 | 0.59 ± 0.02 | 2.86 ± 0.31 | 1.85 ± 0.10 | <0.001 | 0.005 | ns |
| Muc6 | 1.00 ± 0.20 | 0.96 ± 0.36 | 1.17 ± 0.17 | 0.90 ± 0.15 | ns | ns | ns |
| Tff3 | 1.00 ± 0.16 | 0.97 ± 0.09 | 0.42 ± 0.08 | 0.92 ± 0.13 | 0.036 | ns | 0.053 |
| WT | KO | p Value | |||||
|---|---|---|---|---|---|---|---|
| CTL | SDP | CTL | SDP | Colitis | Diet | Int. | |
| Cell count (×106) | 1.55 ± 0.17 | 1.14 ± 0.19 | 16.0 ± 1.07 | 11.1 ± 0.97 | <0.001 | 0.003 | 0.011 |
| Monocytes | 8.10 ± 0.58 | 7.74 ± 0.79 | 13.0 ± 0.49 | 10.5 ± 0.83 | <0.001 | 0.044 | ns |
| Act. monocytes | 29.8 ± 2.72 | 27.1 ± 1.74 | 60.3 ± 1.70 | 49.3 ± 1.89 | <0.001 | 0.002 | ns |
| Neutrophils | 19.0 ± 2.19 | 17.5 ± 2.52 | 38.9 ± 1.39 | 31.4 ± 2.08 | <0.001 | 0.034 | ns |
| Act. neutrophils | 31.1 ± 2.78 | 25.7 ± 2.30 | 57.8 ± 2.21 | 51.5 ± 2.53 | <0.001 | 0.024 | ns |
| Dendritic cells | 19.7 ± 1.50 | 23.7 ± 1.38 | 9.49 ± 0.41 | 12.2 ± 0.60 | <0.001 | <0.001 | ns |
| Act. dendritic cells | 4.51 ± 0.79 | 9.29 ± 1.29 | 6.36 ± 0.46 | 10.7 ± 1.34 | 0.063 | <0.001 | ns |
| Act. Th lymphocytes | 6.80 ± 0.69 | 5.32 ± 0.58 | 17.0 ± 0.85 | 13.8 ± 0.60 | <0.001 | 0.009 | ns |
| Treg lymphocytes | 3.26 ± 0.45 | 3.34 ± 0.53 | 5.77 ± 0.52 | 8.81 ± 0.55 | <0.001 | 0.017 | 0.022 |
| Act. Th/Treg lym. ratio | 2.51 ± 0.22 | 1.66 ± 0.19 | 3.09 ± 0.27 | 1.60 ± 0.09 | ns | <0.001 | 0.040 |
| WT | KO | p Value | |||||
|---|---|---|---|---|---|---|---|
| CTL | SDP | CTL | SDP | Colitis | Diet | Int. | |
| Cell count (×106) | 0.26 ± 0.06 | 0.19 ± 0.05 | 1.76 ± 0.22 | 0.81 ± 0.10 | <0.001 | <0.001 | 0.003 |
| Monocytes | 9.39 ± 0.60 | 8.21 ± 1.55 | 12.8 ± 0.78 | 11.1 ± 0.91 | <0.001 | ns | ns |
| Act. monocytes | 5.90 ± 1.17 | 3.88 ± 0.49 | 19.6 ± 1.71 | 13.1 ± 0.58 | <0.001 | 0.007 | ns |
| Neutrophils | 8.09 ± 0.75 | 5.61 ± 0.54 | 15.5 ± 0.94 | 9.82 ± 0.54 | <0.001 | <0.001 | ns |
| Act. neutrophils | 4.43 ± 1.13 | 6.31 ± 0.99 | 21.7 ± 1.83 | 12.7 ± 0.83 | <0.001 | 0.030 | 0.002 |
| Dendritic cells | 20.1 ± 2.55 | 19.1 ± 2.22 | 15.0 ± 1.42 | 16.4 ± 0.76 | 0.025 | ns | ns |
| Act. dendritic cells | 20.9 ± 2.78 | 20.0 ± 2.02 | 13.8 ± 0.68 | 17.7 ± 0.63 | 0.003 | ns | ns |
| Act. Th lymphocytes | 6.08 ± 0.18 | 6.37 ± 0.76 | 16.1 ± 0.94 | 13.0 ± 0.74 | <0.001 | ns | ns |
| Treg lymphocytes | 3.10 ± 0.43 | 5.33 ± 0.26 | 3.39 ± 0.21 | 5.27 ± 0.47 | ns | <0.001 | ns |
| Act. Th/Treg lym. ratio | 2.09 ± 0.31 | 1.19 ± 0.09 | 4.83 ± 0.26 | 2.58 ± 0.18 | <0.001 | <0.001 | 0.015 |
| Ingredients | Control Diet | Ingredients |
|---|---|---|
| SDP 1 | - | 80 |
| Dried skim milk | 530.7 | 340.5 |
| Corn starch | 199.3 | 308.8 |
| Sucrose | 94.5 | 94.5 |
| Soybean oil | 70 | 70 |
| Cellulose | 50 | 50 |
| AIN-93-G-MX 2 | 35 | 35 |
| AIN-93 VX 2 | 15 | 15 |
| Choline bitartrate | 3 | 3 |
| Methionine | 2.5 | 3.2 |
| Population | Gate |
|---|---|
| Leukocytes | CD45+ |
| B lymphocytes | CD19+CD45+ |
| T helper cells | CD4+CD45+ |
| Activated Th lymphocytes | CD25+FOXP3−CD4+ |
| Regulatory Th lymphocytes | CD25+FOXP3+CD4+ |
| Neutrophils | CD45+ non-lymphocytic leucocytes were separated by forward/side scatter Ly6G+ |
| Monocytes | CD45+ non-lymphocytic leucocytes were separated by forward/side scatter CD68+ |
| Activated neutrophils | CD45+ non-lymphocytic leucocytes were separated by forward/side scatter Ly6G+CD14+ |
| Activated monocytes | CD45+ non-lymphocytic leucocytes were separated by forward/side scatter CD68+CD14+ |
| Dendritic cells | CD11c+CD45+ |
| Activated dendritic cells | CD45+CD11c+CD86+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miró, L.; Amat, C.; Rosell-Cardona, C.; Campbell, J.M.; Polo, J.; Pérez-Bosque, A.; Moretó, M. Dietary Supplementation with Spray-Dried Porcine Plasma Attenuates Colon Inflammation in a Genetic Mouse Model of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 6760. https://doi.org/10.3390/ijms21186760
Miró L, Amat C, Rosell-Cardona C, Campbell JM, Polo J, Pérez-Bosque A, Moretó M. Dietary Supplementation with Spray-Dried Porcine Plasma Attenuates Colon Inflammation in a Genetic Mouse Model of Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2020; 21(18):6760. https://doi.org/10.3390/ijms21186760
Chicago/Turabian StyleMiró, Lluïsa, Concepció Amat, Cristina Rosell-Cardona, Joy M. Campbell, Javier Polo, Anna Pérez-Bosque, and Miquel Moretó. 2020. "Dietary Supplementation with Spray-Dried Porcine Plasma Attenuates Colon Inflammation in a Genetic Mouse Model of Inflammatory Bowel Disease" International Journal of Molecular Sciences 21, no. 18: 6760. https://doi.org/10.3390/ijms21186760
APA StyleMiró, L., Amat, C., Rosell-Cardona, C., Campbell, J. M., Polo, J., Pérez-Bosque, A., & Moretó, M. (2020). Dietary Supplementation with Spray-Dried Porcine Plasma Attenuates Colon Inflammation in a Genetic Mouse Model of Inflammatory Bowel Disease. International Journal of Molecular Sciences, 21(18), 6760. https://doi.org/10.3390/ijms21186760





