Genetic and Epigenetic Mechanisms Underlying Vascular Smooth Muscle Cell Phenotypic Modulation in Abdominal Aortic Aneurysm
Abstract
1. Introduction
2. Genetics of AAA
3. VSMC Development and AAA
4. VSMC-Mediated Extracellular Matrix Production and Degradation in AAA
4.1. MMPs in AAA
4.2. Other Mediators of ECM Degradation: Cathepsins, Osteoprotegerin and Kallikreins
4.3. TIMPs
4.4. miRs Regulating ECM Degradation
4.4.1. miR-29b
4.4.2. miR-205
4.5. HDAC Inhibitors Regulating MMPs-2 and -9
4.5.1. MS-275 and MC-1568
4.5.2. Metacept (MCT)
4.5.3. HATs in AAA
5. Smooth Muscle Cells and Inflammation in AAA
5.1. Upregulated Cytokines in AAA
5.2. Inflammation-Associated Genes
5.3. Reduced Contractile Phenotype/Anti-Inflammatory Inducing miRNA in AAA
5.3.1. miR-24
5.3.2. miR-143/145
6. Smooth Muscle Cell Plasticity and Apoptosis in AAA
6.1. Genetic and Epigenetic Mechanisms Promoting the Synthetic SMC Phenotype
6.1.1. Cyclin-Dependent Kinase 2B Antisense (CDKN2BAS)
6.1.2. Disabled Homologue 2 Interacting Protein (DAB2IP)
6.1.3. Lipoprotein Receptor-Related Protein 1 (LRP1)
6.1.4. Receptor-Interacting Serine/Threonine-Protein Kinase 3 (RIPK3)
6.1.5. Notch Homolog 1 Translocation-Associated (NOTCH1)
6.1.6. HIF-1α
6.2. Elevated miRNA Promoting Synthetic Phenotype
6.2.1. miR-21
6.2.2. miR-146a
6.2.3. miR-26a
6.2.4. miR-221/222
6.3. lncRNAs Associated with Pro-Proliferative SMC Upregulated Alongside Apoptosis
6.3.1. H19
6.3.2. Plasmacytoma Variant Translocation 1 (PVT1)
6.4. Downregulation of Protective Genes in AAA
6.4.1. Peroxisome Proliferative-Activated Receptor Gamma (PPARG)
6.4.2. Serpin Proteinase Inhibitor B9 (SERPINB9)
6.5. Reduced miRNAs Promoting Contractile Phenotype
miR-143/145
7. Smooth Muscle and ROS in AAA
7.1. NADPH Oxidase
7.2. Low Antioxidant Gene Expression in AAA
7.3. ROS and Histone Acetylation
8. DNA Methylation in AAA
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAA | Abdominal aortic aneurysm |
| ACTA | Actin alpha 2 |
| ADCY101 | Adenylate cyclase 10 pseudogene 1 |
| ADD3 | Adducin-3 |
| Ang | Angiotensin |
| ApoB100 | Apolipoprotein B100 |
| Bcl-2 | B cell lymphoma 2 |
| C>T | Cytosine to Thymine single nucleotide polymorphism |
| CaCl2 | Calcium chloride |
| CAD | Coronary artery disease |
| CatK/G | Cathepsin K/G |
| CDKN | Cyclin-dependent kinase |
| Chi311 | Chitinase 3-like 1 |
| CNN | Calponin |
| COL | Collagen |
| CpG | Cytosine-guanine dinucleotide cluster |
| DNA | Deoxyribonucleic acid |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| EDHF | Endothelium-derived hyperpolarising factor |
| ELN | Elastin |
| ERG | E26 oncogene homolog |
| ERK | Extracellular signal regulated kinase |
| G>C | Guanine to Cytosine single nucleotide polymorphism |
| H2O2 | Hydrogen peroxide |
| H3K4me2 | Dimethylation of lysin residue 4 on histone 3 |
| HDAC | Histone deacetylases |
| hESC | Human embryonic stem cell |
| HIF1α | Hypoxia-inducible factor 1 α |
| Hsp90 | Heat shock protein 90 |
| ID3 | Inhibitor of differentiation 3 |
| Il-1β | Interleukin-1β |
| Il-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide |
| Jag1 | Jagged1 |
| JNK | c-Jun N-terminal kinase |
| KAT | Lysine histone acetylase transferase |
| KLF | Kruppel-like factor 4 |
| KLHL35 | Kelch-like family member 35 |
| LKL | Kallikrein |
| LncRNA | Long non-coding ribonucleic acid |
| LRP1 | Lipoprotein receptor-related protein 1 |
| MAPK | Mitogen-activated protein kinase |
| MBD | Methyl-cytosine binding |
| Mcl-1 | Myeloid cell leukemia 1 |
| Mdm2 | Mouse 3T3 cell double minute 2 |
| mIR | Micro ribonucleic acid |
| miRNA | Micro ribonucleic acid |
| MMP | Matrix metallopeptidase |
| mRNA | Messenger ribonucleic acid |
| MRTF-B | Myocardin-related transcription factor-B |
| MYH11 | Myosin heavy chain 11 |
| NADPH | Reduced Nicotinamide adenine dinucleotide phosphate |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nkx2-5 | NK-2 transcription factor |
| NOX | NADPH oxidase |
| Nt | Nucleotide |
| OPG | Osteoprotegerin |
| PCSK9 | Propretein convertase subtilisin/kexin type 9 |
| PDGF | Platelet-derived growth factor |
| PI3k | Phosphoinositide 3-kinase |
| PPARγ | Peroxisome proliferative-activated receptor gamma |
| PPE | Porcine pancreatic elastase |
| PTEN | Phosphatase and tensin homolog |
| PVT1 | Plasmacytoma variant translocation 1 |
| RECK | Reversion inducing cysteine-rich protein with kazal motifs |
| RIPK3 | Receptor-interacting serine/threonine-protein kinase 3 |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SERPINA1 | Serine peptidase inhibitor, clade A, member 1 |
| Shh | Sonic hedgehog |
| shRNA | Short hairpin RNA |
| SMYD2 | SET and MYND domain-containing 2 |
| SNP | Single nucleotide polymorphism |
| SOD | Superoxide dismutase |
| SRF | Serum response factor |
| STAT | Signal transducer and activator of transcription proteins |
| TAA | Thoracic aortic aneurysm |
| TGFBR2 | Transforming growth factor beta receptor 2 |
| TGF-β | Transforming growth factor β |
| TIMP | Tissue inhibitor of metallopeptidase |
| TNFα | Tumor necrosis factor α |
| UCP | Uncoupling protein |
| UTR | Untranslated region |
| VSMC | Vascular smooth muscle cell |
| α1-AT | α1-antitrypsin |
References
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s choice–European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Grøndal, N.; Søgaard, R.; Lindholt, J.S. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65–74 years from a population screening study (VIVA trial). Br. J. Surg. 2015, 102, 902–906. [Google Scholar] [CrossRef]
- Lee, E.S.; Pickett, E.; Hedayati, N.; Dawson, D.L.; Pevec, W.C. Implementation of an aortic screening program in clinical practice: Implications for the screen for abdominal aortic aneurysms very efficiently (SAAAVE) Act. J. Vasc. Surg. 2009, 49, 1107–1111. [Google Scholar] [CrossRef]
- Forsdahl, S.H.; Solberg, S.; Singh, K.; Jacobsen, B.K. Abdominal aortic aneurysms, or a relatively large diameter of non-aneurysmal aortas, increase total and cardiovascular mortality: The Tromsø study. Int. J. Epidemiol. 2010, 39, 225–232. [Google Scholar] [CrossRef] [PubMed]
- DE Rango, P.; Farchioni, L.; Fiorucci, B.; Lenti, M. Diabetes and abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2013, 47, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, M.J.; Thompson, S.G.; Brown, L.C.; Powell, J.T. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br. J. Surg. 2012, 99, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W. Basic science of abdominal aortic aneurysms: Emerging therapeutic strategies for an unresolved clinical problem. Curr. Opin. Cardiol. 1996, 11, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation involvement of vitamin K-dependent processes. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef]
- Pfaltzgraff, E.R.; Bader, D.M. Heterogeneity in vascular smooth muscle cell embryonic origin in relation to adult structure, physiology, and disease. Dev. Dyn. 2015, 244, 410–416. [Google Scholar] [CrossRef]
- Bicknell, C.D.; Kiru, G.; Falaschetti, E.; Powell, J.T.; Poulter, N.R.; Ashby, D.; Brown, L.; Dhanjil, S.; Davies, M.; Earnshaw, J.; et al. An evaluation of the effect of an angiotensinconverting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: A randomized placebo-controlled trial (AARDVARK). Eur. Heart J. 2016, 37, 3213–3221. [Google Scholar] [CrossRef]
- Kokje, V.B.C.; Hamming, J.F.; Lindeman, J.H.N. Editor’s choice-Pharmaceutical management of small abdominal aortic aneurysms: A systematic review of the clinical evidence. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 702–713. [Google Scholar] [CrossRef]
- Baxter, B.T.; Matsumura, J.; Curci, J.A.; McBride, R.; Larson, L.; Blackwelder, W.; Lam, D.; Wijesinha, M.; Terrin, M. Effect of doxycycline on aneurysm growth among patients with small infrarenal abdominal aortic aneurysms: A randomized clinical trial. JAMA 2020, 323, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, N.; Meekel, J.P.; Micha, D.; Blankensteijn, J.D. Impaired smooth muscle cell contractility as a novel concept of abdominal aortic aneurysm pathophysiology. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, C.M.; Larsson, E.; Magnusson, P.K.E.; Hultgren, R.; Swedenborg, J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J. Vasc. Surg. 2010, 51, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Svensjö, S.; Björck, M.; Gürtelschmid, M.; Djavani Gidlund, K.; Hellberg, A.; Wanhainen, A. Low prevalence of abdominal aortic aneurysm among 65-year-old swedish men indicates a change in the epidemiology of the disease. Circulation 2011, 124, 1118–1123. [Google Scholar] [CrossRef]
- Larsson, E.; Granath, F.; Hultgren, R.; Swedenborg, J. A population-based case-control study of the familial risk of abdominal aortic aneurysm. J. Vasc. Surg. 2009, 49, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.; Koepsell, T. Familial tendency for abdominal aortic aneurysms. JAMA 1986, 256, 1934–1936. [Google Scholar] [CrossRef]
- Ogata, T.; Mackean, G.L.; Cole, C.W.; Arthur, C.; Andreou, P.; Tromp, G.; Kuivaniemi, H. NIH public access. J. Vasc. Surg. 2005, 42, 891–897. [Google Scholar] [CrossRef]
- Jones, G.T.; Hill, B.G.; Curtis, N.; Kabir, T.D.; Wong, L.E.; Tilyard, M.W.; Williams, M.J.A.; Rij, A.M. Van comparison of three targeted approaches to screening for abdominal aortic aneurysm based on cardiovascular risk. Br. J. Surg. 2016, 103, 1139–1146. [Google Scholar] [CrossRef]
- Sandford, R.M.; Bown, M.J.; London, N.J.; Sayers, R.D. The genetic basis of abdominal aortic aneurysm: A review. Eur. J. Vasc. Endovasc. Surg. 2007, 390, 381–390. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Defraigne, J.O.; Kerstenne, M.A.; Cheramy-Bien, J.P.; Smelser, D.T.; Tromp, G.; Kuivaniemi, H. Family members of patients with abdominal aortic aneurysms are at increased risk for aneurysms: Analysis of 618 probands and their families from the liège AAA family study. Ann. Vasc. Surg. 2014, 28, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Van de Luijtgaarden, K.M.; Bastos Gonçalves, F.; Hoeks, S.E.; Majoor-Krakauer, D.; Rouwet, E.V.; Stolker, R.J.; Verhagen, H.J.M. Familial abdominal aortic aneurysm is associated with more complications after endovascular aneurysm repair. J. Vasc. Surg. 2014, 59, 275–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beckman, J.A. Aortic aneurysm: Pathophysiology, epidmiology and prognosis. In Vascular Medicine: A Companion to Braunwald’s Heart Disease; Creager, M., Dzau, V., Loscalzo, J., Eds.; Saunders Elsevier Inc.: Philadephia, PA, USA, 2006; pp. 457–470. [Google Scholar]
- Gomez, D.; Swiatlowska, P.; Owens, G.K. Epigenetic control of smooth muscle cell identity and lineage memory. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, F.; Ruigrok, Y.; Lee, C.; Ripke, S.; Anderson, G.; de Andrade, M.; Baas, A.; Blankensteijn, J.; Bottinger, E.; Bown, M.J.; et al. shared genetic risk factors of intracranial, abdominal, and thoracic aneurysms. J. Am. Hear. Assoc. 2016, 5, 1–19. [Google Scholar] [CrossRef]
- Helgadottir, A.; Thorleifsson, G.; Magnusson, K.P.; Grétarsdottir, S.; Steinthorsdottir, V.; Manolescu, A.; Jones, G.T.; Rinkel, G.J.E.; Blankensteijn, J.D.; Ronkainen, A.; et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat. Genet. 2008, 40, 217–224. [Google Scholar] [CrossRef]
- Yao, Q.; Renault, M.A.; Chapouly, C.; Vandierdonck, S.; Belloc, I.; Jaspard-Vinassa, B.; Daniel-Lamazière, J.M.; Laffargue, M.; Merched, A.; Desgranges, C.; et al. Sonic hedgehog mediates a novel pathway of PDGF-BB-dependent vessel maturation. Blood 2014, 123, 2429–2437. [Google Scholar] [CrossRef]
- High, F.A.; Min, M.L.; Pear, W.S.; Loomes, K.M.; Kaestner, K.H.; Epstein, J.A. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. USA 2008, 105, 1955–1959. [Google Scholar] [CrossRef]
- Campa, J.S.; Greenhalgh, R.M.; Powell, J.T. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis 1987, 65, 13–21. [Google Scholar] [CrossRef]
- Cheung, C.; Bernardo, A.S.; Trotter, M.W.B.; Pedersen, R.A.; Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origing-dependent disease susceptibility. Nat. Biotechnol. 2012, 30, 165–173. [Google Scholar] [CrossRef]
- Wiegreffe, C.; Christ, B.; Huang, R.; Scaal, M. Remodeling of aortic smooth muscle during avian embryonic development. Dev. Dyn. 2009, 238, 624–631. [Google Scholar] [CrossRef]
- Tromp, G.; Kuivaniemi, H.; Hinterseher, I.; Carey, D.J. Novel genetic mechanisms for aortic aneurysms. Curr. Atheroscler. Rep. 2010, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.M.; Jones, J.A.; Ikonomidis, J.S. Pathophysiology of thoracic aortic aneurysm (TAA): Is it not one uniform aorta? Role of embryologic origin. Prog. Cardiovasc. Dis. 2013, 56, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.C.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.L.; Gadson, P.F.; Wrenn, R.W.; Rosenquist, T.H. Homocysteine signal cascade: Production of phospholipids, activation of protein kinase C, and the induction of c-fos and c-myb in smooth muscle cells. FASEB J. 1997, 11, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Gadson, P.F.; Dalton, M.L.; Patterson, E.; Svoboda, D.D.; Hutchinson, L.; Schram, D.; Rosenquist, T.H. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-β1: Regulation of c-myb and α1 (I) procollagen genes. Exp. Cell Res. 1997, 230, 169–180. [Google Scholar] [CrossRef]
- Rateri, D.L.; Howatt, D.A.; Moorleghen, J.J.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Prolonged infusion of angiotensin II in apoE-/-mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am. J. Pathol. 2011, 179, 1542–1548. [Google Scholar] [CrossRef]
- Owens, A.P.; Subramanian, V.; Moorleghen, J.J.; Guo, Z.; McNamara, C.A.; Cassis, L.A.; Daugherty, A. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differntiation 3. Circ. Res. 2010, 106, 611–619. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Michel, J.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 2018, 4, 34. [Google Scholar] [CrossRef]
- Airhart, N.; Brownstein, B.H.; Cobb, J.P.; Schierding, W.; Arif, B.; Ennis, T.L.; Thompson, R.W.; Curci, J.A.; Louis, S. From the society for vascular surgery smooth muscle cells from abdominal aortic aneurysms are unique and can independently and synergistically degrade insoluble elastin. J. Vasc. Surg. 2013, 60, 1033–1042. [Google Scholar] [CrossRef]
- Ailawadi, G.; Moehle, C.W.; Pei, H.; Walton, S.P.; Yang, Z.; Kron, I.L.; Lau, C.L.; Owens, G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009, 138, 1392–1399. [Google Scholar] [CrossRef]
- Michel, J.B. Anoïkis in the cardiovascular system: Known and unknown extracellular mediators. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2146–2154. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Ren, J.; Morgan, S.; Assa, C.; Liu, B. Receptor-interacting protein Kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ. Res. 2015, 116, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.A.; Taylor, W.R. Cellular mechanisms of aortic aneurysm formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Liapis, C.D.; Kadoglou, N.P.; Liapis, C.D. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms Matrix metalloproteinases: Contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr. Med. Res. Opin. 2004, 20, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.M.; Jean-Claude, J.; Li, H.; Scholes, J.V.; Ogata, Y.; Nagase, H.; Tilson, M.D. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J. Vasc. Surg. 1994, 20, 814–820. [Google Scholar] [CrossRef][Green Version]
- Crowther, M.; Goodall, S.; Jones, J.L.; Bell, P.R.F.; Thompson, M.M. Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J. Vasc. Surg. 2000, 32, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Tamarina, N.A.; McMillan, W.D.; Shively, V.P.; Pearce, W.H. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery 1997, 122, 264–272. [Google Scholar] [CrossRef]
- Goodall, S.; Porter, K.E.; Bell, P.R.; Thompson, M.M. Enhanced invasive properties exhibited by smooth muscle cells are associated with elevated production of MMP-2 in patients with aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 72–80. [Google Scholar] [CrossRef][Green Version]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Timothy Baxter, B. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef]
- Saracini, C.; Bolli, P.; Sticchi, E.; Pratesi, G.; Pulli, R.; Sofi, F.; Pratesi, C.; Gensini, G.F.; Abbate, R.; Giusti, B. Polymorphisms of genes involved in extracellular matrix remodeling and abdominal aortic aneurysm. J. Vasc. Surg. 2012, 55, 171–179. [Google Scholar] [CrossRef]
- Jones, G.T.; Phillips, V.L.; Harris, E.L.; Rossaak, J.I.; Van Rij, A.M. Functional matrix metalloproteinase-9 polymorphism (C-1562T) associated with abdominal aortic aneurysm. J. Vasc. Surg. 2003, 38, 1363–1367. [Google Scholar] [CrossRef]
- Thompson, R.W.; Holmes, D.R.; Mertens, R.A.; Liao, S.; Botney, M.D.; Mecham, R.P.; Welgus, H.G.; Parks, W.C. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. Am. Soc. Clin. Investig. 1995, 96, 318–326. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Curci, J.A.; Liao, S.; Huffman, M.D.; Shapiro, S.D.; Thompson, R.W. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Investig. 1998, 102, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xing, L.; Zeng, C.; Wu, T.; Gui, Y.; Li, W.; Lan, T.; Yang, Y.; Gu, Q.; Qi, C.; et al. Inactivation of PI3Kδ induces vascular injury and promotes aneurysm development by upregulating the AP-1/MMP-12 pathway in macrophages. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 368–377. [Google Scholar] [CrossRef]
- Lizarbe, T.R.; Tarín, C.; Gómez, M.; Lavin, B.; Aracil, E.; Orte, L.M.; Zaragoza, C. Nitric oxide induces the progression of abdominal aortic aneurysms through the matrix metalloproteinase inducer EMMPRIN. Am. J. Pathol. 2009, 175, 1421–1430. [Google Scholar] [CrossRef]
- Nollendorfs, A.; Greiner, T.C.; Nagase, H.; Baxter, B.T. The expression and localization of membrane type-1 matrix metalloproteinase in human abdominal aortic aneurysms. J. Vasc. Surg. 2001, 34, 316–322. [Google Scholar] [CrossRef]
- Xiong, W.; Knispel, R.; MacTaggart, J.; Greiner, T.C.; Weiss, S.J.; Baxter, B.T. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J. Biol. Chem. 2009, 284, 1765–1771. [Google Scholar] [CrossRef]
- Sun, J.; Sukhova, G.K.; Zhang, J.; Chen, H.; Sjoberg, S.; Libby, P.; Xia, M.; Xiong, N.; Gelb, B.D.; Shi, G.-P. Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arter. Thromb. Vasc. Biol. 2012, 32, 15–23. [Google Scholar] [CrossRef]
- Wang, J.; Kukhova, G.K.; Liu, J.; Ozaki, K.; Lesner, A.; Libby, P.; Kovanen, P.T.; Shi, G.-P. Cathepsin G deficiency reduces peri-aortic calcium chloride injury-induced abdominal aortic aneurysms in mice. Physiol. Behav. 2015, 62, 1615–1624. [Google Scholar] [CrossRef]
- Carretero, O.A. Vascular remodelling and the kallikrein-kinin system. J. Clin. Investig. 2005, 115, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Biros, E.; Norman, P.E.; Walker, P.J.; Nataatmadja, M.; West, M.; Golledge, J. A single nucleotide polymorphism in exon 3 of the kallikrein 1 gene is associated with large but not small abdominal aortic aneurysm. Atherosclerosis 2011, 217, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Defawe, O.D.; Colige, A.; Lambert, C.A.; Munaut, C.; Delvenne, P.; Lapière, C.M.; Limet, R.; Nusgens, B.V.; Sakalihasan, N. TIMP-2 and PAI-1 mRNA levels are lower in aneurysmal as compared to athero-occlusive abdominal aortas. Cardiovasc. Res. 2003, 60, 205–213. [Google Scholar] [CrossRef]
- Brophy, C.M.; Marks, W.H.; Reilly, J.M.; David Tilson, M. Decreased tissue inhibitor of metalloproteinases (TIMP) in abdominal aortic aneurysm tissue: A preliminary report. J. Surg. Res. 1991, 50, 653–657. [Google Scholar] [CrossRef]
- Eskandari, M.K.; Vijungco, J.D.; Flores, A.; Borensztajn, J.; Shively, V.; Pearce, W.H. Enhanced abdominal aortic aneurysm in TIMP-1-deficient mice. J. Surg. Res. 2005, 123, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Tilson, M.D.; Reilly, J.M.; Brophy, C.M.; Webster, E.L.; Barnett, T.R. Expression and sequence of the gene for tissue inhibitor of metalloproteinases in patients with abdominal aortic aneurysms. J. Vasc. Surg. 1993, 18, 266–270. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Tromp, G.; Cole, C.W.; Verloes, A.; Sakalihasan, N.; Yoon, S.; Kuivaniemi, H. Analysis of coding sequences for tissue inhibitor of metalloproteinases 1 (TIMP1)) and 2 (TIMP2) in patients with aneurysms. Matrix Biol. 1999, 18, 121–124. [Google Scholar] [CrossRef]
- Hinterseher, I.; Krex, D.; Kuhlisch, E.; Schmidt, K.G.; Pilarsky, C.; Schneiders, W.; Saeger, H.D.; Bergert, H. Tissue inhibitor of metalloproteinase-1 (TIMP-1) polymorphisms in a Caucasian population with abdominal aortic aneurysm. World J. Surg. 2007, 31, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Annabi, B.; Shédid, D.; Ghosn, P.; Kenigsberg, R.L.; Desrosiers, R.R.; Bojanowski, M.W.; Beaulieu, E.; Nassif, E.; Moumdjian, R.; Béliveau, R. Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J. Vasc. Surg. 2002, 35, 539–546. [Google Scholar] [CrossRef]
- Hinterseher, I.; Tromp, G.; Kuivaniemi, H. Genes and abdominal aortic aneurysm. Ann. Vasc. Surg. 2011, 25, 388–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Juttermann, R.; Soloway, P.D. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. 2000, 275, 26411–26415. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Knispel, R.; Mactaggart, J.; Baxter, B.T. Effects of tissue inhibitor of metalloproteinase 2 deficiency on aneurysm formation. J. Vasc. Surg. 2006, 44, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Fan, D.; Kandalam, V.; Lee, J.; Das, S.K.; Wang, X.; Baldwin, T.A.; Oudit, G.Y.; Kassiri, Z. Loss of Timp3 gene leads to abdominal aortic aneurysm formation in response to angiotensin II *. J. Biol. Chem. 2012, 287, 44083–44096. [Google Scholar] [CrossRef]
- Narayanan, N.; Tyagi, N.; Shah, A.; Pagni, S.; Tyagi, S.C. Hyperhomocysteinemia during aortic aneurysm, a plausible role of epigenetics. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 32–42. [Google Scholar]
- Mangum, K.D.; Farber, M.A. Genetic and epigenetic regulation of abdominal aortic aneurysms. Clin. Genet. 2020, 97, 1–12. [Google Scholar] [CrossRef]
- Kim, V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005, 6, 376–385. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Merk, D.R.; Deng, A.; Chin, J.T.; Raaz, U.; Schoelmerich, A.M.; Raiesdana, A.; Leeper, N.J.; et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Investig. 2012, 122, 497–506. [Google Scholar] [CrossRef]
- Merk, D.R.; Chin, J.T.; Dake, B.A.; Maegdefessel, L.; Miller, M.O.; Kimura, N.; Tsao, P.S.; Iosef, C.; Berry, G.J.; Mohr, F.W.; et al. miR-29b participates in early aneurysm development in marfan syndrome. Circ. Res. 2012, 110, 312–324. [Google Scholar] [CrossRef]
- Kim, C.W.; Kumar, S.; Son, D.J.; Jang, I.-H.; Griendling, K.K.; Jo, H. Prevention of abdominal aortic aneurysm by anti-miRNA-712 or anti-miR-205 in Angiotensin II infused mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1412–1421. [Google Scholar] [CrossRef]
- Pons, D.; De Vries, F.R.; Van Den Elsen, P.J.; Heijmans, B.T.; Quax, P.H.A.; Jukema, J.W. Epigenetic histone acetylation modifiers in vascular remodelling: New targets for therapy in cardiovascular disease. Eur. Heart J. 2009, 30, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Tekwani, B.L. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol. 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Galán, M.; Varona, S.; Orriols, M.; Rodríguez, J.A.; Aguiló, S.; Dilmé, J.; Camacho, M.; Martínez-González, J.; Rodriguez, C. Induction of histone deacetylases (HDACs) in human abdominal aortic aneurysm: Therapeutic potential of HDAC inhibitors. DMM Dis. Model. Mech. 2016, 9, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Jovinge, S.; Hultgårdh-Nilsson, A.; Regnström, J.; Nilsson, J. Tumor necrosis factor-α activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tanios, F.; Reeps, C.; Zhang, J.; Schwamborn, K.; Eckstein, H.H.; Zernecke, A.; Pelisek, J. Histone acetylation and histone acetyltransferases show significant alterations in human abdominal aortic aneurysm. Clin. Epigenetics 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Zhang, S.L.; Du, X.; Chen, Y.Q.; Tan, Y.S.; Liu, L. Potential medication treatment according to pathological mechanisms in abdominal aortic aneurysm. J. Cardiovasc. Pharmacol. 2018, 71, 46–57. [Google Scholar] [CrossRef]
- Hadi, T.; Boytard, L.; Silvestro, M.; Alebrahim, D.; Jacob, S.; Feinstein, J.; Barone, K.; Spiro, W.; Hutchison, S.; Simon, R.; et al. Macrophage-derived netrin-1 promotes abdominal aortic aneurysm formation by activating MMP3 in vascular smooth muscle cells. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Boyle, J.J.; Weissberg, P.L.; Bennett, M.R. Tumor necrosis factor-α promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1553–1558. [Google Scholar] [CrossRef]
- Middleton, R.K.; Lloyd, G.M.; Bown, M.J.; Cooper, N.J.; London, N.J.; Sayers, R.D. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: A protein array study. J. Vasc. Surg. 2007, 45, 574–580. [Google Scholar] [CrossRef]
- Akerman, A.W.; Stroud, R.E.; Bans, R.W.; Grespin, R.T.; McDonald, L.T.; LaRue, R.A.C.; Mukerjee, R.; Ikonomidis, J.S.; Jones, J.A.; Ruddy, J.M. Elevated wall tension initiates interleukin-6 expression and abdominal aortic dilation. Ann. Vasc. Surg. 2018, 46, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The interface of atherosclerosis and thrombosis: Basic mechanisms. Vasc. Med. 1998, 3, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Beasley, D.; McGuiggin, M.E.; Dinarello, C.A. Human vascular smooth muscle cells produce an intracellular form of interleukin-1 receptor antagonist. Am. J. Physiol. Cell Physiol. 1995, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Castresana, M.R.; Newman, W.H. NF-κB is required for TNF-α-directed smooth muscle cell migration. FEBS Lett. 2001, 508, 360–364. [Google Scholar] [CrossRef]
- Clarke, M.C.H.; Talib, S.; Figg, N.L.; Bennett, M.R. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: Effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ. Res. 2010, 106, 363–372. [Google Scholar] [CrossRef]
- Geng, Y.J.; Wu, Q.; Muszynski, M.; Hansson, G.K.; Libby, P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-γ, tumor necrosis factor-α, and interleukin-1β. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 19–27. [Google Scholar] [CrossRef]
- Juvonen, J.; Surcel, H.M.; Satta, J.; Teppo, A.M.; Bloigu, A.; Syrjälä, H.; Airaksinen, J.; Leinonen, M.; Saikku, P.; Juvonen, T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2843–2847. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Johnson, S.H.; Delain, L.P.; Haedt, M.J.; Noll, A.L.; Sandbo, N.; Jarjour, N.N. Matrix Metalloproteinase-9-Dependent Release of IL-1β by Human Eosinophils. Mediators Inflamm. 2019, 2019, 7479107. [Google Scholar] [CrossRef]
- Bauer, J.; Huy, C.; Brenmoehl, J.; Obermeier, F.; Bock, J. Matrix metalloproteinase-1 expression induced by IL-1β requires acid sphingomyelinase. FEBS Lett. 2009, 583, 915–920. [Google Scholar] [CrossRef]
- Xie, S.; Issa, R.; Sukkar, M.B.; Oltmanns, U.; Bhavsar, P.K.; Papi, A.; Caramori, G.; Adcock, I.; Chung, K.F. Induction and regulation of matrix metalloproteinase-12 in human airway smooth muscle cells. Respir. Res. 2005, 6, 1–11. [Google Scholar] [CrossRef]
- Raymond, L.; Eck, S.; Mollmark, J.; Hays, E.; Tomek, I.; Kantor, S.; Elliott, S.; Vincenti, M. Interleukin-1 beta induction of matrix metalloproteinase-1 transcription in chondrocytes requires ERK-dependent activation of CCAAT enhancer-binding protein-beta. J. Cell. Physiol. 2006, 207, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, W.; Huwiler, A.; Beck, K.-F.; Walpen, S.; Pfeilschifter, J. Amplification of IL-1β-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-κB and activating protein-1 and involves activation of the mitogen-activated protein kinase path. J. Immunol. 2000, 165, 5788–5797. [Google Scholar] [CrossRef] [PubMed]
- Marculescu, R.; Sodeck, G.; Domanovits, H.; Hobusch, G.; Exner, M.; Heinzl, H.; Huber, K.; Mannhalter, C.; Minar, E.; Wagner, O.; et al. Interleukin-1 gene cluster variants and abdmominal aortic aneurysms. Thromb. Haemost. 2005, 94, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Bown, M.J.; Burton, P.R.; Horsburgh, T.; Nicholson, M.L.; Bell, P.R.F.; Sayers, R.D. The role of cytokine gene polymorphisms in the pathogenesis of abdominal aortic aneurysms: A case-control study. J. Vasc. Surg. 2003, 37, 999–1005. [Google Scholar] [CrossRef]
- Thompson, A.R.; Drenos, F.; Hafez, H.; Humphries, S.E. Candidate gene association studies in abdominal aortic aneurysm disease: A review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 19–30. [Google Scholar] [CrossRef][Green Version]
- Tang, M.; Fang, J. TNF-α regulates apoptosis of human vascular smooth muscle cells through gap junctions. Mol. Med. Rep. 2017, 15, 1407–1411. [Google Scholar] [CrossRef]
- Wilson, A.G.; Symons, J.A.; Mcdowell, T.L.; Mcdevitt, H.O.; Duff, G.W. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA 1997, 94, 3195–3199. [Google Scholar] [CrossRef]
- Smallwood, L.; Allcock, R.; Van Bockxmeer, F.; Warrington, N.; Palmer, L.J.; Iacopetta, B.; Golledge, J.; Norman, P.E. Polymorphisms of the matrix metalloproteinase 9 gene and abdominal aortic aneurysm. Br. J. Surg. 2008, 95, 1239–1244. [Google Scholar] [CrossRef]
- Jones, G.; Brull, D.; Brown, L.; Sian, M.; Greenhalgh, R.; Humphries, S.; Powell, J. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation 2001, 103, 2260–2265. [Google Scholar] [CrossRef]
- Doyle, A.J.; Redmond, E.M.; Gillespie, D.L.; Knight, P.A.; Cullen, J.P.; Cahill, P.A.; Morrow, D.J. Differential expression of Hedgehog/Notch and transforming growth factor-β in human abdominal aortic aneurysms. Physiol. Behav. 2015, 62, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Rateri, D.L.; Daugherty, A.; Arbor, A. Abdominal aortic aneurysm: Novel mechanisms and therapies. Curr. Opin. Cardiol. 2015, 30, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tedgui, A.; Mallat, Z.; Wang, Y.; Ait-oufella, H.; Herbin, O.; Bonnin, P.; Ramkhelawon, B. TGF- b activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II –infused mice Find the latest version: TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotens. J. Clin. Investig. 2010, 120, 422–432. [Google Scholar] [CrossRef]
- Dai, X.; Shen, J.; Priyanka Annam, N.; Jiang, H.; Levi, E.; Schworer, C.M.; Tromp, G.; Arora, A.; Higgins, M.; Wang, X.F.; et al. SMAD3 deficiency promotes vessel wall remodeling, collagen fiber reorganization and leukocyte infiltration in an inflammatory abdominal aortic aneurysm mouse model. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Seto, S.W.; Jose, R.J.; Biros, E.; Moran, C.S.; Wang, Y.; Clancy, P.; Golledge, J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Clancy, P.; Jones, G.T.; Cooper, M.; Palmer, L.J.; Van Rij, A.M.; Norman, P.E. Possible association between genetic polymorphisms in transforming growth factor β receptors, serum transforming growth factor β1 concentration and abdominal aortic aneurysm. Br. J. Surg. 2009, 96, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Baas, A.F.; Medic, J.; Van ’T Slot, R.; De Kovel, C.G.; Zhernakova, A.; Geelkerken, R.H.; Kranendonk, S.E.; Van Sterkenburg, S.M.; Grobbee, D.E.; Boll, A.P.; et al. Association of the TGF-Β receptor genes with abdominal aortic aneurysm. Eur. J. Hum. Genet. 2010, 18, 240–244. [Google Scholar] [CrossRef][Green Version]
- Du, S.J.; Tan, X.; Zhang, J. SMYD proteins: Key regulators in skeletal and cardiac muscle development and function. Anat. Rec. 2014, 297, 1650–1662. [Google Scholar] [CrossRef]
- Qi, J.; Yang, P.; Yi, B.; Huo, Y.; Chen, M.; Zhang, J.; Sun, J. Heat shock protein 90 inhibition by 17-DMAG attenuates abdominal aortic aneurysm formation in mice. Am. J. Physiol. Hear. Circ. Physiol. 2015, 308, H841–H852. [Google Scholar] [CrossRef]
- Xu, G.; Liu, G.; Xiong, S.; Liu, H.; Chen, X.; Zheng, B. The histone methyltransferase Smyd2 is a negative regulator of macrophage activation by suppressing interleukin 6 (IL-6) and tumor necrosis factor α (tnf-α). J. Biol. Chem. 2015, 290, 5414–5423. [Google Scholar] [CrossRef]
- Jones, G.T.; Tromp, G.; Kuivaniemi, H.; Gretarsdottir, S.; Baas, A.F.; Giusti, B.; Strauss, E.; Van’T Hof, F.N.G.; Webb, T.R.; Erdman, R.; et al. Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ. Res. 2017, 120, 341–353. [Google Scholar] [CrossRef]
- Jones, G.T.; Bown, M.J.; Gretarsdottir, S.; Romaine, S.P.R.; Helgadottir, A.; Yu, G.; Tromp, G.; Norman, P.E.; Jin, C.; Baas, A.F.; et al. A sequence variant associated with sortilin-1 (SORT1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum. Mol. Genet. 2013, 22, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Strong, A.; Frank-Kamenetsky, M.; Lee, N.E.; Ahfeldt, T.; Sachs, K.V.; Li, X.; Li, H.; Kuperwasser, N.; Vera, M.; et al. Cholesterol Locus. Nature 2010, 466, 714–719. [Google Scholar] [CrossRef]
- Kjolby, M.; Andersen, O.M.; Breiderhoff, T.; Fjorback, A.W.; Pedersen, K.M.; Madsen, P.; Jansen, P.; Heeren, J.; Willnow, T.E.; Nykjaer, A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, W.; Yang, H.; Lin, Q.; Qin, X. The miR-182/SORT1 axis regulates vascular smooth muscle cell calcification in vitro and in vivo. Exp. Cell Res. 2018, 362, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Maegdefessel, L.; Dalman, R.L.; Tsao, P.S. Pathogenesis of Abdominal Aortic Aneurysms: MicroRNAs, Proteases, Genetic Associations. Annu. Rev. Med. 2014, 65, 49–62. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Y.; Shi, Z.; Huang, H.; Fang, Z.; Chen, J.; Xiu, Q.; Li, B. YKL-40 Induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-κB pathways, causing bronchial smooth muscle proliferation and migration. J. Immunol. 2013, 190, 438–446. [Google Scholar] [CrossRef]
- Elia, L.; Quintavalle, M.; Zhang, J.; Contu, R.; Cossu, L.; Latronico, M.V.G.; Peterson, K.L.; Indolfi, C.; Catalucci, D.; Chen, J.; et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell Death Differ. 2009, 16, 1590–1598. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef]
- Rangrez, A.Y.; Massy, Z.A.; Meuth, V.M.-L.; Metzinger, L. MiR-143 and miR-145 molecular keys to switch the phenotype of vascular smooth muscle cells. Circ. Cardiovasc. Genet. 2011, 4, 197–205. [Google Scholar] [CrossRef]
- Riches, K.; Angelini, T.G.; Mudhar, G.S.; Kaye, J.; Clark, E.; Bailey, M.A.; Sohrabi, S.; Korossis, S.; Walker, P.G.; Scott, D.J.A.; et al. Exploring smooth muscle phenotype and function in a bioreactor model of abdominal aortic aneurysm. J. Transl. Med. 2013, 11, 1. [Google Scholar] [CrossRef]
- Thompson, A.R.; Golledge, J.; Cooper, J.A.; Hafez, H.; Norman, P.E.; Humphries, S.E. Sequence variant on 9p21 is associated with the presence of abdominal aortic aneurysm disease but does not have an impact on aneurysmal expansion. Eur. J. Hum. Genet. 2009, 17, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Bown, M.J.; Braund, P.S.; Thompson, J.; London, N.J.M.; Samani, N.J.; Sayers, R.D. Association between the coronary artery disease risk locus on chromosome 9p21.3 and abdominal aortic aneurysm. Circ. Cardiovasc. Genet. 2008, 1, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Biros, E.; Cooper, M.; Palmer, L.J.; Walker, P.; Norman, P.E.; Golledge, J. Association of an allele on chromosome 9 and abdominal aortic aneurysm aneurysm. Atherosclerosis 2010, 212, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Leeper, N.J.; Raiesdana, A.; Kojima, Y.; Kundu, R.K.; Cheng, H.; Maegdefessel, L.; Toh, R.; Ahn, G.O.; Ali, Z.A.; Anderson, R.D.; et al. Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Visel, A.; Zhu, Y.; Afzal, V.; Gong, E.; Attanasio, C.; Blow, M.J.B.; Cohen, J.C.; Rubin, E.M.; Pennacchio, L.A. Targeted deletion of the 9p21 noncoding coronary artery disease risk interval in mice. Nature 2010, 464, 409–412. [Google Scholar] [CrossRef]
- Golledge, J.; Kuivaniemi, H. Genetics of abdominal aortic aneurysm. Curr. Opin. Cardiol. 2013, 28, 290–296. [Google Scholar] [CrossRef]
- Gretarsdottir, S.; Baas, A.F.; Thorleifsson, G.; Holm, H.; Den Heijer, M.; De Vries, J.P.P.M.; Kranendonk, S.E.; Zeebregts, C.J.A.M.; Van Sterkenburg, S.M.; Geelkerken, R.H.; et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat. Genet. 2010, 42, 692–697. [Google Scholar] [CrossRef]
- Yu, L.; Qin, L.; Zhang, H.; He, Y.; Chen, H.; Pober, J.S.; Tellides, G.; Min, W. AIP1 prevents graft arteriosclerosis by inhibiting interferon-γ- dependent smooth muscle cell proliferation and intimal expansion. Circ. Res. 2011, 109, 418–427. [Google Scholar] [CrossRef]
- Bown, M.J.; Jones, G.T.; Harrison, S.C.; Wright, B.J.; Bumpstead, S.; Baas, A.F.; Gretarsdottir, S.; Badger, S.A.; Bradley, D.T.; Burnand, K.; et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am. J. Hum. Genet. 2011, 89, 619–627. [Google Scholar] [CrossRef]
- Boucher, P.; Gotthardt, M.; Li, W.P.; Anderson, R.G.W.; Herz, J. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science 2003, 300, 329–332. [Google Scholar] [CrossRef]
- Wild, J.B.; Stather, P.W.; Sylvius, N.; Choke, E.; Sayers, R.D.; Bown, M.J. Low Density Lipoprotein Receptor Related Protein 1 and Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Giusti, C.; Luciani, M.F.; Klein, G.; Aubry, L.; Tresse, E.; Kosta, A.; Golstein, P. Necrotic cell death: From reversible mitochondrial uncoupling to irreversible lysosomal permeabilization. Exp. Cell Res. 2009, 315, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Koenig, S.N.; Huang, N.; Chen, J.; Beceiro, S.; Guggilam, A.; Kuivaniemi, H.; Partida-Sanchez, S.; Garg, V. Inhibition of Notch1 signaling reduces abdominal aortic aneurysm in mice by attenuating macrophage-mediated inflammation. Arter. Thromb. Vasc. Biol. 2012, 32, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Hinterseher, I.; Elmore, J.R.; Cindric, M.C.; Bowen, W.D.; Schworer, M.; Chernousov, M.A.; Franklin, P.; Gray, J.L.; Garvin, P.; Gatalica, Z.; et al. Novel pathways in the pathobiology of human abdominal aortic aneurysms. Pathobiology 2013, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.C.; Shah, K.A.; Han, C.; Campo, L.; Turley, H.; Leek, R.; Corbridge, R.J.; Cox, G.J.; Harris, A.L. The relation between hypoxia-inducible factor (HIF)=1α and HIF-2α expression with anemia and outcome in surgically treated head and neck cancer. Cancer 2006, 107, 757–766. [Google Scholar] [CrossRef]
- Imanishi, M.; Chiba, Y.; Tomita, N.; Matsunaga, S.; Nakagawa, T. Hypoxia-inducible factor-1 α in smooth muscle cells protects against aortic aneurysms—Brief report. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2158–2162. [Google Scholar] [CrossRef]
- Nakayama, T.; Kurobe, H.; Sugasawa, N.; Kinoshita, H.; Higashida, M.; Matsuoka, Y.; Yoshida, Y.; Hirata, Y.; Sakata, M.; Maxfield, M.W.; et al. Role of macrophage-derived hypoxia-inducible factor (HIF)-1α as a mediator of vascular remodelling. Cardiovasc. Res. 2013, 99, 705–715. [Google Scholar] [CrossRef]
- Gäbel, G.; Northoff, B.H.; Weinzierl, I.; Ludwig, S.; Hinterseher, I.; Wilfert, W.; Teupser, D.; Doderer, S.A.; Bergert, H.; Schönleben, F.; et al. Molecular fingerprint for terminal abdominal aortic aneurysm disease. J. Am. Heart Assoc. 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Deng, A.; Merk, D.R.; Leeper, N.J.; Raaz, U.; Schoelmerich, A.M.; Michael, V.; Dalman, R.L.; et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci. Transl. Med. 2012, 4, 1–27. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, B.; Han, M.; Fang, X.; Li, H.; Miao, S.; Su, M.; Han, Y.; Shi, H.; Wen, J. miR-146a and Kru¨ppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011, 12, 56–62. [Google Scholar] [CrossRef]
- Leeper, N.J.; Raiesdana, A.; Kojima, Y.; Chun, H.J.; Azuma, J.; Maegdefessel, L.; Kundu, R.K.; Quertermous, T.; Tsao, P.S.; Spin, J.M. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J. Cell. Physiol. 2011, 226, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.B.; Deng, D.Y.; Beppu, H.; Hong, C.C.; Lai, C.; Hoyng, S.A.; Kawai, N.; Bloch, K.D. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J. Biol. Chem. 2008, 283, 3877–3888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hou, S.; Chen, J.; Zhang, J.; Lin, F.; Ju, R.; Cheng, X.; Ma, X.; Song, Y.; Zhang, Y.; et al. Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ. Res. 2016, 118, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Y.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. A Necessary Role of miR-221 and miR-222 in Vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 2009, 104, 476–487. [Google Scholar] [CrossRef]
- Davis, B.N.; Hilyard, A.C.; Nguyen, P.H.; Lagna, G.; Hata, A. Induction of MicroRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J. Biol. Chem. 2009, 284, 3728–3738. [Google Scholar] [CrossRef]
- Li, D.Y.; Busch, A.; Jin, H.; Chernogubova, E.; Pelisek, J.; Karlsson, J.; Sennblad, B.; Lao, S.; Hofmann, P.; Suzanne, M.; et al. H19 induces abdominal aortic aneurysm development and progression. Circulation 2018, 138, 1551–1568. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, G.; Chen, X.; Lu, W.; Liu, J.; Zhai, S. Molecules and cells knockdown of lncRNA PVT1 inhibits vascular smooth muscle cell apoptosis and extracellular matrix disruption in a murine abdominal aortic aneurysm model. Mol. Cells 2019, 42, 218–227. [Google Scholar]
- Kleinhenz, J.M.; Murphy, T.C.; Pokutta-Paskaleva, A.P.; Gleason, R.L.; Lyle, A.N.; Taylor, W.R.; Blount, M.A.; Cheng, J.; Yang, Q.; Sutliff, R.L.; et al. Smooth muscle-targeted overexpression of peroxisome proliferator activated receptor-γ disrupts vascular wall structure and function. PLoS ONE 2015, 10, 1–25. [Google Scholar] [CrossRef][Green Version]
- Fu, M.; Zhang, J.; Lin, Y.G.; Zhu, X.; Willson, T.M.; Chen, Y.E. Activation of peroxisome proliferator-activated receptor γ inhibits osteoprotegerin gene expression in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2002, 294, 597–601. [Google Scholar] [CrossRef]
- Moran, C.S.; Clancy, P.; Biros, E.; Blanco-Martin, B.; McCaskie, P.; Palmer, L.J.; Coomans, D.; Norman, P.E.; Golledge, J. Association of PPARγ allelic variation, osteoprotegerin and abdominal aortic aneurysm. Clin. Endocrinol. Oxf. 2010, 72, 128–132. [Google Scholar] [CrossRef]
- Hansmann, G.; Perez, V.A.D.J.; Alastalo, T.; Alvira, C.M.; Guignabert, C.; Bekker, J.M.; Schellong, S.; Urashima, T.; Wang, L.; Morrell, N.W.; et al. An antiproliferative BMP-2 / PPARγ / apoE axis in human and murine SMCs and its role in pulmonary hypertension. J. Clin. Investig. 2008, 118, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Villacorta, L.; Zhang, J.; Garcia-Barrio, M.T.; Yang, K.; Hamblin, M.; Whitesall, S.E.; D’Alecy, L.G.; Chen, Y.E. Vascular smooth muscle cell-selective PPARγ deletion leads to hypotension. Circulation 2009, 119, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Beyer, A.M.; Baumbach, G.L.; Halabi, C.M.; Modrick, M.L.; Cynthia, M.; Gerhold, T.D.; Ghoneim, S.M.; De Lange, W.J.; Keen, H.L.; Tsai, Y.; et al. Interference with PPARγ signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension 2008, 51, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Halabi, C.M.; Beyer, A.M.; de Lange, W.J.; Keen, H.L.; Baumbach, G.L.; Faraci, F.M.; Sigmund, C.D. Interference with PPARγ function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008, 7, 215–226. [Google Scholar] [CrossRef]
- Ortiz-Muñoz, G.; Houard, X.; Martín-Ventura, J.-L.; Ishida, B.Y.; Loyau, S.; Rossignol, P.; Moreno, J.-A.; Kane, J.P.; Chalkley, R.J.; Burlingame, A.L.; et al. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. 2009, 23, 3129–3139. [Google Scholar] [CrossRef]
- Young, J.L.; Sukhova, G.K.; Foster, D.; Kisiel, W.; Libby, P.; Schönbeck, U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1β-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J. Exp. Med. 2000, 191, 1535–1544. [Google Scholar] [CrossRef]
- Ryer, E.J.; Ronning, K.E.; Erdman, R.; Schworer, C.M.; Elmore, J.R.; Peeler, T.C.; Nevius, C.D.; Lillvis, J.H.; Garvin, R.P.; Franklin, D.P.; et al. The potential role of DNA methylation in abdominal aortic aneurysms. Int. J. Mol. Sci. 2015, 16, 11259–11275. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, S.; Nordström, I.; Hellstrand, P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J. Biol. Chem. 2004, 279, 34849–34855. [Google Scholar] [CrossRef]
- Xin, M.; Small, E.M.; Sutherland, L.B.; Qi, X.; McAnally, J.; Plato, C.F.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009, 23, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.-H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, X.; Yang, J.; Lin, Y.; Xu, D.Z.; Lu, Q.; Deitch, E.A.; Huo, Y.; Delphin, E.S.; Zhang, C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009, 105, 158–166. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.Z.; Hockemeyer, D.; McAnally, J.; Nordheim, A.; Olson, E.N. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 2004, 428, 185–189. [Google Scholar] [CrossRef]
- Wang, X.; Hu, G.; Zhou, J. Repression of versican expression by microRNA-143. J. Biol. Chem. 2010, 285, 23241–23250. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, K.; Barrientos, T.; Pipes, G.C.T.; Li, S.; Olson, E.N. Muscle-Specific Signaling Mechanism That Links Actin Dynamics to Serum Response Factor. Mol. Cell. Biol. 2005, 25, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Miralles, F.; Posern, G.; Zaromytidou, A.I.; Treisman, R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003, 113, 329–342. [Google Scholar] [CrossRef]
- Barkalow, K.L.; Italiano, J.E.; Chou, D.E.; Matsuoka, Y.; Bennett, V.; Hartwig, J.H. α-adducin dissociates from F-actin and spectrin during platelet activation. J. Cell Biol. 2003, 161, 557–570. [Google Scholar] [CrossRef]
- Gardner, K.; Bennett, V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature 1987, 328, 359–362. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Spin, J.M.; Raaz, U.; Eken, S.M.; Toh, R.; Azuma, J.; Adam, M.; Nagakami, F.; Heymann, H.M.; Chernugobova, E.; et al. MiR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Venkatesh, P.; Phillippi, J.; Chukkapalli, S.; Rivera-Kweh, M.; Velsko, I.; Gleason, T.; VanRyzin, P.; Aalaei-Andabili, S.H.; Ghanta, R.K.; Beaver, T.; et al. Aneurysm-specific miR-221 and miR-146a participates in human thoracic and abdominal aortic aneurysms. Int. J. Mol. Sci. 2017, 18, 875. [Google Scholar] [CrossRef]
- Kin, K.; Miyagawa, S.; Fukushima, S.; Shirakawa, Y.; Torikai, K.; Shimamura, K.; Daimon, T.; Kawahara, Y.; Kuratani, T.; Sawa, Y. Tissue-and plasma-specific microRNA signatures for atherosclerotic abdominal aortic aneurysm. Am. Hear. Assoc. J. 2012, 1, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Yang, J.; Xu, L.; Zhang, C. Cell-specific effects of miR-221/222 in vessels: Molecular mechanism and therapeutic application. J. Mol. Cell. Cardiol. 2012, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.J.; Sharp, W.J.; Fang, X.; Oberley, L.W.; Oberley, T.D.; Weintraub, N.L. Oxidative stress in human abdominal aortic aneurysms: A potential mediator of aneurysmal remodeling. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Gavrila, D.; Li, W.G.; Mccormick, M.L.; Thomas, M.; Daugherty, A.; Cassis, L.A.; Miller, F.J., Jr.; Oberley, L.W.; Dellsperger, K.C.; Weintraub, N.L. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II–Infused apolipoprotein E–Deficient mice. Arterioscler. Thromb. 2005, 25, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Maiellaro-Rafferty, K.; Weiss, D.; Joseph, G.; Wan, W.; Gleason, R.L.; Taylor, W.R. Catalase overexpression in aortic smooth muscle prevents pathological mechanical changes underlying abdominal aortic aneurysm formation. Am. J. Physiol. Hear. Circ. Physiol. 2011, 301, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.L.; Gavrila, D.; Weintraub, N.L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 461–469. [Google Scholar] [CrossRef]
- Grote, K.; Flach, I.; Luchtefeld, M.; Akin, E.; Holland, S.M.; Drexler, H.; Schieffer, B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ. Res. 2003, 92, 1–7. [Google Scholar] [CrossRef]
- Hishikawa, K.; Oemar, B.S.; Yang, Z.; Lüscher, T.F. Pulsatile stretch stimulates superoxide production and activates nuclear factor-κb in human coronary smooth muscle. Circ. Res. 1997, 81, 797–803. [Google Scholar] [CrossRef]
- Brandes, R.P.; Kreuzer, J. Vascular NADPH oxidases: Molecular mechanisms of activation. Cardiovasc. Res. 2005, 65, 16–27. [Google Scholar] [CrossRef]
- De Keulenaer, G.W.; Alexander, R.W.; Ushio-Fukai, M.; Ishizaka, N.; Griendling, K.K. Tumour necrosis factor α activates a p22phox -based NADH oxidase in vascular smooth muscle. Biochem. J. 1998, 329, 653–657. [Google Scholar] [CrossRef]
- Thomas, M.; Gavrila, D.; McCormick, M.L.; Miller, F.J.; Daugherty, A.; Cassis, L.A.; Dellsperger, K.C.; Weintraub, N.L. Deletion of p47phox attenuates angiotensin II–Induced abdominal aortic aneurysm formation in apolipoprotein E–deficient mice. Circulation 2006, 114, 404–413. [Google Scholar] [CrossRef]
- Siu, K.L.; Miao, X.N.; Cai, H. Recoupling of eNOS with folic acid prevents abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E null mice. PLoS ONE 2014, 9, e0088899. [Google Scholar] [CrossRef] [PubMed]
- Kigawa, Y.; Miyazaki, T.; Lei, X.F.; Nakamachi, T.; Oguchi, T.; Kim-Kaneyama, J.R.; Taniyama, M.; Tsunawaki, S.; Shioda, S.; Miyazaki, A. NADPH oxidase deficiency exacerbates angiotensin II-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.L.; Li, Q.; Zhang, Y.; Guo, J.; Youn, J.Y.; Du, J.; Cai, H. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol. 2017, 11, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.L.; Carraro, C.C.; Belló-Klein, A.; Kalil, A.N.; Aerts, N.R.; Carvalho, F.B.; Fernandes, M.C.; Zettler, C.G. Oxidative stress in aortas of patients with advanced occlusive and aneurysmal diseases. Ann. Vasc. Surg. 2018, 52, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Dubick, M.A.; Keen, C.L.; DiSilvestro, R.A.; Eskelson, C.D.; Ireton, J.; Hunter, G.C. Antioxidant enzyme activity in human abdominal aortic aneurysmal and occlusive disease. Proc. Soc. Exp. Biol. Med. 1999, 220, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.; Pearce, C.G.; Cho, B.S.; Hannawa, K.K.; Roelofs, K.J.; Stanley, J.C.; Henke, P.K.; Upchurch, G.R. Differential regulation of the superoxide dismutase family in experimental aortic aneurysms and rat aortic explants. J. Surg. Res. 2007, 138, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Yajima, N.; Masuda, M.; Miyazaki, M.; Nakajima, N.; Chien, S.; Shyy, J.Y.J. Oxidative stress is involved in the development of experimental abdominal aortic aneurysm: A study of the transcription profile with complementary DNA microarray. J. Vasc. Surg. 2002, 36, 379–385. [Google Scholar] [CrossRef]
- Pecqueur, C.; Alves-Guerra, C.; Ricquier, D.; Bouillaud, F. UCP2, a metabolic sensor coupling glucose oxidation to mitochondrial metabolism? IUBMB Life 2009, 61, 762–767. [Google Scholar] [CrossRef]
- Bouillaud, F.; Alves-Guerra, M.C.; Ricquier, D. UCPs, at the interface between bioenergetics and metabolism. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2443–2456. [Google Scholar] [CrossRef]
- Yan, P.; Chen, K.; Wang, Q.; Yang, D.; Li, D.; Yang, Y. UCP-2 is involved in angiotensin-II-induced abdominal aortic aneurysm in apolipoprotein E-knockout mice. PLoS ONE 2017, 12, e0179743. [Google Scholar] [CrossRef]
- Kim, H.W.; Stansfield, B.K. Genetic and Epigenetic Regulation of Aortic Aneurysms. BioMed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Soe, N.N.; Sowden, M.; Baskaran, P.; Kim, Y.; Nigro, P.; Smolock, E.M.; Berk, B.C. Acetylation of cyclophilin A is required for its secretion and vascular cell activation. Cardiovasc. Res. 2014, 101, 444–453. [Google Scholar] [CrossRef]
- Manea, S.A.; Antonescu, M.L.; Fenyo, I.M.; Raicu, M.; Simionescu, M.; Manea, A. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol. 2018, 16, 332–343. [Google Scholar] [CrossRef]
- Krishna, S.M.; Dear, A.E.; Norman, P.E.; Golledge, J. Genetic and epigenetic mechanisms and their possible role in abdominal aortic aneurysm. Atherosclerosis 2010, 212, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.L.H.; Yan, M.S.C.; Marsden, P.A. Epigenetics and cardiovascular disease. Can. J. Cardiol. 2013, 29, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Matouk, C.C.; Marsden, P.A. Epigenetic regulation of vascular endothelial gene expression. Circ. Res. 2008, 102, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Bestor, T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000, 9, 2395–2402. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A Landscape Takes Shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef]
- Yang, X.; Han, H.; de Carvalho, D.D.; Lay, F.D.; Jones, P.A.; Liang, G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 2014, 26, 577–590. [Google Scholar] [CrossRef]
- Toghill, B.J.; Saratzis, A.; Freeman, P.J.; Sylvius, N.; Bown, M.J. SMYD2 promoter DNA methylation is associated with abdominal aortic aneurysm (AAA) and SMYD2 expression in vascular smooth muscle cells. Clin. Epigenetics 2018, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; He, X.; Si, X.; Wang, H.; Li, B.; Hu, Y.; Li, M.; Chen, X.; Liao, W.; Liao, Y.; et al. SM22 (smooth muscle 22) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-κB (nuclear factor-κB). Arterioscler. Thromb. Vasc. Biol. 2019, 39, e10–e25. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Zykovich, A.; Hubbard, A.; Flynn, J.M.; Tarnopolsky, M.; Fraga, M.F.; Kerksick, C.; Ogborn, D.; MacNeil, L.; Mooney, S.D.; Melov, S. Genome-wide DNA methylation changes with age in disease-free human skeletal muscle. Aging Cell 2014, 13, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Toghill, B.J.; Saratzis, A.; Harrison, S.C.; Verissimo, A.R.; Mallon, E.B.; Bown, M.J. The potential role of DNA methylation in the pathogenesis of abdominal aortic aneurysm. Atherosclerosis 2015, 241, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tsaprouni, L.G.; Yang, T.P.; Bell, J.; Dick, K.J.; Kanoni, S.; Nisbet, J.; Viñuela, A.; Grundberg, E.; Nelson, C.P.; Meduri, E.; et al. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics 2014, 9, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, C.; Voelter-Mahlknecht, S.; Dahmke, I.; Mahlknecht, U. On the epigenetics of vascular regulation and disease. Clin. Epigenetics 2012, 4, 7. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Karimi, M.; Johansson, S.; Axelsson, J.; Suliman, M.; Lindholm, B.; Heimbürger, O.; Barany, P.; Alvestrand, A.; Nordfors, L.; et al. Impact of inflammation on epigenetic DNA methylation-A novel risk factor for cardiovascular disease? J. Intern. Med. 2007, 261, 488–499. [Google Scholar] [CrossRef]
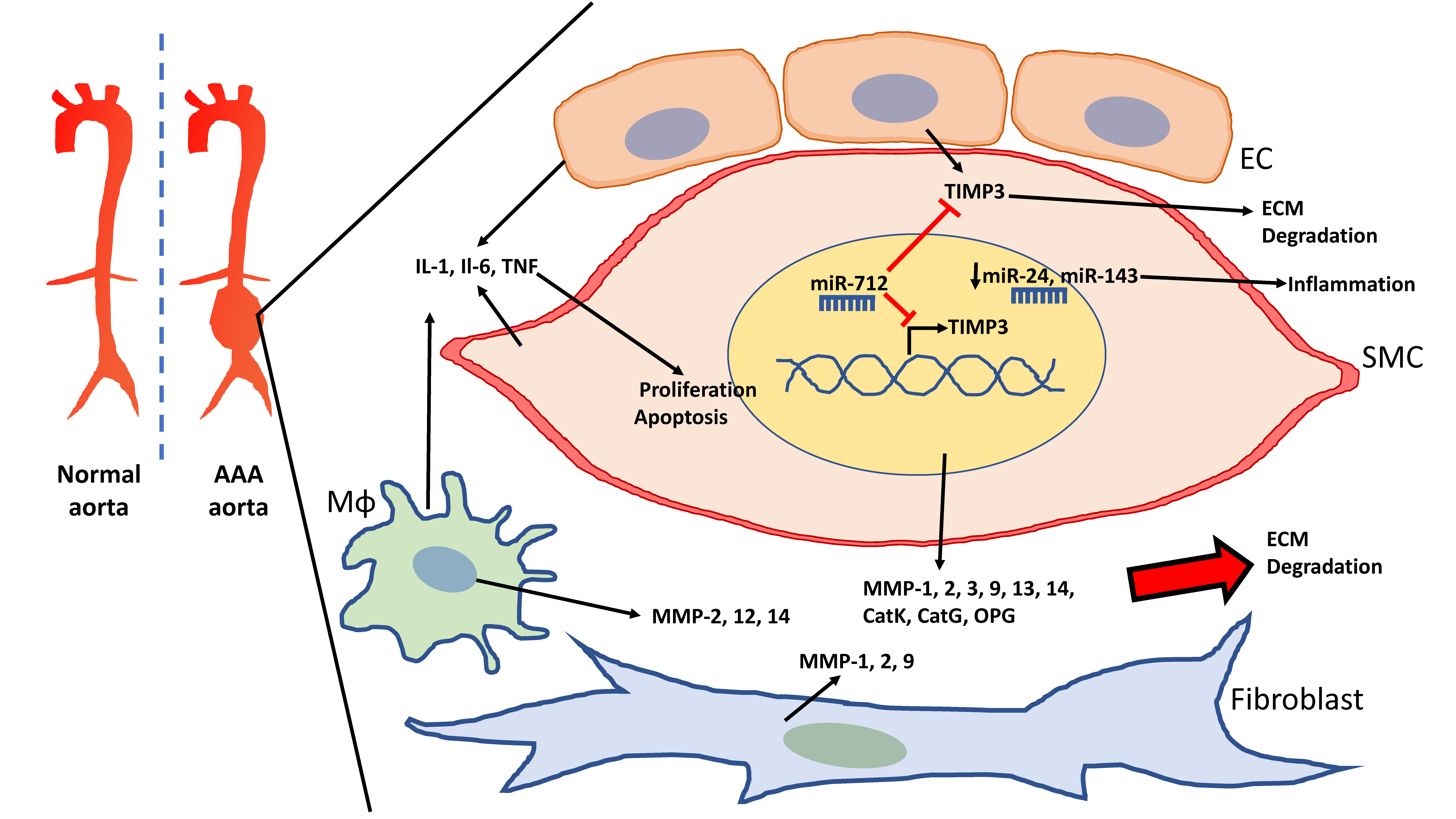

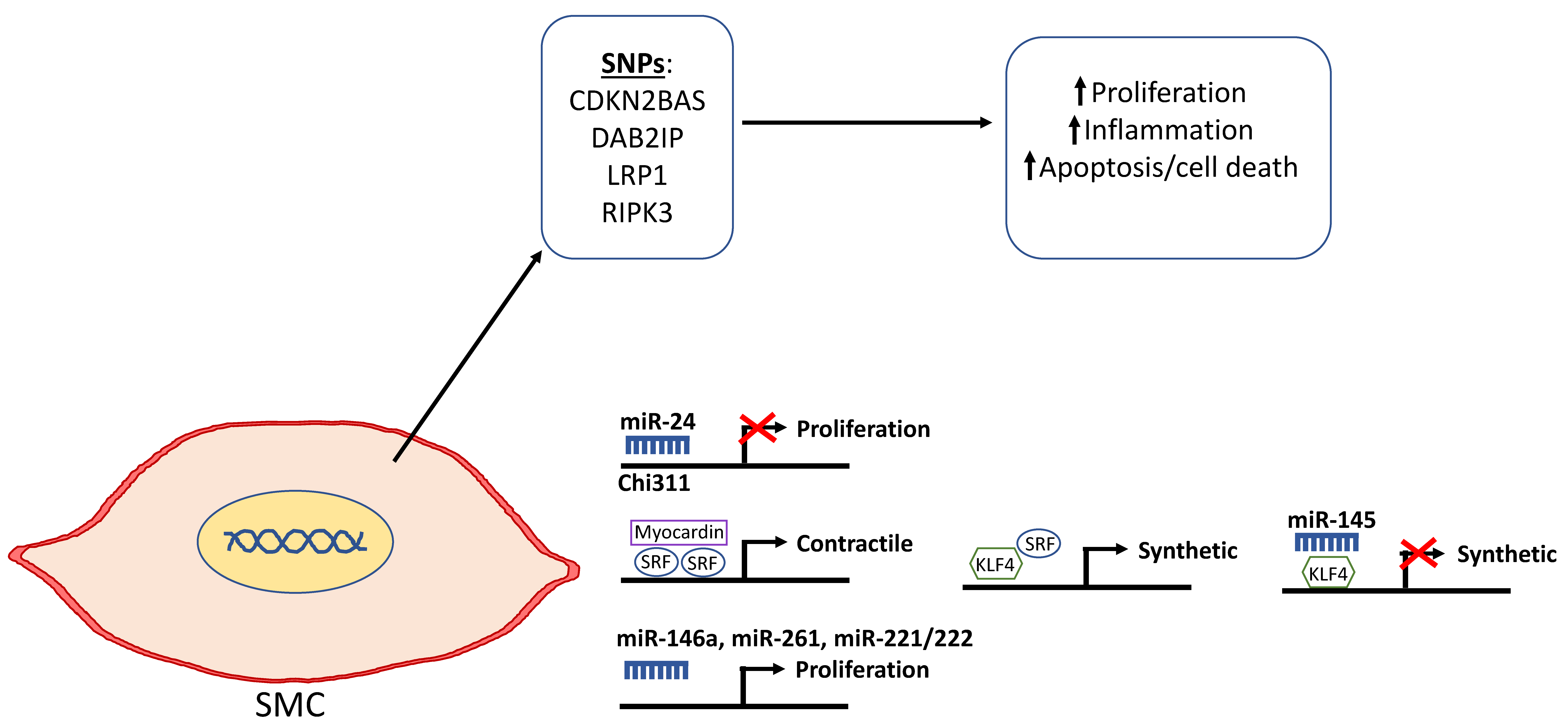
| Expression | Enzyme Group | Substrate | Function | |
|---|---|---|---|---|
| MMP-1 | VSMCs, fibroblast, leukocytes | Collagenase | Collagen (I, II, III, VII, VIII, X), MMP-2, MMP-9, gelatin, proteoglycans | Released predominantly by mesenchymal cells [45,46]. Relies on presence of active MMP3 and plasmin to promote transition of proMMP-1 to active MMP-1 [46] |
| MMP-2 | VSMCs, fibroblasts, macrophages | Gelatinase | Gelatin, collagens (I, IV, V, VII, X, XI, XIV), MMP-1, MMP-9, MMP-13, elastin, fibronectin, laminin | Degrades elastin and fibrillar collagen. Largely expressed by VSMCs [47,48]. Transition of contractile to synthetic VSMC phenotype (as seen in AAA) induces MMP-2 production and enables migratory properties [49]. Mediated by other MMPs (1, 7, membrane type MMPs) [50] |
| MMP-3 | Fibroblasts, epithelial cells, macrophages | Stromelysin | Collagens (III, IV, V, IX, X), MMPs (1, 7, 8, 9, 13), fibronectin, gelatin, laminin | The 5A/6A polymorphism on the MMP-3 gene promoter region increased MMP-3 transcriptional activity and is an independent risk factor for AAA development [51] |
| MMP-9 | VSMCs, fibroblasts, infiltrating macrophages | Gelatinase | Collagens (I, IV, V, VII, X, XI, XIV), elastin, fibronectin, plasminogen | Comprises the predominant elastases present in human AAA. Also exhibits collagenolytic and gelatinolytic activity [52]. Very low concentrations in cell cultures from normal aortic tissues [53]. Works in concert with MMP-2 and MMP-12 to promote aneurysmal degeneration [54,55]. MMP9 C-1562T polymorphism significantly more common in AAA compared to PVD patients and control subjects [52] |
| MMP-12 | Macrophages | Collagenase | Collagen IV, MMP-2, gelatin, elastin, fibronectin, casein, plasminogen, fibrinogen | Increased in human AAA and not seen in atherosclerotic or normal media tissues. Activity is localized in the tunica media [56]. Genetic inactivation or pharmacological inhibition of PI3-kinase delta increased MMP-12 expression and macrophage migration [57] |
| MMP-13 | VSMCs | Collagenase | Collagens (I, II, III, IV, IX, X, XIV), gelatin, MMP-9 | Enzymatic activity is localized to VSMCs of aneurysms. -77A/G polymorphism was an independent risk factor for AAA formation [51]. Nitric oxide-induced CD147 production led to increased MMP13 expression in PPE-induced AAA mice [58] |
| MMP-14 | VSMCs, macrophages | Membrane type | Collagens (I, II, III), MMP-2, gelatin, casein, elastin, vitronectin, fibronectin, laminin | Prominent activator of proMMP-2 [59]. Primarily degrades collagens type I, II, and III. To a lesser degree, degrades gelatin, casein, elastin, fibronectin, vitronectin, and laminin causing degradation of the ECM in the tunica media and adventitia [45,59,60] |
| Category | miRs: | Key Targets | Expression | Function |
|---|---|---|---|---|
| ECM degradation inducing | miR-29b | ELN, Col1A1, COL3A1, COL5A1, Bcl-2, Mcl-1 | Decreased in AngII and PPE perfusion-induced AAA mice and Marfan syndrome mice with aortic root aneurysm [81] Decreased in AAA samples [80] | Increased apoptosis. Activity was repressed by NFκB signaling |
| miR-712/205 | TIMP3, RECK | Increased miR-712 in AngII-induced AAA mouse SMCs and endothelial cells Increased miR-205 in AAA human samples [82] | Suppress TIMP activity in response to AngII-induced enhanced MMP activity | |
| Contractile/anti-inflammatory phenotype inducing | miR-24 | Chi3l1, Mmp14, Stac2, Limd2, Marcksl1, Bcl2l11, Vav1, Prdm1 | Decreased in PPE and AngII-induced AAA in mice Decreased in AAA human plasma [179] | Blocked IL-8 and CCL production by VSMCs and M1 macrophages. Inhibited macrophage recruitment and survival. Expression was downregulated by IL-6 via NFκB signaling. Inhibited CHi3l1-induced VSMC migration |
| miR-143 | Elk1, Versican, protein kinase C-ε, PDGFR-α | Decreased in TAC and AngII ApoE-/- AAA mice Decreased in human aortic aneurysms [128,130] | VSMC proliferation and differentiation, actin remodeling, contractility, podosome formation and migration [130,171] | |
| miR-145 | Myocd, Klf4, Klf5, Calmodulin kinase II-δ, Slit-Robo GTPase-activating protein 1 & 2, Fascin, Adducin-3 | |||
| miR-143/145 | Myocd, related transcription factor-B, Sling-shot 2, tropomyosin 4, ACE | |||
| Synthetic phenotype inducing | miR-21 | PTEN, Bcl-2 | Increased in PPE- and angII-induced AAA in mice Increased in human AAA aortic samples [126,150] | VSMC proliferation and apoptosis via phosphorylation of AKT |
| miR-26a | Smad1, Smad4, Loxl, Inhbb, BAK1, PAK2, SULF1 | Decreased in PPE and AngII-induced AAA mouse models [152] | Increased proliferation/migration, apoptosis, cytokine production, TGF-β receptor pathway signaling | |
| miR-146a | KLF4 | Increased in human AAA aortic samples [151,180] | VSMC proliferation, neointimal hyperplasia | |
| miR-221/-222 | P27, p57, c-Kit | Increased in human AAA aortic tissue [181] | VSMC proliferation, neointimal hyperplasia [155,156,182] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurung, R.; Choong, A.M.; Woo, C.C.; Foo, R.; Sorokin, V. Genetic and Epigenetic Mechanisms Underlying Vascular Smooth Muscle Cell Phenotypic Modulation in Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2020, 21, 6334. https://doi.org/10.3390/ijms21176334
Gurung R, Choong AM, Woo CC, Foo R, Sorokin V. Genetic and Epigenetic Mechanisms Underlying Vascular Smooth Muscle Cell Phenotypic Modulation in Abdominal Aortic Aneurysm. International Journal of Molecular Sciences. 2020; 21(17):6334. https://doi.org/10.3390/ijms21176334
Chicago/Turabian StyleGurung, Rijan, Andrew Mark Choong, Chin Cheng Woo, Roger Foo, and Vitaly Sorokin. 2020. "Genetic and Epigenetic Mechanisms Underlying Vascular Smooth Muscle Cell Phenotypic Modulation in Abdominal Aortic Aneurysm" International Journal of Molecular Sciences 21, no. 17: 6334. https://doi.org/10.3390/ijms21176334
APA StyleGurung, R., Choong, A. M., Woo, C. C., Foo, R., & Sorokin, V. (2020). Genetic and Epigenetic Mechanisms Underlying Vascular Smooth Muscle Cell Phenotypic Modulation in Abdominal Aortic Aneurysm. International Journal of Molecular Sciences, 21(17), 6334. https://doi.org/10.3390/ijms21176334





