DHA Attenuates Cerebral Edema Following Traumatic Brain Injury via the Reduction in Blood–Brain Barrier Permeability
Abstract
1. Introduction
2. Results
2.1. DHA Reduces Sensorimotor Deficits after TBI
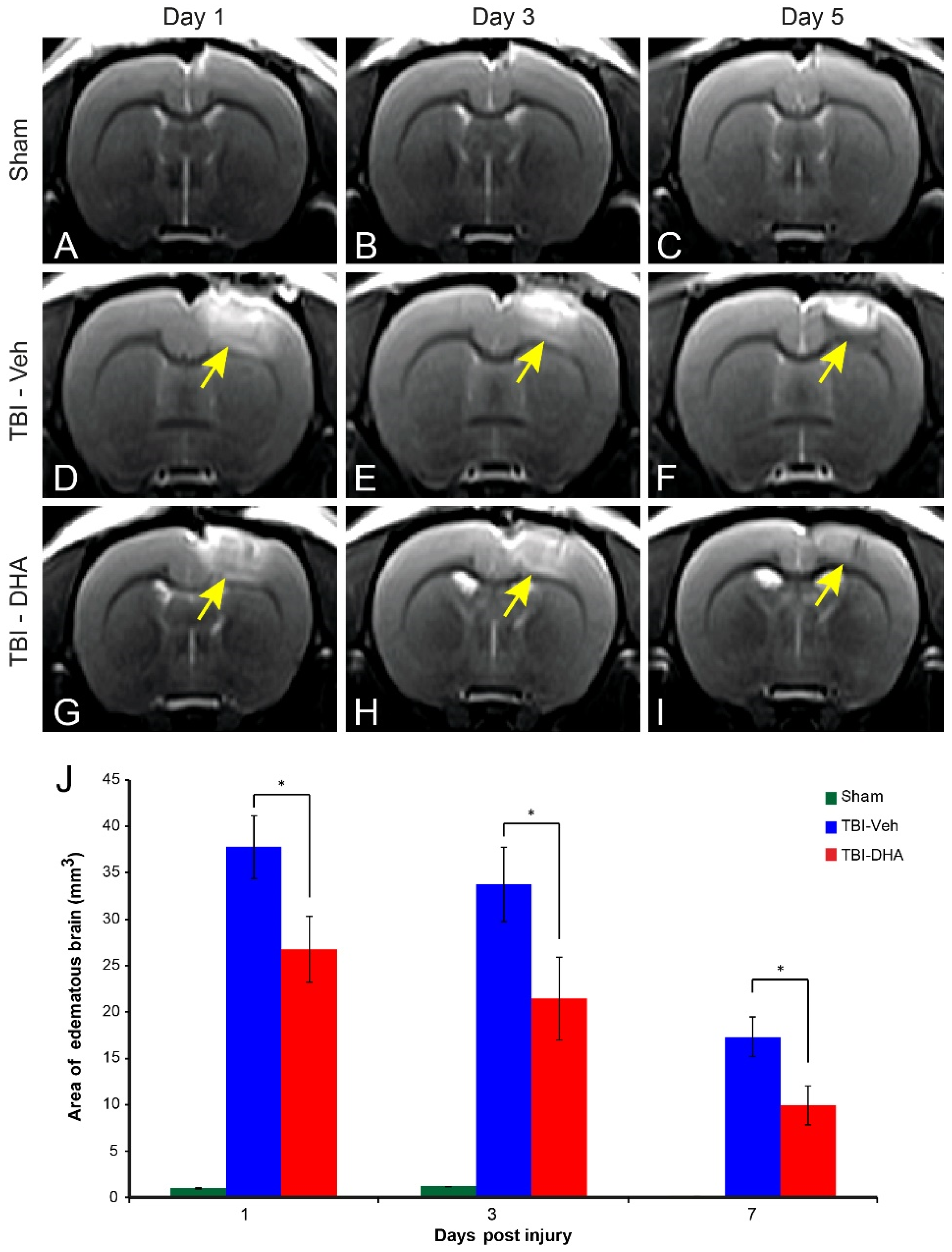
2.2. DHA Attenuates Cerebral Edema after TBI
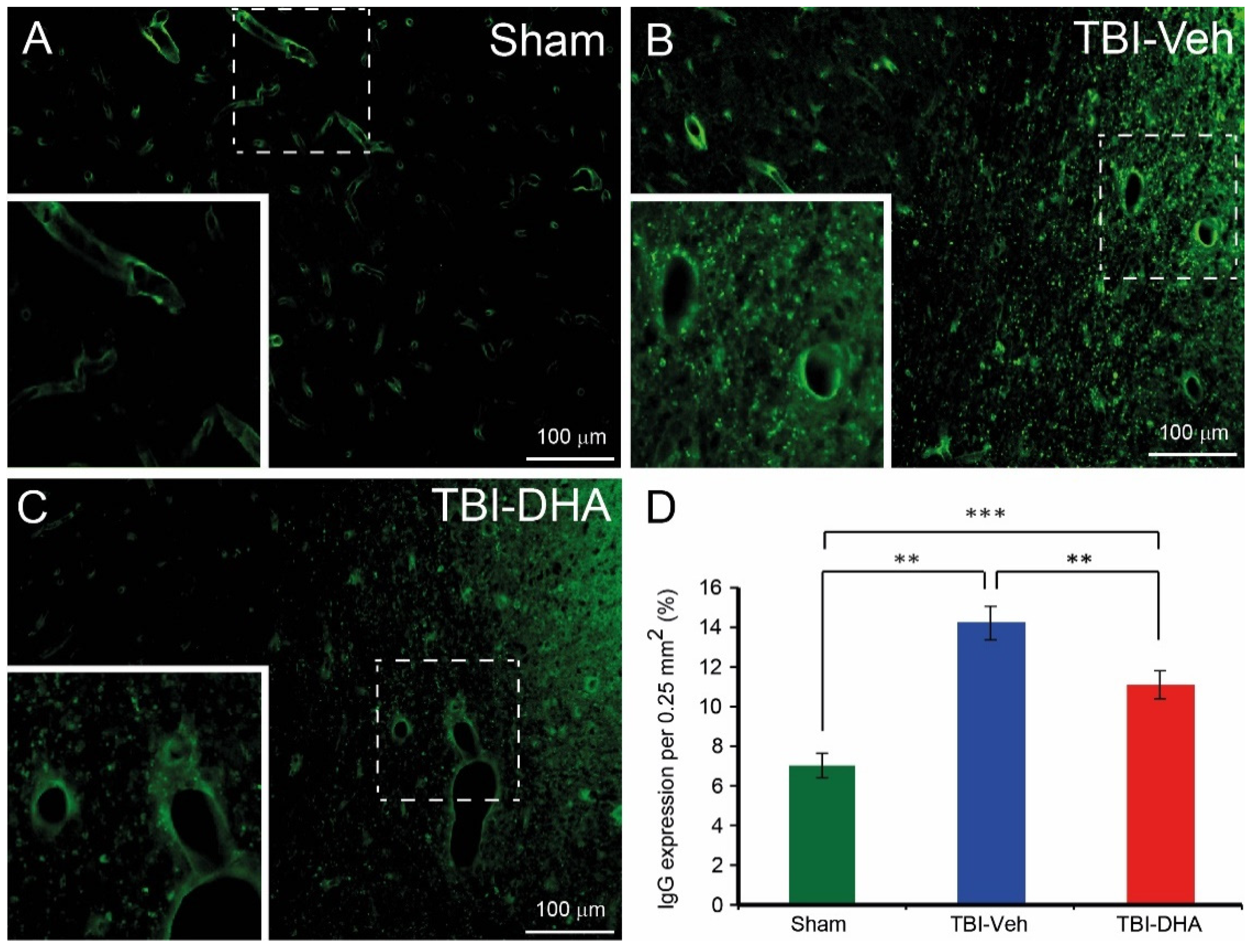
2.3. DHA Attenuates Extravasation after TBI
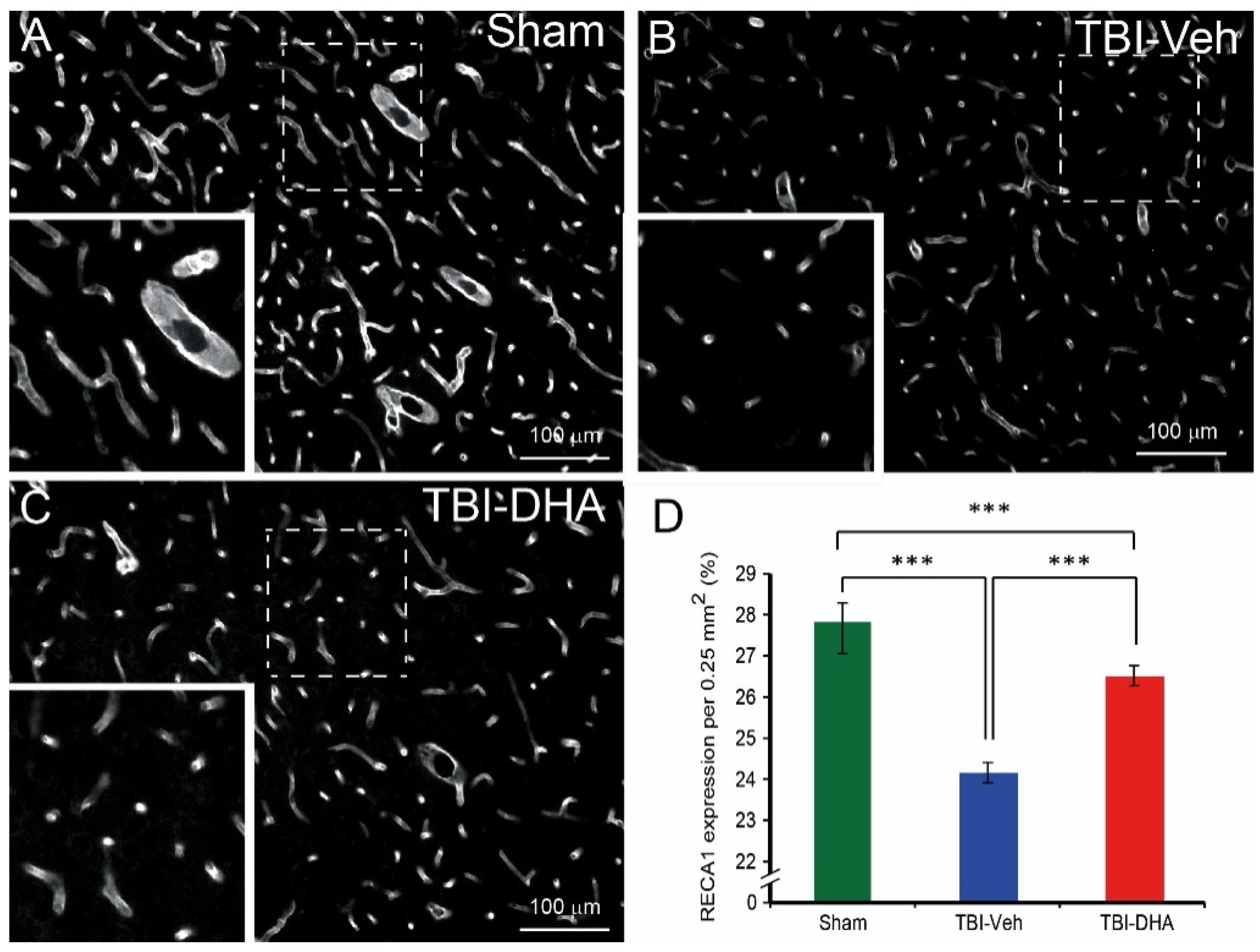
2.4. DHA Alleviates BBB Damage after TBI
2.5. DHA Reduces Aquaporin4 Expression after TBI
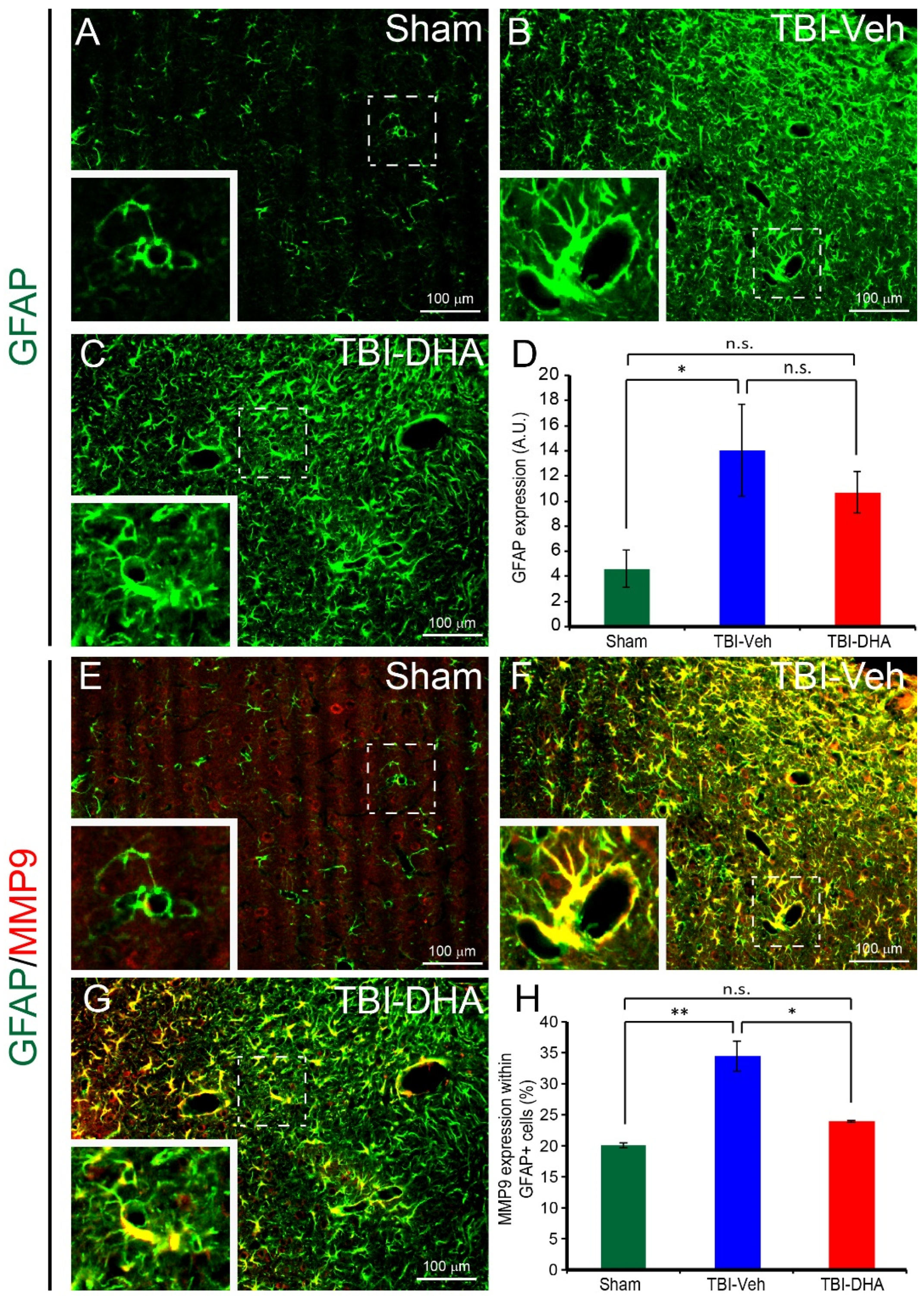
2.6. DHA Reduces Astrocytic MMP9 Expression after TBI
2.7. DHA Increases Endothelial Occludin Expression after TBI
3. Discussion
4. Materials and Methods
4.1. Surgical Procedure
4.2. Drug Administration Procedure
4.3. Modified Neurological Severity Scores
4.4. Forelimb Asymmetry Test
4.5. MRI Image Evaluation
4.6. Immunohistochemical Staining
4.7. Histological Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Laskowitz, D.; Grant, G. Translational Research in Traumatic Brain Injury; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Xiong, Y.; Mahmood, A.; Chopp, M. Emerging treatments for traumatic brain injury. Expert Opin. Emerg. Drugs 2009, 14, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Michael-Titus, A.T. Omega-3 fatty acids: Their neuroprotective and regenerative potential in traumatic neurological injury. Clin. Lipidol. 2009, 4, 343–353. [Google Scholar] [CrossRef]
- Desai, A.; Kevala, K.; Kim, H.Y. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS ONE 2014, 9, e86472. [Google Scholar] [CrossRef]
- Mills, J.D.; Bailes, J.E.; Sedney, C.L.; Hutchins, H.; Sears, B. Omega-3 fatty acid supplementation and reduction of traumatic axonal injury in a rodent head injury model. J. Neurosurg. 2011, 114, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, Z.; Fang, Z.; Lin, W.; Wu, S.; Yang, F.; Li, Y.; Fu, H.; Gao, H.; Li, S. Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1. J. Neuroinflamm. 2018, 15, 310. [Google Scholar] [CrossRef]
- Hall, J.C.; Priestley, J.V.; Perry, V.H.; Michael-Titus, A.T. Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J. Neurochem. 2012, 121, 738–750. [Google Scholar] [CrossRef]
- Yip, P.K.; Pizzasegola, C.; Gladman, S.; Biggio, M.L.; Marino, M.; Jayasinghe, M.; Ullah, F.; Dyall, S.C.; Malaspina, A.; Bendotti, C.; et al. The omega-3 fatty acid eicosapentaenoic acid accelerates disease progression in a model of amyotrophic lateral sclerosis. PLoS ONE 2013, 8, e61626. [Google Scholar] [CrossRef]
- Zhu, W.; Chi, N.; Zou, P.; Chen, H.; Tang, G.; Zhao, W. Effect of docosahexaenoic acid on traumatic brain injury in rats. Exp. Ther. Med. 2017, 14, 4411–4416. [Google Scholar] [CrossRef]
- Tang, R.; Lin, Y.M.; Liu, H.X.; Wang, E.S. Neuroprotective effect of docosahexaenoic acid in rat traumatic brain injury model via regulation of TLR4/NF-κB signaling pathway. Int. J. Biochem. Cell Biol. 2018, 99, 64–71. [Google Scholar] [CrossRef]
- Thau-Zuchman, O.; Ingram, R.; Harvey, G.G.; Cooke, T.; Palmas, F.; Pallier, P.N.; Brook, J.; Priestley, J.V.; Dalli, J.; Tremoleda, J.L.; et al. A Single Injection of Docosahexaenoic Acid Induces a Pro-Resolving Lipid Mediator Profile in the Injured Tissue and a Long-Lasting Reduction in Neurological Deficit after Traumatic Brain Injury in Mice. J. Neurotrauma 2020, 37, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef]
- Marmarou, A. Pathophysiology of traumatic brain edema: Current concepts. Acta Neurochir. Suppl. 2003, 86, 7–10. [Google Scholar] [PubMed]
- Adams, C.A.; Stein, D.M.; Morrison, J.J.; Scalea, T.M. Does intracranial pressure management hurt more than it helps in traumatic brain injury? Trauma Surg. Acute Care Open 2018, 3, e000142. [Google Scholar] [CrossRef]
- Fenn, N.E., 3rd; Sierra, C.M. Hyperosmolar Therapy for Severe Traumatic Brain Injury in Pediatrics: A Review of the Literature. J. Pediatr. Pharmacol. Ther. 2019, 24, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Badenes, R.; Hutton, B.; Citerio, G.; Robba, C.; Aguilar, G.; Alonso-Arroyo, A.; Taccone, F.S.; Tornero, C.; Catala-Lopez, F. Hyperosmolar therapy for acute brain injury: Study protocol for an umbrella review of meta-analyses and an evidence mapping. BMJ Open 2020, 10, e033913. [Google Scholar] [CrossRef]
- Boone, M.D.; Oren-Grinberg, A.; Robinson, T.M.; Chen, C.C.; Kasper, E.M. Mannitol or hypertonic saline in the setting of traumatic brain injury: What have we learned? Surg. Neurol. Int. 2015, 6, 177. [Google Scholar] [CrossRef]
- Leonardo, C.C.; Pennypacker, K.R. Neuroinflammation and MMPs: Potential therapeutic targets in neonatal hypoxic-ischemic injury. J. Neuroinflamm. 2009, 6, 13. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Potential utility of aquaporin modulators for therapy of brain disorders. Prog. Brain Res. 2008, 170, 589–601. [Google Scholar]
- Mahmood, A.; Goussev, A.; Lu, D.; Qu, C.; Xiong, Y.; Kazmi, H.; Chopp, M. Long-lasting benefits after treatment of traumatic brain injury (TBI) in rats with combination therapy of marrow stromal cells (MSCs) and simvastatin. J. Neurotrauma 2008, 25, 1441–1447. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, X.; Liao, Z.; Xiang, H.; Ren, M.; Xu, M.; Zhao, H. Novel-graded traumatic brain injury model in rats induced by closed head impacts. Neuropathology 2018, 38, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Trueman, R.C.; Diaz, C.; Farr, T.D.; Harrison, D.J.; Fuller, A.; Tokarczuk, P.F.; Stewart, A.J.; Paisey, S.J.; Dunnett, S.B. Systematic and detailed analysis of behavioural tests in the rat middle cerebral artery occlusion model of stroke: Tests for long-term assessment. J. Cereb. Blood Flow Metab. 2017, 37, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Koyama, Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int. J. Mol. Sci. 2015, 16, 9949–9975. [Google Scholar] [CrossRef] [PubMed]
- Michalak, Z.; Lebrun, A.; di Miceli, M.; Rousset, M.C.; Crespel, A.; Coubes, P.; Henshall, D.C.; Lerner-Natoli, M.; Rigau, V. IgG leakage may contribute to neuronal dysfunction in drug-refractory epilepsies with blood-brain barrier disruption. J. Neuropathol. Exp. Neurol. 2012, 71, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Moore, A.N.; Redell, J.B.; Dash, P.K. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 2007, 27, 10240–10248. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bacha, R.S.; Baez, E.; Garcia-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef]
- Zador, Z.; Stiver, S.; Wang, V.; Manley, G.T. Role of aquaporin-4 in cerebral edema and stroke. Handb. Exp. Pharmacol. 2009, 190, 159–170. [Google Scholar]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef]
- Kamat, P.K.; Swarnkar, S.; Rai, S.; Kumar, V.; Tyagi, N. Astrocyte mediated MMP-9 activation in the synapse dysfunction: An implication in Alzheimer disease. Ther. Targets Neurol. Dis. 2014, 1, e243. [Google Scholar] [CrossRef]
- Zhu, W.; Ding, Y.; Kong, W.; Li, T.; Chen, H. Docosahexaenoic Acid (DHA) Provides Neuroprotection in Traumatic Brain Injury Models via Activating Nrf2-ARE Signaling. Inflammation 2018, 41, 1182–1193. [Google Scholar] [CrossRef]
- Roth, T.L.; Nayak, D.; Atanasijevic, T.; Koretsky, A.P.; Latour, L.L.; McGavern, D.B. Transcranial amelioration of inflammation and cell death after brain injury. Nature 2014, 505, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Baeza, A.; Reina-de la Torre, F.; Poca, A.; Marti, M.; Garnacho, A. Morphological features in human cortical brain microvessels after head injury: A three-dimensional and immunocytochemical study. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 273, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Villalba, N.; Sackheim, A.M.; Nunez, I.A.; Hill-Eubanks, D.C.; Nelson, M.T.; Wellman, G.C.; Freeman, K. Traumatic Brain Injury Causes Endothelial Dysfunction in the Systemic Microcirculation through Arginase-1-Dependent Uncoupling of Endothelial Nitric Oxide Synthase. J. Neurotrauma 2017, 34, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Bell, J.D.; Siddiq, I.P.; Baker, A.J. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: A role for hypoxia-inducible factors in traumatic brain injury. J. Cereb. Blood Flow Metab. 2009, 29, 575–584. [Google Scholar] [CrossRef]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rama Rao, K.V.; Chandra, N. Synergistic Role of Oxidative Stress and Blood-Brain Barrier Permeability as Injury Mechanisms in the Acute Pathophysiology of Blast-induced Neurotrauma. Sci. Rep. 2019, 9, 7717. [Google Scholar] [CrossRef]
- Kyyriainen, J.; Ekolle Ndode-Ekane, X.; Pitkanen, A. Dynamics of PDGFRβ expression in different cell types after brain injury. Glia 2017, 65, 322–341. [Google Scholar] [CrossRef]
- Muellner, A.; Benz, M.; Kloss, C.U.; Mautes, A.; Burggraf, D.; Hamann, G.F. Microvascular basal lamina antigen loss after traumatic brain injury in the rat. J. Neurotrauma 2003, 20, 745–754. [Google Scholar] [CrossRef]
- Yamagata, K.; Suzuki, S.; Tagami, M. Docosahexaenoic acid prevented tumor necrosis factor alpha-induced endothelial dysfunction and senescence. Prostaglandins Leukot. Essent. Fatty Acids 2016, 104, 11–18. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Malinowski, K.; Golightly, M.; Jesty, J.; Goligorsky, M.S. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation 2002, 106, 2372–2378. [Google Scholar] [CrossRef]
- Kataoka, H.; Kume, N.; Miyamoto, S.; Minami, M.; Moriwaki, H.; Murase, T.; Sawamura, T.; Masaki, T.; Hashimoto, N.; Kita, T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 1999, 99, 3110–3117. [Google Scholar] [CrossRef]
- Collins, M.A.; Moon, K.H.; Tajuddin, N.; Neafsey, E.J.; Kim, H.Y. Docosahexaenoic acid (DHA) prevents binge ethanol-dependent aquaporin-4 elevations while inhibiting neurodegeneration: Experiments in rat adult-age entorhino-hippocampal slice cultures. Neurotox. Res. 2013, 23, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Tajuddin, N.; Moon, K.H.; Marshall, S.A.; Nixon, K.; Neafsey, E.J.; Kim, H.Y.; Collins, M.A. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: Abrogation by docosahexaenoic acid. PLoS ONE 2014, 9, e101223. [Google Scholar] [CrossRef] [PubMed]
- Pirici, I.; Balsanu, T.A.; Bogdan, C.; Margaritescu, C.; Divan, T.; Vitalie, V.; Mogoanta, L.; Pirici, D.; Carare, R.O.; Muresanu, D.F. Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways. Int. J. Mol. Sci. 2017, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Uchida, K.; Papadopoulos, M.C.; Zador, Z.; Manley, G.T.; Verkman, A.S. Mildly Reduced Brain Swelling and Improved Neurological Outcome in Aquaporin-4 Knockout Mice following Controlled Cortical Impact Brain Injury. J. Neurotrauma 2015, 32, 1458–1464. [Google Scholar] [CrossRef]
- Sun, M.C.; Honey, C.R.; Berk, C.; Wong, N.L.; Tsui, J.K. Regulation of aquaporin-4 in a traumatic brain injury model in rats. J. Neurosurg. 2003, 98, 565–569. [Google Scholar] [CrossRef]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow Metab. 2002, 22, 367–378. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Lu, H. Expression of aquaporin-4 and pathological characteristics of brain injury in a rat model of traumatic brain injury. Mol. Med. Rep. 2015, 12, 7351–7357. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; di Paola, R.; Esposito, E.; Gladman, S.; Yip, P.; Priestley, J.V.; Michael-Titus, A.T.; Cuzzocrea, S. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: In-vivo and in-vitro studies. J. Neuroinflamm. 2014, 11, 6. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Navratil, M.; Barone, F.; Feuerstein, G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J. Cereb. Blood Flow Metab. 1996, 16, 360–366. [Google Scholar] [CrossRef]
- Shigemori, Y.; Katayama, Y.; Mori, T.; Maeda, T.; Kawamata, T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir. Suppl. 2006, 96, 130–133. [Google Scholar]
- Wang, X.; Jung, J.; Asahi, M.; Chwang, W.; Russo, L.; Moskowitz, M.A.; Dixon, C.E.; Fini, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J. Neurosci. 2000, 20, 7037–7042. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kaneko, Y.; Yu, S.; Bae, E.; Stahl, C.E.; Kawase, T.; van Loveren, H.; Sanberg, P.R.; Borlongan, C.V. Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res. 2009, 1280, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Reeves, T.M.; Phillips, L.L. Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury. Exp. Neurol. 2014, 261, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.L.; Chan, J.L.; Doperalski, A.E.; Reeves, T.M. Time dependent integration of matrix metalloproteinases and their targeted substrates directs axonal sprouting and synaptogenesis following central nervous system injury. Neural. Regen. Res. 2014, 9, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.W.; Krekoski, C.A.; Bou, S.S.; Chapman, K.R.; Edwards, D.R. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci. Lett. 1997, 238, 53–56. [Google Scholar] [CrossRef]
- Grossetete, M.; Phelps, J.; Arko, L.; Yonas, H.; Rosenberg, G.A. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery 2009, 65, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef]
- Lindberg, R.L.; de Groot, C.J.; Montagne, L.; Freitag, P.; van der Valk, P.; Kappos, L.; Leppert, D. The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain 2001, 124, 1743–1753. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Yamamoto, S.; Steers, J.; Sevlever, D.; Lin, W.; Shimojima, N.; Castanedes-Casey, M.; Genco, P.; Golde, T.; Richelson, E.; et al. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J. Hepatol. 2006, 44, 1105–1114. [Google Scholar] [CrossRef]
- Diaz-Sanchez, M.; Williams, K.; DeLuca, G.C.; Esiri, M.M. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 2006, 111, 289–299. [Google Scholar] [CrossRef]
- Newman, T.A.; Woolley, S.T.; Hughes, P.M.; Sibson, N.R.; Anthony, D.C.; Perry, V.H. T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: Involvement of metalloproteinases. Brain 2001, 124, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Chao, C.Y.; Lin, L.L.; Lu, C.Y.; Liu, K.L.; Lii, C.K.; Li, C.C. Inhibition of matrix metalloproteinase-9 expression by docosahexaenoic acid mediated by heme oxygenase 1 in 12-O-tetradecanoylphorbol-13-acetate-induced MCF-7 human breast cancer cells. Arch. Toxicol. 2013, 87, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Yu, H.N.; Noh, E.M.; Kim, J.M.; Hong, O.Y.; Youn, H.J.; Jung, S.H.; Kwon, K.B.; Kim, J.S.; Lee, Y.R. DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-γ/NF-κB pathway in MCF-7 cells. Oncol. Lett. 2017, 13, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Mu, H.; Zhu, W.; Jiang, X.; Hu, X.; Shi, Y.; Leak, R.K.; Dong, Q.; Chen, J.; et al. Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury. Neurobiol. Dis. 2016, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Brilha, S.; Ong, C.W.M.; Weksler, B.; Romero, N.; Couraud, P.O.; Friedland, J.S. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci. Rep. 2017, 7, 16031. [Google Scholar] [CrossRef]
- Chen, F.; Ohashi, N.; Li, W.; Eckman, C.; Nguyen, J.H. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 2009, 50, 1914–1923. [Google Scholar] [CrossRef]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594. [Google Scholar] [CrossRef]
- Wang, T.M.; Chen, C.J.; Lee, T.S.; Chao, H.Y.; Wu, W.H.; Hsieh, S.C.; Sheu, H.H.; Chiang, A.N. Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells. J. Nutr. Biochem. 2011, 22, 187–194. [Google Scholar] [CrossRef]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69, 7–21. [Google Scholar] [CrossRef]
- Chmurzynska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Sanchez, R.; Sali, A.; Heintz, N. Ligand specificity of brain lipid-binding protein. J. Biol. Chem. 1996, 271, 24711–24719. [Google Scholar] [PubMed]
- Balendiran, G.K.; Schnutgen, F.; Scapin, G.; Borchers, T.; Xhong, N.; Lim, K.; Godbout, R.; Spener, F.; Sacchettini, J.C. Crystal structure and thermodynamic analysis of human brain fatty acid-binding protein. J. Biol. Chem. 2000, 275, 27045–27054. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Yip, P.K.; Adams, L.; Davies, M.; Lee, J.W.; Michael, G.J.; Priestley, J.V.; Michael-Titus, A.T. A Single Bolus of Docosahexaenoic Acid Promotes Neuroplastic Changes in the Innervation of Spinal Cord Interneurons and Motor Neurons and Improves Functional Recovery after Spinal Cord Injury. J. Neurosci. 2015, 35, 12733–12752. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sanberg, P.R.; Li, Y.; Wang, L.; Lu, M.; Willing, A.E.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001, 32, 2682–2688. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Mahmood, A.; Meng, Y.; Liu, Z.; Qu, C.; Chopp, M. Sprouting of corticospinal tract axons from the contralateral hemisphere into the denervated side of the spinal cord is associated with functional recovery in adult rat after traumatic brain injury and erythropoietin treatment. Brain Res. 2010, 1353, 249–257. [Google Scholar] [CrossRef]



| Motor Tests | Points |
|---|---|
| Raising rat by the tail | 3 |
| 1 Flexion of forelimb | |
| 1 Flexion of hindlimb | |
| 1 Head moved > 10° to vertical axis within 30 s | |
| Placing rat on the floor (normal = 0; maximum = 3) | 3 |
| 0 Normal walk | |
| 1 Inability to walk straight | |
| 2 Circling toward the paretic side | |
| 3 Fall down to the paretic side | |
| Sensory tests | 2 |
| 1 Placing balance tests (visual and tactile test) | |
| 2 Proprioceptive test (deep sensation, pushing the paw against the table edge to stimulate limb muscles) | |
| Beam balance tests (normal = 0; maximum = 6) | 6 |
| 0 Balances with steady posture | |
| 1 Grasps side of beam | |
| 2 Hugs the beam and one limb falls down from the beam | |
| 3 Hugs the beam and two limbs fall down from the beam, or spins on beam (>60 s) | |
| 4 Attempts to balance on the beam but falls off (>40 s) | |
| 5 Attempts to balance on the beam but falls off (>20 s) | |
| 6 Falls off: No attempt on the balance or hang on to the beam (<20 s) | |
| Reflexes absent and abnormal movements | 4 |
| 1 Pinna reflex (head sake when touching the auditory meatus) | |
| 1 Corneal reflex (eye blink when lightly touching the cornea with cotton) | |
| 1 Startle reflex (motor response to a brief noise from snapping a clipboard paper) | |
| 1 Seizure, myoclonus, myodystony | |
| Maximum points | 18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-H.; Chen, N.-Y.; Tu, P.-h.; Wu, C.-T.; Chiu, S.-C.; Huang, Y.-C.; Lim, S.-N.; Yip, P.K. DHA Attenuates Cerebral Edema Following Traumatic Brain Injury via the Reduction in Blood–Brain Barrier Permeability. Int. J. Mol. Sci. 2020, 21, 6291. https://doi.org/10.3390/ijms21176291
Liu Z-H, Chen N-Y, Tu P-h, Wu C-T, Chiu S-C, Huang Y-C, Lim S-N, Yip PK. DHA Attenuates Cerebral Edema Following Traumatic Brain Injury via the Reduction in Blood–Brain Barrier Permeability. International Journal of Molecular Sciences. 2020; 21(17):6291. https://doi.org/10.3390/ijms21176291
Chicago/Turabian StyleLiu, Zhuo-Hao, Nan-Yu Chen, Po-hsun Tu, Chen-Te Wu, Shao-Chieh Chiu, Ying-Cheng Huang, Siew-Na Lim, and Ping K. Yip. 2020. "DHA Attenuates Cerebral Edema Following Traumatic Brain Injury via the Reduction in Blood–Brain Barrier Permeability" International Journal of Molecular Sciences 21, no. 17: 6291. https://doi.org/10.3390/ijms21176291
APA StyleLiu, Z.-H., Chen, N.-Y., Tu, P.-h., Wu, C.-T., Chiu, S.-C., Huang, Y.-C., Lim, S.-N., & Yip, P. K. (2020). DHA Attenuates Cerebral Edema Following Traumatic Brain Injury via the Reduction in Blood–Brain Barrier Permeability. International Journal of Molecular Sciences, 21(17), 6291. https://doi.org/10.3390/ijms21176291






