Identification of Novel Targets of Knee Osteoarthritis Shared by Cartilage and Synovial Tissue
Abstract
1. Introduction
2. Results
2.1. Initial Evaluation of the Synovial and Cartilage RNA-seq Data Sets
2.2. Identification of the Common OA-Responsive Genes in Both Cartilage and Synovium
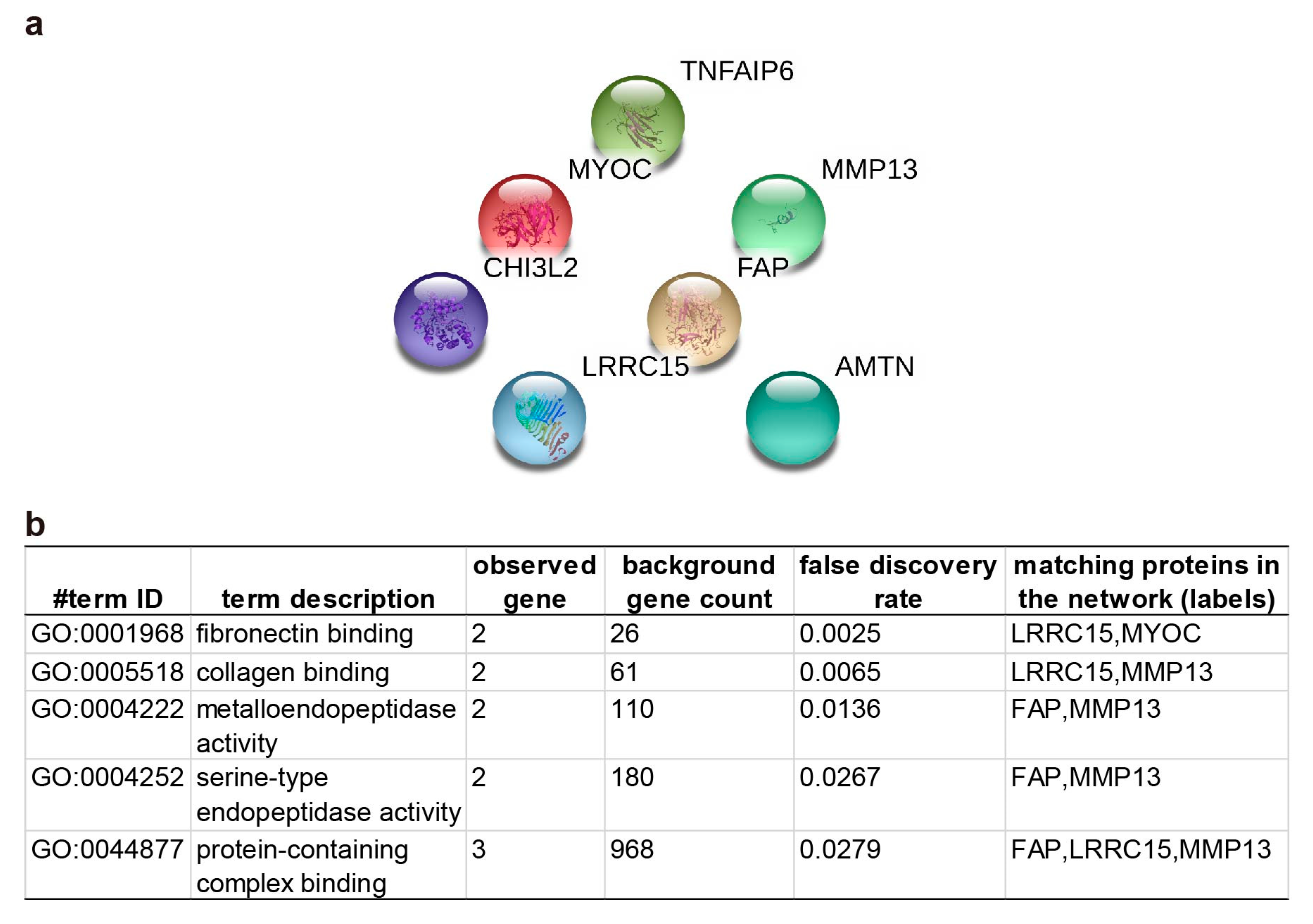
2.3. Pathway Enrichment and Protein-Protein Interaction Cluster
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- National Health Service, UK, Arthritis. Available online: https://www.nhs.uk/conditions/arthritis/ (accessed on 19 August 2020).
- Centres for Disease Control and Prevention, USA, Arthritis Types. Available online: https://www.cdc.gov/arthritis/basics/types.html (accessed on 19 August 2020).
- Arthritis Foundation. Understanding Arthritis. Available online: https://www.arthritis.org/about-arthritis/understanding-arthritis/ (accessed on 19 August 2020).
- Osteoarthritis Research Society International. Osteoarthritis: A serious Disease. 2016, pp. 1–103. Available online: https://www.oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf (accessed on 19 August 2020).
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Kopec, J.A.; Rahman, M.M.; Berthelot, J.M.; Le Petit, C.; Aghajanian, J.; Sayre, E.C.; Cibere, J.; Anis, A.H.; Badley, E.M. Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J. Rheumatol. 2007, 34, 386–393. [Google Scholar] [PubMed]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef]
- Puig-Junoy, J.; Ruiz, A. Socio-economic costs of osteoarthritis: A systematic review of cost-of-illness studies. Semin. Arthritis Rheu. 2015, 44, 531–541. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Wenham, C.Y.; Conaghan, P.G. The role of synovitis in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 349–359. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Caplan, L.; Wolfe, F.; Russell, A.S.; Michaud, K. Corticosteroid use in rheumatoid arthritis: Prevalence, predictors, correlates, and outcomes. J. Rheumatol. 2007, 34, 696–705. [Google Scholar] [PubMed]
- Hammer, M.; Schwarz, T.; Ganser, G. Intra-articular injection of cortisone. Z. Rheumatol. 2015, 74, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Chandrappa, M.; Biswas, S. Glucocorticoids in Management of Adult Rheumatoid Arthritis-Current Prescribing Practices and Perceptions of Physicians in India: GLUMAR Survey. Rheumatology 2017, 7, 1000220. [Google Scholar] [CrossRef]
- Saltychev, M.; Mattie, R.; McCormick, Z.; Laimi, K. The Magnitude and Duration of the Effect of Intra-articular Corticosteroid Injections on Pain Severity in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2020, 99, 617–625. [Google Scholar] [CrossRef]
- Arroll, B.; Goodyear-Smith, F. Corticosteroid injections for osteoarthritis of the knee: Meta-analysis. BMJ 2004, 328, 869. [Google Scholar] [CrossRef]
- Savvidou, O.; Milonaki, M.; Goumenos, S.; Flevas, D.; Papagelopoulos, P.; Moutsatsou, P. Glucocorticoid signaling and osteoarthritis. Mol. Cell Endocrinol. 2019, 480, 153–166. [Google Scholar] [CrossRef]
- Cooper, C.; Bardin, T.; Brandi, M.L.; Cacoub, P.; Caminis, J.; Civitelli, R.; Cutolo, M.; Dere, W.; Devogelaer, J.P.; Diez-Perez, A.; et al. Balancing benefits and risks of glucocorticoids in rheumatic diseases and other inflammatory joint disorders: New insights from emerging data. An expert consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Aging Clin. Exp. Res. 2016, 28, 1–16. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Scott, D.L. Arthritis in the Elderly. In Brocklehurst’s Textbook of Geriatric Medicine and Gerontology, 7th ed.; Fillit, H.M., Rockwood, K., Woodhouse, K., Eds.; Saunders, Elsevier: Philadelphia, PA, USA, 2010; pp. 566–576. [Google Scholar]
- Brosseau, L.; Taki, J.; Desjardins, B.; Thevenot, O.; Fransen, M.; Wells, G.A.; Mizusaki Imoto, A.; Toupin-April, K.; Westby, M.; Alvarez Gallardo, I.C.; et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: Strengthening exercise programs. Clin. Rehabil. 2017, 31, 596–611. [Google Scholar] [CrossRef]
- Verbruggen, G.; Wittoek, R.; Cruyssen, B.V.; Elewaut, D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: A double blind, randomised trial on structure modification. Ann. Rheum. Dis. 2012, 71, 891–898. [Google Scholar] [CrossRef]
- Chevalier, X.; Ravaud, P.; Maheu, E.; Baron, G.; Rialland, A.; Vergnaud, P.; Roux, C.; Maugars, Y.; Mulleman, D.; Lukas, C.; et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double- blind, placebo-controlled trial. Ann. Rheum. Dis. 2015, 74, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular Injection of Anakinra in Osteoarthritis of the Knee: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthrit. Rheum.-Arthr. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.T. Osteoarthritis year in review 2017: Biology. Osteoarthr. Cartil. 2018, 26, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, 498–503. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, 506–515. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef]
- Wang, M.; Sampson, E.R.; Jin, H.; Li, J.; Ke, Q.H.; Im, H.J.; Chen, D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 2013, 15, 5. [Google Scholar] [CrossRef]
- Steck, E.; Breit, S.; Breusch, S.J.; Axt, M.; Richter, W. Enhanced expression of the human chitinase 3-like 2 gene (YKL-39) but not chitinase 3-like 1 gene (YKL-40) in osteoarthritic cartilage. Biochem. Biophys. Res. Commun. 2002, 299, 109–115. [Google Scholar] [CrossRef]
- Knorr, T.; Obermayr, F.; Bartnik, E.; Zien, A.; Aigner, T. YKL-39 (chitinase 3-like protein 2), but not YKL-40 (chitinase 3-like protein 1), is up regulated in osteoarthritic chondrocytes. Ann. Rheum Dis 2003, 62, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, K.; Tsuji, K.; Yamaga, M.; Yamada, J.; Matsukura, Y.; Abula, K.; Sekiya, I.; Muneta, T. Human YKL39 (chitinase 3-like protein 2), an osteoarthritis-associated gene, enhances proliferation and type II collagen expression in ATDC5 cells. Biochem. Biophys. Res. Commun. 2013, 431, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Ranok, A.; Khunkaewla, P.; Suginta, W. Human cartilage chitinase 3-like protein 2: Cloning, expression, and production of polyclonal and monoclonal antibodies for osteoarthritis detection and identification of potential binding partners. Monoclon. Antibodies Immunodiagn. Immunother. 2013, 32, 317–325. [Google Scholar] [CrossRef]
- Tsuruha, J.; Masuko-Hongo, K.; Kato, T.; Sakata, M.; Nakamura, H.; Sekine, T.; Takigawa, M.; Nishioka, K. Autoimmunity against YKL-39, a human cartilage derived protein, in patients with osteoarthritis. J. Rheumatol. 2002, 29, 1459–1466. [Google Scholar] [PubMed]
- Waldele, S.; Koers-Wunrau, C.; Beckmann, D.; Korb-Pap, A.; Wehmeyer, C.; Pap, T.; Dankbar, B. Deficiency of fibroblast activation protein alpha ameliorates cartilage destruction in inflammatory destructive arthritis. Arthritis Res. Ther. 2015, 17, 12. [Google Scholar] [CrossRef]
- Van der Geest, T.; Laverman, P.; Gerrits, D.; Walgreen, B.; Helsen, M.M.; Klein, C.; Nayak, T.K.; Storm, G.; Metselaar, J.M.; Koenders, M.I.; et al. Liposomal Treatment of Experimental Arthritis Can Be Monitored Noninvasively with a Radiolabeled Anti-Fibroblast Activation Protein Antibody. J. Nucl. Med. 2017, 58, 151–155. [Google Scholar] [CrossRef]
- Dorst, D.N.; Rijpkema, M.; Boss, M.; Walgreen, B.; Helsen, M.M.A.; Bos, D.L.; Brom, M.; Klein, C.; Laverman, P.; van der Kraan, P.M.; et al. Targeted photodynamic therapy selectively kills activated fibroblasts in experimental arthritis. Rheumatology 2020. [Google Scholar] [CrossRef]
- Gorlier, C.; Gottenberg, J.E.; Laurans, L.; Simon, T.; Ait-Oufella, H.; Sellam, J. Serum level of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) is a biomarker of synovitis in rheumatoid arthritis. Int. J. Rheum. Dis. 2019, 22, 1616–1618. [Google Scholar] [CrossRef]
- Gamez-Nava, J.I.; Bonilla-Lara, D.; Ponce-Guarneros, J.M.; Zuniga-Mora, J.A.; Perez-Guerrero, E.E.; Murillo-Vazquez, J.D.; Becerra-Alvarado, I.N.; Rodriguez-Jimenez, N.A.; Saldana-Cruz, A.M.; Vazquez-Villegas, M.L.; et al. Utility of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as biomarker to predict therapeutic response to methotrexate in rheumatoid arthritis. Innate Immun. 2017, 23, 606–614. [Google Scholar] [CrossRef]
- Tang, J.; Dong, Q. Knockdown of TREM-1 suppresses IL-1beta-induced chondrocyte injury via inhibiting the NF-kappaB pathway. Biochem. Biophys. Res. Commun. 2017, 482, 1240–1245. [Google Scholar] [CrossRef]
- Shen, Z.T.; Sigalov, A.B. Rationally designed ligand-independent peptide inhibitors of TREM-1 ameliorate collagen-induced arthritis. J. Cell. Mol. Med. 2017, 21, 2524–2534. [Google Scholar] [CrossRef]
- Haasken, S.; Auger, J.L.; Taylor, J.J.; Hobday, P.M.; Goudy, B.D.; Titcombe, P.J.; Mueller, D.L.; Binstadt, B.A. Macrophage scavenger receptor 1 (Msr1, SR-A) influences B cell autoimmunity by regulating soluble autoantigen concentration. J. Immunol. 2013, 191, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Hartlova, A.; Gierlinski, M.; Prescott, A.; Castellvi, J.; Losa, J.H.; Petersen, S.K.; Wenzel, U.A.; Dill, B.D.; Emmerich, C.H.; et al. Triggering MSR1 promotes JNK-mediated inflammation in IL-4-activated macrophages. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzoglou, E.; Griffin, T.M.; Humphrey, M.B. Innate Immune Responses and Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Millerand, M.; Berenbaum, F.; Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol 2019, 37, 48–56. [Google Scholar]
- Lopes, E.B.P.; Filiberti, A.; Husain, S.A.; Humphrey, M.B. Immune Contributions to Osteoarthritis. Curr. Osteoporos. Rep. 2017, 15, 593–600. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78–79, 60–83. [Google Scholar] [CrossRef]
- Tellier, L.E.; Trevino, E.A.; Brimeyer, A.L.; Reece, D.S.; Willett, N.J.; Guldberg, R.E.; Temenoff, J.S. Intra-articular TSG-6 delivery from heparin-based microparticles reduces cartilage damage in a rat model of osteoarthritis. Biomater. Sci. 2018, 6, 1159–1167. [Google Scholar] [CrossRef]
- Chou, C.H.; Attarian, D.E.; Wisniewski, H.G.; Band, P.A.; Kraus, V.B. TSG-6—A double-edged sword for osteoarthritis (OA). Osteoarthr. Cartil. 2018, 26, 245–254. [Google Scholar] [CrossRef]
- Qu, J.; Lu, D.; Guo, H.; Miao, W.; Wu, G.; Zhou, M. PFKFB3 modulates glycolytic metabolism and alleviates endoplasmic reticulum stress in human osteoarthritis cartilage. Clin. Exp. Pharmacol. Physiol. 2016, 43, 312–318. [Google Scholar] [CrossRef]
- Zou, Y.; Zeng, S.; Huang, M.; Qiu, Q.; Xiao, Y.; Shi, M.; Zhan, Z.; Liang, L.; Yang, X.; Xu, H. Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Br. J. Pharmacol. 2017, 174, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Tew, S.R.; Clegg, P.D.; Brew, C.J.; Redmond, C.M.; Hardingham, T.E. SOX9 transduction of a human chondrocytic cell line identifies novel genes regulated in primary human chondrocytes and in osteoarthritis. Arthritis Res. Ther. 2007, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Salkowska, A.; Karas, K.; Karwaciak, I.; Walczak-Drzewiecka, A.; Krawczyk, M.; Sobalska-Kwapis, M.; Dastych, J.; Ratajewski, M. Identification of Novel Molecular Markers of Human Th17 Cells. Cells 2020, 9. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Jiang, Y.; Liu, X.; Huang, L.; Wei, Q.; Huang, Y.; Wu, W.; Gu, J. Alterations in peripheral T cell and B cell subsets in patients with osteoarthritis. Clin. Rheumatol. 2020, 39, 523–532. [Google Scholar] [CrossRef]
- Desmarais, F.; Bergeron, K.F.; Lacaille, M.; Lemieux, I.; Bergeron, J.; Biron, S.; Rassart, E.; Joanisse, D.R.; Mauriege, P.; Mounier, C. High ApoD protein level in the round ligament fat depot of severely obese women is associated with an improved inflammatory profile. Endocrine 2018, 61, 248–257. [Google Scholar] [CrossRef]
- Trouw, L.A.; Daha, N.; Kurreeman, F.A.; Bohringer, S.; Goulielmos, G.N.; Westra, H.J.; Zhernakova, A.; Franke, L.; Stahl, E.A.; Levarht, E.W.; et al. Genetic variants in the region of the C1q genes are associated with rheumatoid arthritis. Clin. Exp. Immunol. 2013, 173, 76–83. [Google Scholar] [CrossRef]
- Lubbers, R.; van Schaarenburg, R.A.; Kwekkeboom, J.C.; Levarht, E.W.N.; Bakker, A.M.; Mahdad, R.; Monteagudo, S.; Cherifi, C.; Lories, R.J.; Toes, R.E.M.; et al. Complement component C1q is produced by isolated articular chondrocytes. Osteoarthr. Cartil. 2020, 28, 675–684. [Google Scholar] [CrossRef]
- Li, C.; Ha, P.; Jiang, W.; Haveles, C.S.; Zheng, Z.; Zou, M. Fibromodulin—A New Target of Osteoarthritis Management? Front. Pharmacol. 2019, 10, 1475. [Google Scholar] [CrossRef]
- Huan, X.; Jinhe, Y.; Rongzong, Z. Identification of Pivotal Genes and Pathways in Osteoarthritic Degenerative Meniscal Lesions via Bioinformatics Analysis of the GSE52042 Dataset. Med. Sci. Monit. 2019, 25, 8891–8904. [Google Scholar] [CrossRef]
- Zhang, G.; Gu, M.; Xu, Y.; Wu, Z. A comprehensive analysis on the effects of 1,25(OH)2D3 on primary chondrocytes cultured from patients with osteoarthritis. Gene 2020, 730, 144322. [Google Scholar] [CrossRef] [PubMed]
- Fattah, S.A.; Abdel Fattah, M.A.; Mesbah, N.M.; Saleh, S.M.; Abo-Elmatty, D.M.; Mehanna, E.T. The expression of zinc finger 804a (ZNF804a) and cyclin-dependent kinase 1 (CDK1) genes is related to the pathogenesis of rheumatoid arthritis. Arch. Physiol. Biochem. 2020, 1–6. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017, 85, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Vergelli, C.; Guerrini, G.; Cantini, N.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Parisio, C.; Di Cesare Mannelli, L.; Ghelardini, C.; et al. Novel formyl peptide receptor (FPR) agonists with pyridinone and pyrimidindione scaffolds that are potentially useful for the treatment of rheumatoid arthritis. Bioorg. Chem. 2020, 100, 103880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, M.; Xiao, W. KLF15 Regulates the Expression of MMP-3 in Human Chondrocytes. J. Interferon Cytokine Res. 2018, 38, 356–362. [Google Scholar] [CrossRef]
- Song, Z.; Lian, X.; Wang, Y.; Xiang, Y.; Li, G. KLF15 regulates in vitro chondrogenic differentiation of human mesenchymal stem cells by targeting SOX9. Biochem. Biophys. Res. Commun. 2017, 493, 1082–1088. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Liao, X.; Sangwung, P.; Prosdocimo, D.A.; Zhou, G.; Votruba, A.R.; Brian, L.; Han, Y.J.; Gao, H.; et al. Kruppel-like factor 15 is critical for vascular inflammation. J. Clin. Investig. 2013, 123, 4232–4241. [Google Scholar] [CrossRef]
- Jung, D.Y.; Chalasani, U.; Pan, N.; Friedline, R.H.; Prosdocimo, D.A.; Nam, M.; Azuma, Y.; Maganti, R.; Yu, K.; Velagapudi, A.; et al. KLF15 is a molecular link between endoplasmic reticulum stress and insulin resistance. PLoS ONE 2013, 8, e77851. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Guo, Y.; Revelo, M.P.; Roa-Pena, L.; Miller, T.; Ling, J.; Shankland, S.J.; Bialkowska, A.B.; Ly, V.; Estrada, C.; et al. Kruppel-Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers. J. Am. Soc. Nephrol. 2017, 28, 166–184. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Li, X.D.; Zhou, H.D.; Shao, S.; He, S.; Hong, M.N.; Liu, J.C.; Xu, Y.L.; Wu, Y.J.; Zhu, D.L.; et al. Transactivation domain of Kruppel-like factor 15 negatively regulates angiotensin II-induced adventitial inflammation and fibrosis. FASEB J. 2019, 33, 6254–6268. [Google Scholar] [CrossRef]
- Iwasaki, K.; Bajenova, E.; Somogyi-Ganss, E.; Miller, M.; Nguyen, V.; Nourkeyhani, H.; Gao, Y.; Wendel, M.; Ganss, B. Amelotin—A Novel Secreted, Ameloblast-specific Protein. J. Dent. Res. 2005, 84, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Tsuruya, Y.; Noda, K.; Yamazaki-Takai, M.; Iwai, Y.; Ganss, B.; Ogata, Y. Negative feedback by SNAI2 regulates TGFbeta1-induced amelotin gene transcription in epithelial-mesenchymal transition. J. Cell. Physiol. 2019, 234, 11474–11489. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Mezawa, M.; Noda, K.; Iwai, Y.; Matsui, S.; Takai, H.; Nakayama, Y.; Ogata, Y. Transcriptional regulation of human amelotin gene by interleukin-1beta. FEBS Open Bio 2018, 8, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Nockemann, D.; Rouault, M.; Labuz, D.; Hublitz, P.; McKnelly, K.; Reis, F.C.; Stein, C.; Heppenstall, P.A. The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol. Med. 2013, 5, 1263–1277. [Google Scholar] [CrossRef]
- Tsantoulas, C. Emerging potassium channel targets for the treatment of pain. Curr. Opin. Support. Palliat. Care 2015, 9, 147–154. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Cooper, C.; Kirwan, J.R.; Dieppe, P.A. Knee pain and disability in the community. Br. J. Rheumatol. 1992, 31, 189–192. [Google Scholar] [CrossRef]
- Cho, H.J.; Chang, C.B.; Yoo, J.H.; Kim, S.J.; Kim, T.K. Gender Differences in the Correlation between Symptom and Radiographic Severity in Patients with Knee Osteoarthritis. Clin. Orthop. Relat. Res. 2010, 468, 1749–1758. [Google Scholar] [CrossRef]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, 537–544. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. limma: Linear Models for Microarray Data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Statistics for Biology and Health; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005; Available online: https://doi.org/10.1007/0-387-29362-0_23 (accessed on 19 August 2020).
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Lun, A.T.; Chen, Y.; Smyth, G.K. It’s DE-licious: A Recipe for Differential Expression Analyses of RNA-seq Experiments Using Quasi-Likelihood Methods in edgeR. Methods Mol. Biol. 2016, 1418, 391–416. [Google Scholar] [CrossRef] [PubMed]
- A Language and Environment for Statistical Computing; Version 3.6.3; The R Foundation: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 19 August 2020).
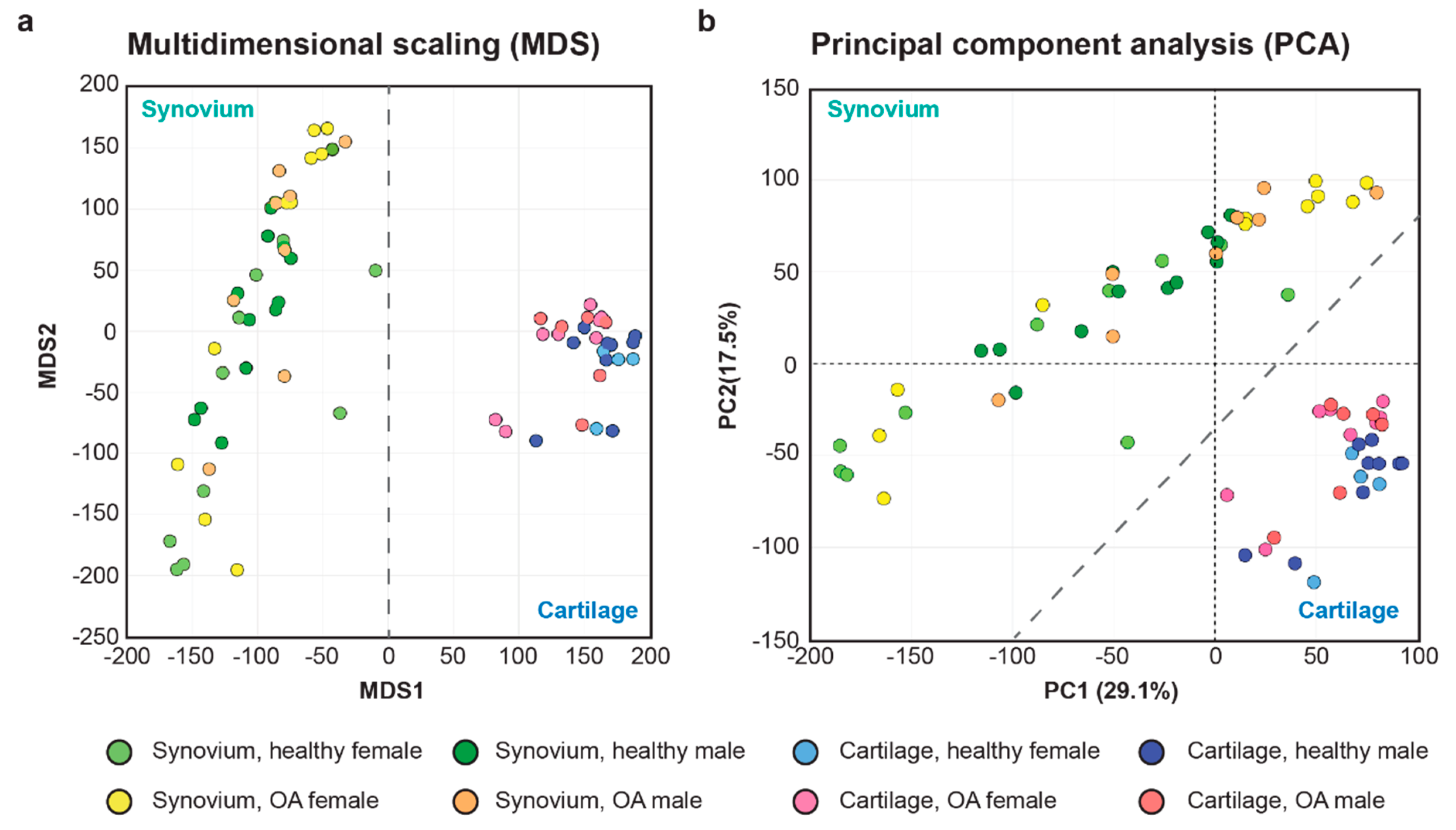

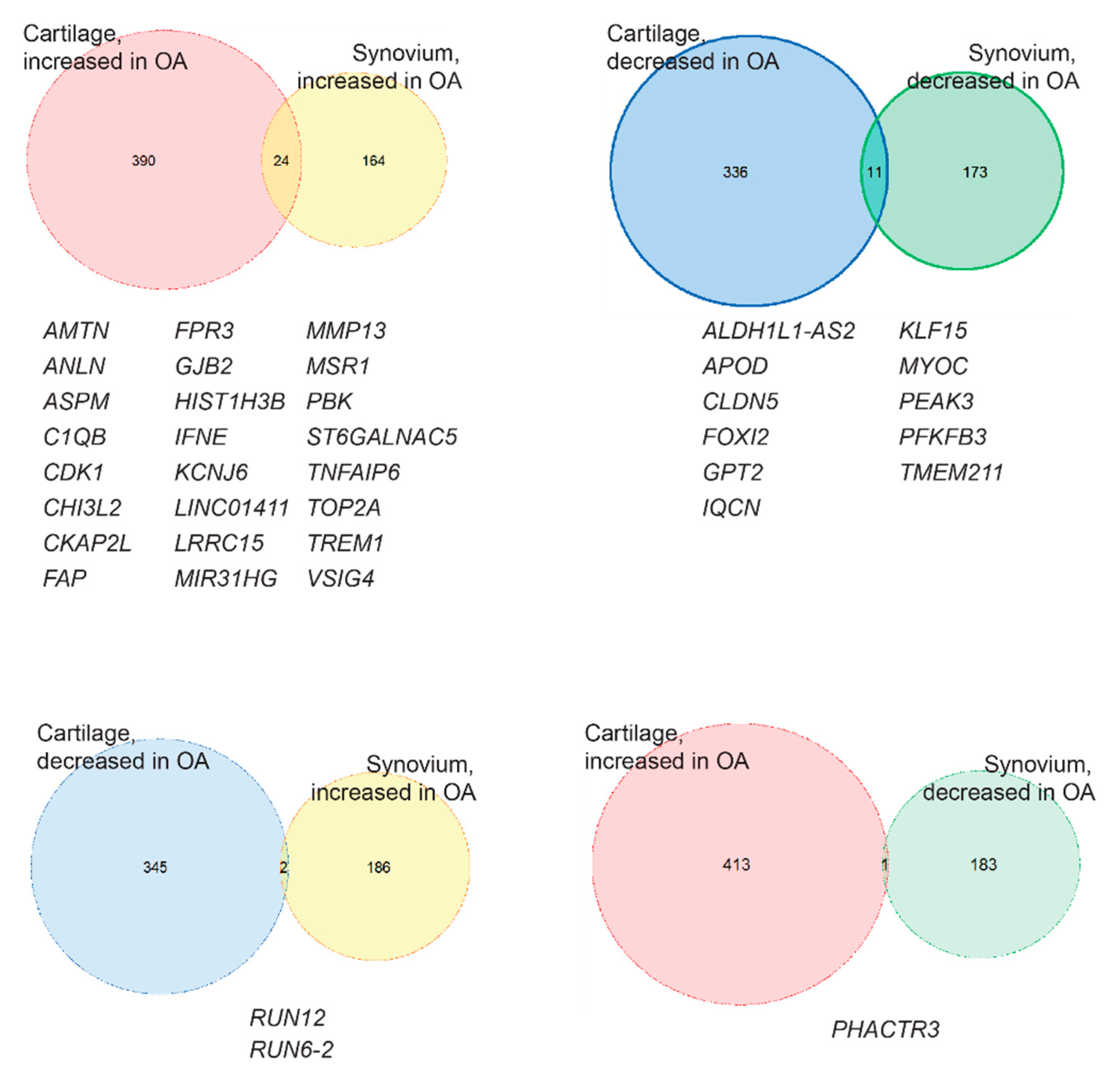
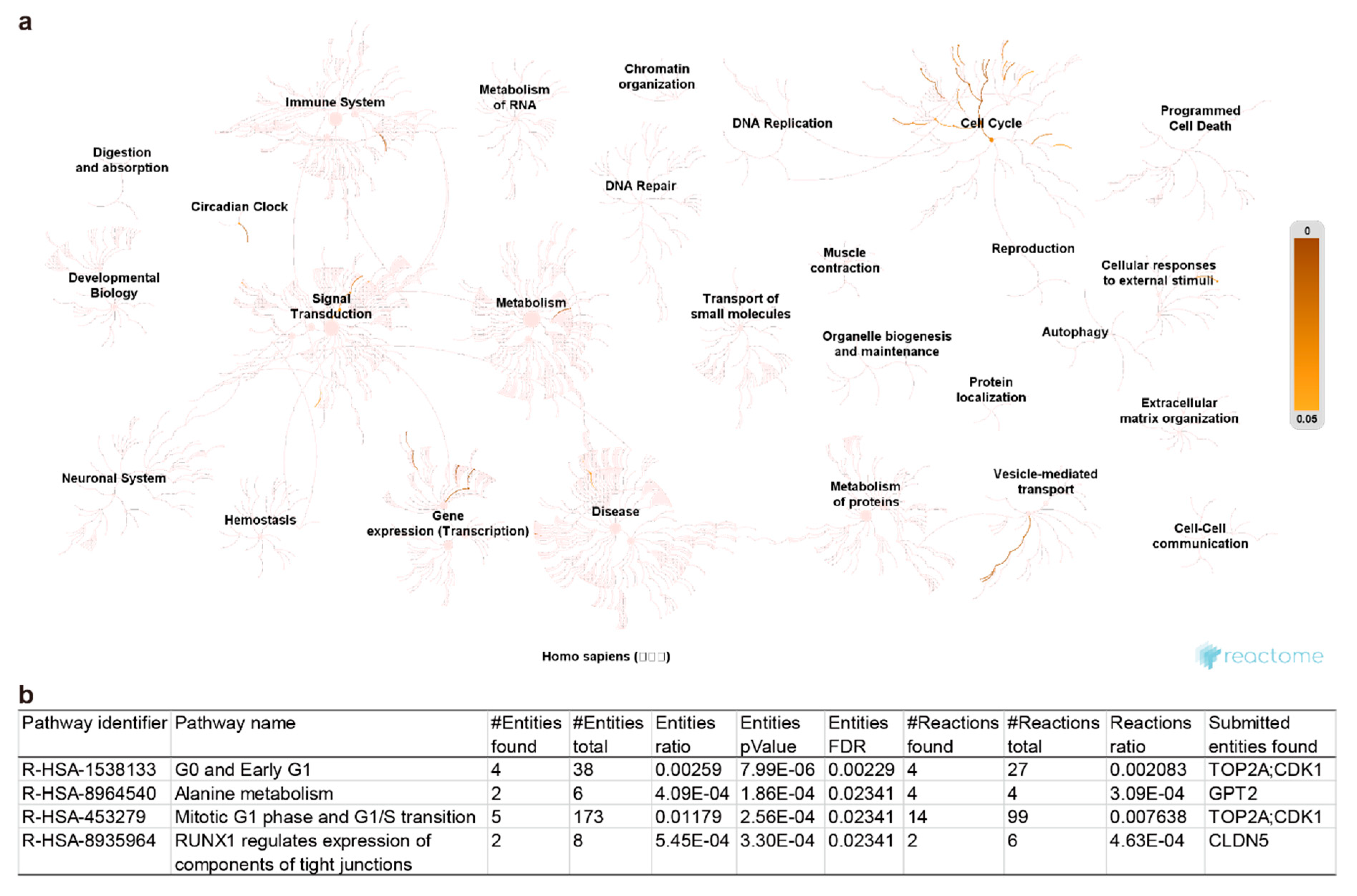
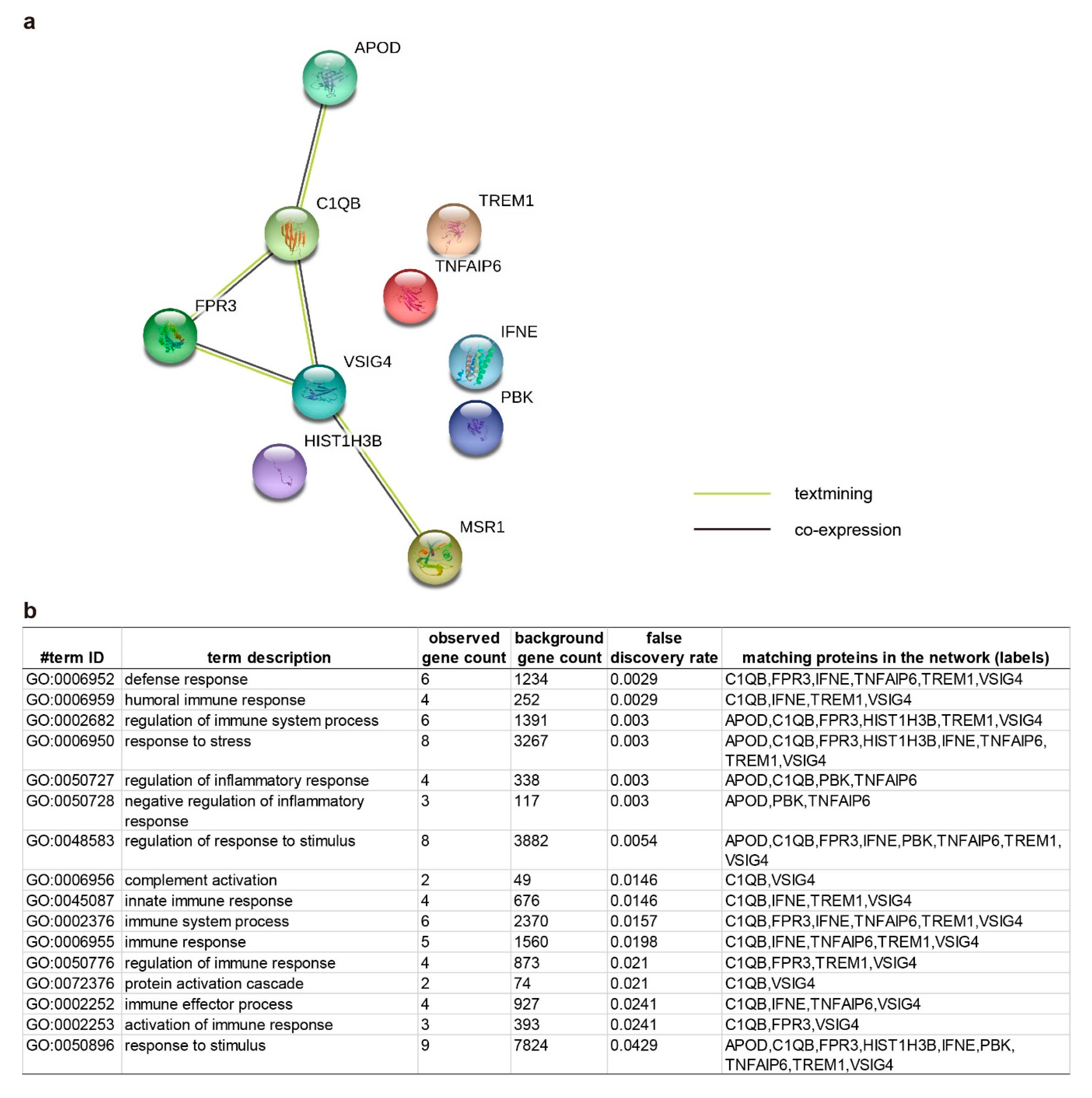
| Symbol | Gene Name | Healthy vs. OA | Expression | Function in Uniprot | |||
|---|---|---|---|---|---|---|---|
| Synovium | Cartilage | ||||||
| logFC | p Value | logFC | p Value | ||||
| APOD | Apolipoprotein D | −2.30916619 | 0.025415 | −3.64045 | 0.000375 | Extracellular region or secreted | Aging, angiogenesis, brain development, glucose metabolic process, lipid metabolic process, negative regulation of cytokine production involved in inflammatory response, negative regulation of focal adhesion assembly, negative regulation of lipoprotein lipid oxidation, negative regulation of monocyte chemotactic protein-1 production, negative regulation of platelet-derived growth factor receptor signaling pathway, negative regulation of protein import into nucleus, negative regulation of smooth muscle cell-matrix adhesion, negative regulation of smooth muscle cell proliferation, negative regulation of T cell migration, peripheral nervous system axon regeneration, response to axon injury, response to drug, response to reactive oxygen species, tissue regeneration |
| C1QB | Complement C1q subcomponent subunit B | 1.71705429 | 0.036863 | 2.74003 | 0.042712 | Extracellular region or secreted | Complement activation, classical pathway; innate immune response, inner ear development, regulation of complement activation, synapse pruning |
| FPR3 | N-formyl peptide receptor 3 | 2.00718964 | 0.004814 | 2.587989 | 0.045465 | Plasma membrane | Chemotaxis, complement receptor mediated signaling pathway, G protein-coupled receptor signaling pathway, inflammatory response, phospholipase C-activating G protein-coupled receptor signaling pathway, positive regulation of cytosolic calcium ion concentration, signal transduction |
| HIST1H3B | Histone cluster 1 H3 family member b | 1.89596286 | 0.033674 | 2.685807 | 0.025722 | Nucleus | Blood coagulation, cellular protein metabolic process, chromatin organization, chromatin silencing at rDNA, DNA replication-dependent nucleosome assembly, interleukin-7 mediated signaling pathway, negative regulation of gene expression, epigenetic, nucleosome assembly, regulation of gene silencing by miRNA, regulation of megakaryocyte differentiation, telomere organization |
| IFNE | Interferon epsilon | 2.74670721 | 0.001932 | 3.545149 | 0.000413 | Extracellular region or secreted | Adaptive immune response, B cell differentiation, B cell proliferation, cytokine-mediated signaling pathway, defense response to bacterium, defense response to virus, humoral immune response, natural killer cell activation involved in immune response, positive regulation of peptidyl-serine phosphorylation of STAT protein, response to exogenous dsRNA, T cell activation involved in immune response |
| MSR1 | Macrophage scavenger receptor types I and II | 2.00838663 | 0.011941 | 3.360986 | 0.015031 | - | Amyloid-beta clearance, cholesterol transport, negative regulation of gene expression, phagocytosis, engulfment, plasma lipoprotein particle clearance, positive regulation of cholesterol storage, positive regulation of macrophage derived foam cell differentiation, receptor-mediated endocytosis |
| PBK | Lymphokine-activated killer T-cell-originated protein kinase | 1.94190515 | 0.035765 | 2.802857 | 0.011073 | Nucleus | Cellular response to UV, mitotic cell cycle, negative regulation of inflammatory response, negative regulation of proteasomal ubiquitin-dependent protein catabolic process, negative regulation of stress-activated MAPK cascade |
| TNFAIP6 | Tumor necrosis factor-inducible gene 6 protein | 2.42594431 | 0.004714 | 4.335193 | 0.000138 | Extracellular region or secreted | Hyaluronic acid binding; cell adhesion, cell-cell signaling, inflammatory response, negative regulation of inflammatory response, neutrophil degranulation, ovulation, positive regulation of cell migration, signal transduction |
| TREM1 | Triggering receptor expressed on myeloid cells 1 | 1.77423941 | 0.042306 | 4.671057 | 3.93 × 10−5 | Extracellular region or secreted, Plasma membrane | Acute inflammatory response, humoral immune response, innate immune response, intracellular signal transduction, leukocyte migration, regulation of immune response |
| VSIG4 | V-set and immunoglobulin domain-containing protein 4 | 1.90947583 | 0.019372 | 3.174618 | 0.024179 | - | Negative regulation of complement activation, alternative pathway; negative regulation of interleukin-2 production, negative regulation of macrophage activation, negative regulation of T cell proliferation |
| Symbol | Gene Name | Healthy vs. OA | Expression | Function in Uniprot | |||
|---|---|---|---|---|---|---|---|
| Synovium | Cartilage | ||||||
| logFC | p Value | logFC | p Value | ||||
| MYOC | Myocilin | −3.2029017 | 0.00068 | −4.11544 | 0.002817 | Extracellular region or secreted, Golgi apparatus, mitochondrion, rough endoplasmic reticulum | Bone development, clustering of voltage-gated sodium channels, ERBB2-ERBB3 signaling pathway, myelination in peripheral nervous system, negative regulation of cell-matrix adhesion, negative regulation of Rho protein signal transduction, negative regulation of stress fiber assembly, neuron projection development, non-canonical Wnt signaling pathway via JNK cascade, osteoblast differentiation, positive regulation of cell migration, positive regulation of focal adhesion assembly, positive regulation of mitochondrial depolarization, positive regulation of phosphatidylinositol 3-kinase signaling, positive regulation of protein kinase B signaling, positive regulation of stress fiber assembly, positive regulation of substrate adhesion-dependent cell spreading, regulation of MAPK cascade, skeletal muscle hypertrophy |
| AMTN | Amelotin | 3.91241415 | 0.002914 | 5.414278 | 0.000259 | Extracellular region or secreted | Biomineral tissue development, cell adhesion, cellular protein metabolic process, odontogenesis of dentin-containing tooth, positive regulation of biomineral tissue development, positive regulation of enamel mineralization, post-translational protein modification |
| CHI3L2 | Chitinase-3-like protein 2 | 2.17945593 | 0.023414 | 3.223409 | 0.000902 | Extracellular region or secreted | Carbohydrate metabolic process, chitin catabolic process |
| FAP | Prolyl endopeptidase FAP/Fibroblast activation protein alpha | 1.68745632 | 0.044592 | 2.264122 | 0.013885 | Extracellular region or secreted, plasma membrane | Angiogenesis, cell adhesion, endothelial cell migration, melanocyte apoptotic process, melanocyte proliferation, mitotic cell cycle arrest, negative regulation of cell proliferation involved in contact inhibition, negative regulation of extracellular matrix disassembly, negative regulation of extracellular matrix organization, positive regulation of cell cycle arrest, positive regulation of execution phase of apoptosis, proteolysis, proteolysis involved in cellular protein catabolic process, regulation of collagen catabolic process, regulation of fibrinolysis |
| LRRC15 | Leucine-rich repeat-containing protein 15 | 2.10204668 | 0.016016 | 5.236099 | 2.11 × 10−5 | Extracellular region or secreted | Collagen binding, fibronectin binding, laminin binding; negative regulation of protein localization to plasma membrane, positive regulation of cell migration, receptor-mediated virion attachment to host cell |
| MMP13 | Matrix metallopeptidase-13/Collagenase 3 | 3.48223529 | 7.54 × 10−5 | 3.194238 | 0.001586 | Extracellular region or secreted | Bone mineralization, bone morphogenesis, cellular protein metabolic process, collagen catabolic process, endochondral ossification, extracellular matrix disassembly, extracellular matrix organization, growth plate cartilage development, proteolysis, response to amyloid-beta |
| TNFAIP6 | Tumor necrosis factor-inducible gene 6 protein | 2.42594431 | 0.004714 | 4.335193 | 0.000138 | Extracellular region or secreted | Hyaluronic acid binding; cell adhesion, cell-cell signaling, inflammatory response, negative regulation of inflammatory response, neutrophil degranulation, ovulation, positive regulation of cell migration, signal transduction |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zheng, Z. Identification of Novel Targets of Knee Osteoarthritis Shared by Cartilage and Synovial Tissue. Int. J. Mol. Sci. 2020, 21, 6033. https://doi.org/10.3390/ijms21176033
Li C, Zheng Z. Identification of Novel Targets of Knee Osteoarthritis Shared by Cartilage and Synovial Tissue. International Journal of Molecular Sciences. 2020; 21(17):6033. https://doi.org/10.3390/ijms21176033
Chicago/Turabian StyleLi, Chenshuang, and Zhong Zheng. 2020. "Identification of Novel Targets of Knee Osteoarthritis Shared by Cartilage and Synovial Tissue" International Journal of Molecular Sciences 21, no. 17: 6033. https://doi.org/10.3390/ijms21176033
APA StyleLi, C., & Zheng, Z. (2020). Identification of Novel Targets of Knee Osteoarthritis Shared by Cartilage and Synovial Tissue. International Journal of Molecular Sciences, 21(17), 6033. https://doi.org/10.3390/ijms21176033






