Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis
Abstract
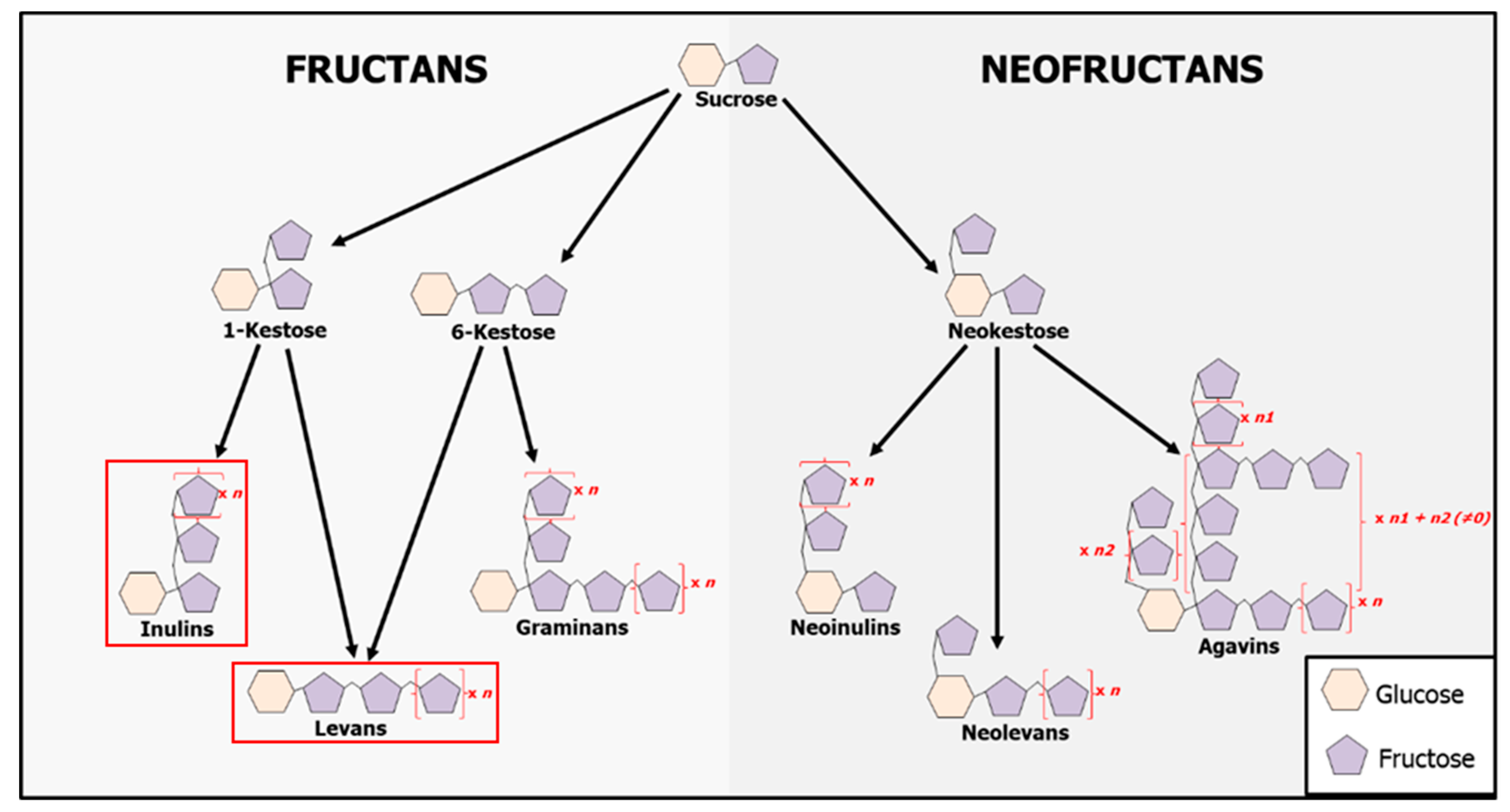
1. Introduction
2. Results
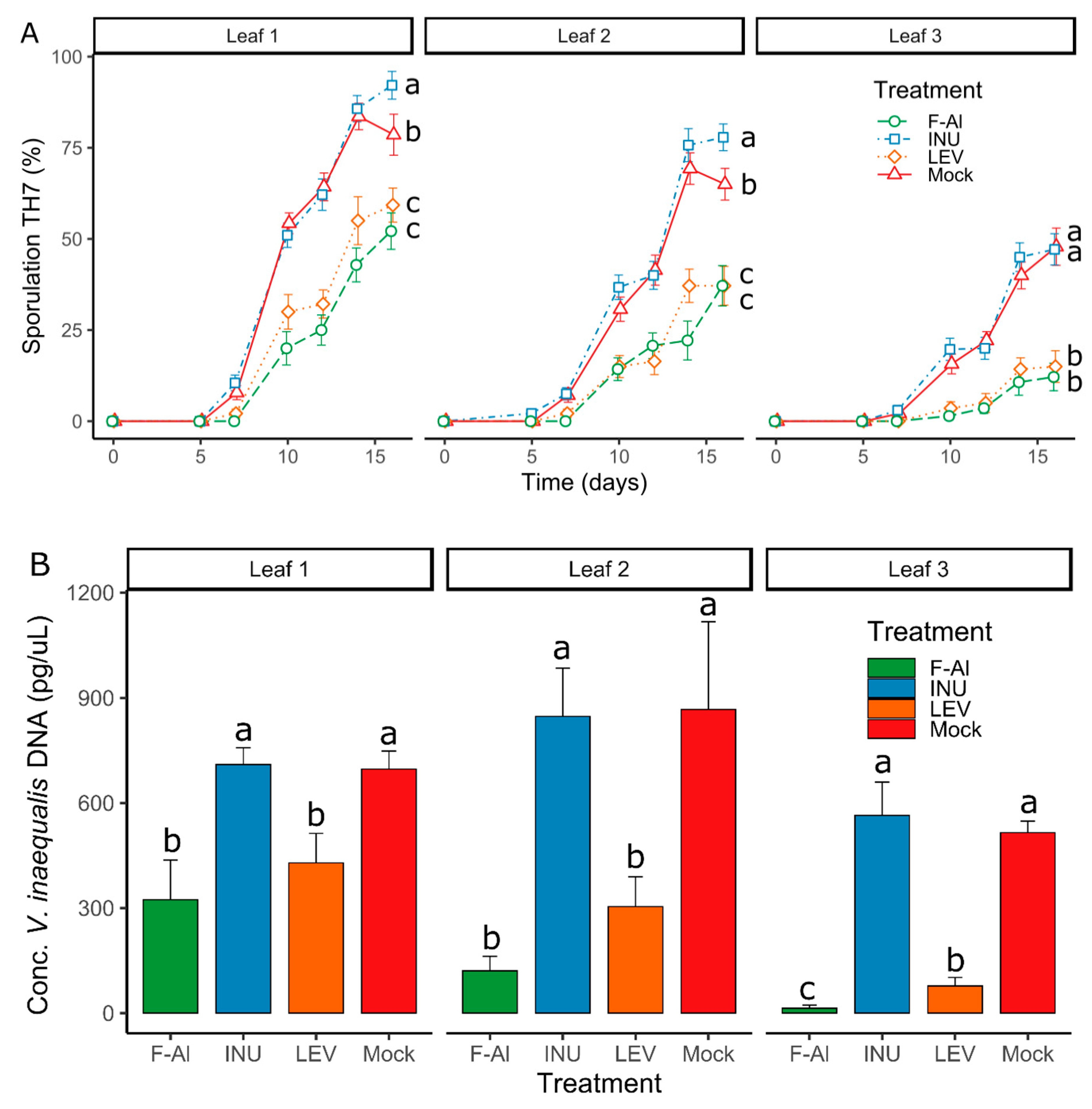
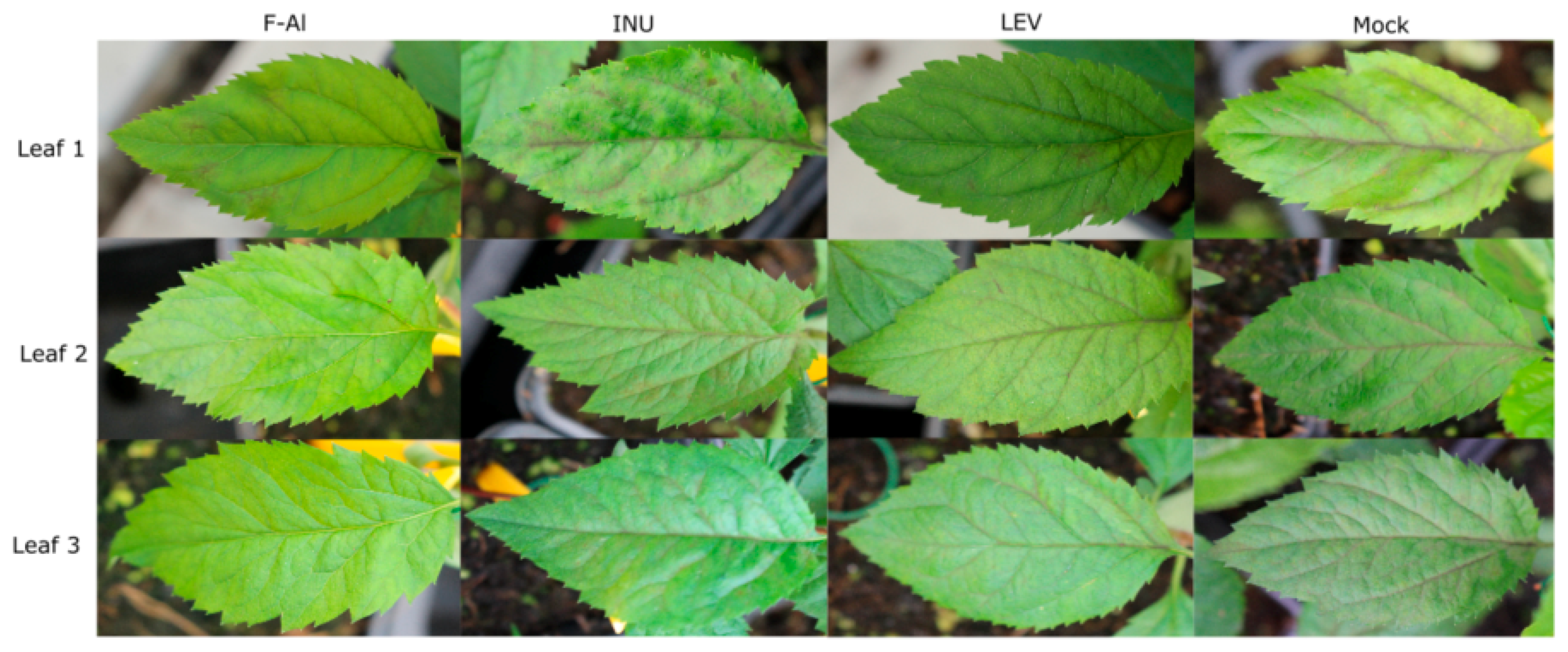
2.1. Levan Application Significantly Decreases V. inaequalis Sporulation on Leaves of Apple Seedlings
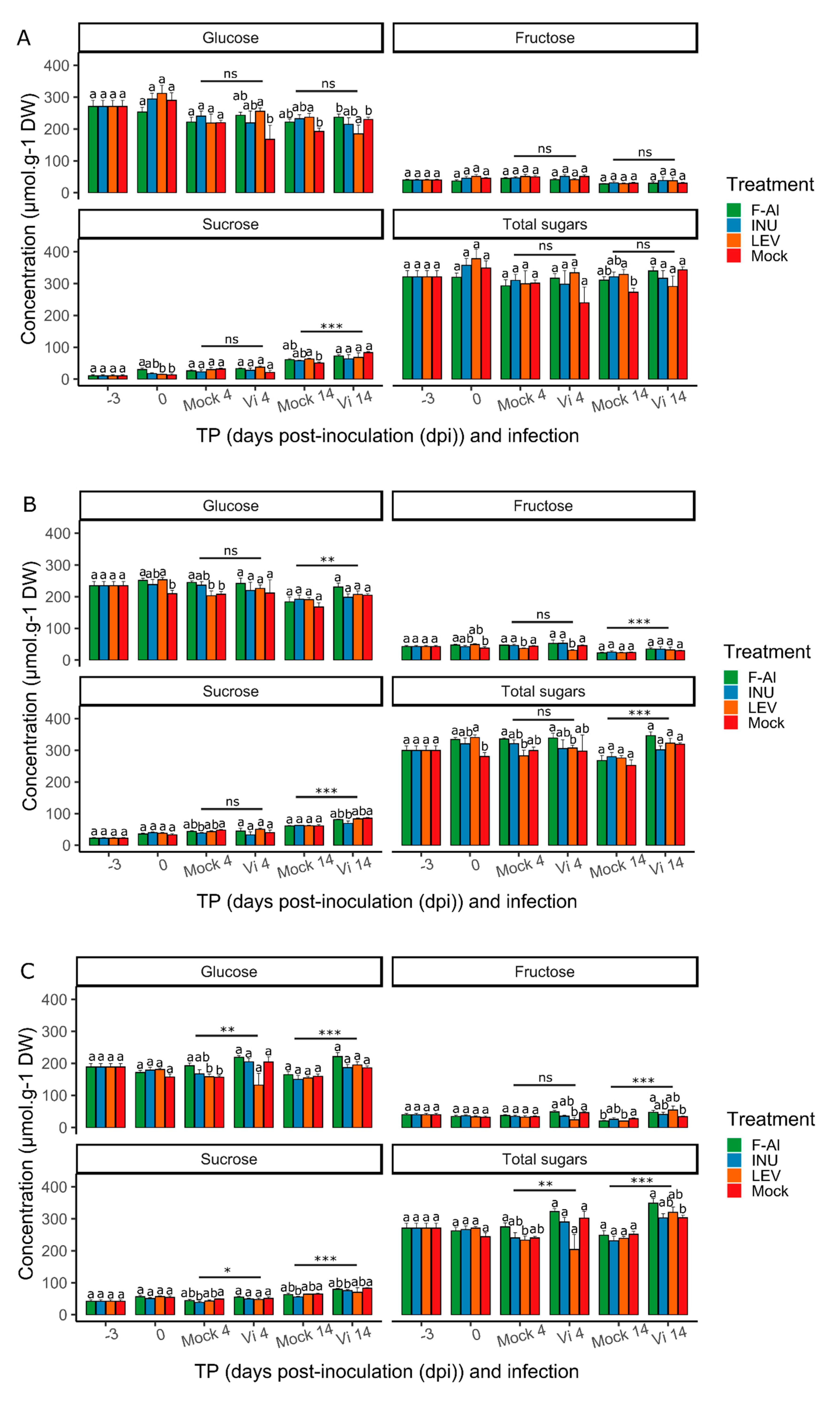
2.2. Infection, but not Priming, Induces Suc Accumulation
2.3. Levan-Type Fructans Directly Inhibit Venturia inaequalis Growth
3. Discussion
4. Materials and Methods
4.1. Plant and Inoculation Materials
4.2. Priming and Infection Assays
4.3. Preparation of Priming Agents and Solutions
4.4. Fungal Material and Artificial Inoculation
4.5. Visual Evaluation Assay of Macroscopic Symptoms
4.6. DNA Extraction and Quantification Assay of Venturia DNA
4.7. Carbohydrate Extraction, Processing and Analysis
4.8. Media Preparation and In Vitro V. inaequalis Growth on the Plate
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT, Division, Food and Agriculture Organization of the United Nations. Statistics. Available online: http://www.fao.org/statistics/en/ (accessed on 1 February 2020).
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The causal agent of apple scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef] [PubMed]
- MacHardy, W.E. Apple Scab: Biology, Epidemiology and Management; The American Phytopathological Society Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Manktelow, D.W.L.; Beresford, R.M.; Batchelor, T.A.; Walker, J.T.S. Use patterns and economics of fungicides for disease control in New Zealand apples. Acta Hortic. 1996, 422, 187–192. [Google Scholar] [CrossRef]
- Bus, V.G.M.; Rikkerink, E.H.A.; Caffier, V.; Durel, C.-E.; Plummer, K.M. Revision of the Nomenclature of the Differential Host-Pathogen Interactions of Venturia inaequalis and Malus. Annu. Rev. Phytopathol. 2011, 49, 391–413. [Google Scholar] [CrossRef] [PubMed]
- Köller, W.; Wilcox, W.F.; Barnard, J.; Jones, A.L.; Braun, P.G. Detection and quantification of resistance of Venturia inaequalis populations to sterol demethylation inhibitors. Phytopathology 1997, 87, 184–190. [Google Scholar] [CrossRef]
- Köller, W.; Parker, D.M.; Turechek, W.W.; Avila-Adame, C.; Cronshaw, K. A two-phase resistance response of Venturia inaequalis populations to the QoI fungicides kresoxim-methyl and trifloxystrobin. Plant. Dis. 2004, 88, 537–544. [Google Scholar] [CrossRef]
- Holb, I.J. Fungal disease management in organic apple orchards: Epidemiological aspects and management approaches. In Proceedings of the 9th International Congress. Plant Pathology in the 21st Century: Recent Developments in Management of Plant Diseases; Gisi, U., Chet, I., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 163–177. [Google Scholar]
- Dewdney, M.; Charest, J.; Paulitz, T.; Carisse, O. Multivariate analysis of apple cultivar susceptibility to Venturia inaequalis under greenhouse conditions. Can. J. Plant Pathol. 2003, 25, 387–400. [Google Scholar] [CrossRef]
- Holb, I.J.; Dremák, P.; Bitskey, K.; Gonda, I. Yield response, pest damage and fruit quality parameters of scab-resistant and scab-susceptible apple cultivars in integrated and organic production systems. Sci. Hortic. (Amsterdam) 2012, 145, 109–117. [Google Scholar] [CrossRef]
- Gessler, C.; Patocchi, A.; Sansavini, S.; Tartarini, S.; Gianfranceschi, L. Venturia inaequalis resistance in apple. CRC. Crit. Rev. Plant. Sci. 2006, 25, 473–503. [Google Scholar] [CrossRef]
- Laurens, F.; Aranzana, M.J.; Arus, P.; Bassi, D.; Bink, M.; Bonany, J.; Caprera, A.; Corelli-Grappadelli, L.; Costes, E.; Durel, C.E.; et al. An integrated approach for increasing breeding efficiency in apple and peach in Europe. Hortic. Res. 2018, 5, 11. [Google Scholar] [CrossRef]
- Muñoz, P.R.; Valle, D.; Huber, D.A.; Butnor, J.R. Phenotypic analysis of first-year traits in a pseudo-backcross {( slash x loblolly ) x slash } and the open-pollinated families of the pure-species progenitors. Tree Genet. Genomes 2011, 183–192. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I. Vf scab resistance of Malus. Trees-Struct. Funct. 2012, 26, 95–108. [Google Scholar] [CrossRef]
- Holb, I.J. Classification of apple cultivar reactions to scab in integrated and organic production systems. Can. J. Plant Pathol. 2007, 29, 251–260. [Google Scholar] [CrossRef]
- Xu, M.; Korban, S.S. A cluster of four receptor-like genes resides in the Vf locus that confers resistance to apple scab disease. Genetics 2002, 162, 1995–2006. [Google Scholar] [PubMed]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant. Heal. Instr. 2006, 1–25. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Smart, L.E.; Birch, A.N.E.; Blok, V.C.; MacKenzie, K.; Guerrieri, E.; Cascone, P.; Luna, E.; Ton, J. Prospects for plant defence activators and biocontrol in IPM—Concepts and lessons learnt so far. Crop. Prot. 2017, 97, 128–134. [Google Scholar] [CrossRef]
- Kim, J.M.; To, T.K.; Matsui, A.; Tanoi, K.; Kobayashi, N.I.; Matsuda, F.; Habu, Y.; Ogawa, D.; Sakamoto, T.; Matsunaga, S.; et al. Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 2017, 3, 4–10. [Google Scholar] [CrossRef]
- van Aubel, G.; Cambier, P.; Dieu, M.; Van Cutsem, P. Plant immunity induced by COS-OGA elicitor is a cumulative process that involves salicylic acid. Plant. Sci. 2016, 247, 60–70. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Moving to the field: Plant innate immunity in crop protection. Int. J. Mol. Sci. 2017, 18, 640. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant. Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Van Hulten, M.; Pelser, M.; Van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef]
- Walters, D.R.; Paterson, L.; Walsh, D.J.; Havis, N.D. Priming for plant defense in barley provides benefits only under high disease pressure. Physiol. Mol. Plant. Pathol. 2008, 73, 95–100. [Google Scholar] [CrossRef]
- Conrath, U.; Pieterse, C.M.J.; Mauch-Mani, B. Priming in plant—pathogen interactions. Trends Plant. Sci. 2002, 7, 210–216. [Google Scholar] [CrossRef]
- Yu, C.; Zeng, L.; Sheng, K.; Chen, F.; Zhou, T.; Zheng, X.; Yu, T. γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014, 159, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Jakab, G.; Toquin, V.; Flors, V.; Iavicoli, A.; Maeder, M.N.; Metrauxt, J.; Mauch-Mani, B. Dissecting the β -aminobutyric acid—induced priming phenomenon in Arabidopsis. Plant. Cell. 2007, 17, 987–999. [Google Scholar] [CrossRef]
- Sadak, M.S.; Orabi, S.A. Improving thermo tolerance of wheat plant by foliar application of citric acid or oxalic acid. Int. J. ChemTech Res. 2015, 8, 333–345. [Google Scholar]
- Petré, R.; Labourdette, G.; Braun, C.A.; Meredith, R.; Hauke, K.; Van Hemelrijck, W.; Schoofs, H.; Deckers, T.; Keulemans, W.; Schoevaerts, C.; et al. Fosetyl-al (Aliette®), a plant defense enhancer with good efficacy on bacteria and on ascomycetes in apples and pears. Acta Hortic. 2015, 1094, 431–438. [Google Scholar] [CrossRef]
- Vivancos, J.; Labbé, C.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)—dependent defence pathway. Mol. Plant. Pathol. 2015, 16, 572–582. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Calzarano, F.; Roberti, R.; Veronesi, A.; Amalfitano, C. Effects of grapevine applications of fosetyl-aluminium formulations for downy mildew control on “esca” and associated fungi. Phytopathol. Mediterr. 2011, 50, 285–299. [Google Scholar] [CrossRef]
- Massoud, K.; Barchietto, T.; le Rudulier, T.; Pallandre, L.; Didierlaurent, L.; Garmier, M.; Ambard-Bretteville, F.; Seng, J.M.; Saindrenan, P. Dissecting Phosphite-induced priming in Arabidopsis infected with Hyaloperonospora Arabidopsidis. Plant. Physiol. 2012, 159, 286–298. [Google Scholar] [CrossRef]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant. Sci. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Chaliha, C.; Rugen, M.D.; Field, R.A.; Kalita, E. Glycans as modulators of plant defense against filamentous pathogens. Front. Plant. Sci. 2018, 9, 928. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant. Sci. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, C.; Zhou, C.M.; Zhang, T.Q.; Lian, H.; Sun, Y.; Wu, J.; Huang, J.; Wang, G.; Wang, J.W. Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. Elife 2013, 2013, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef]
- Broeckx, T.; Hulsmans, S.; Rolland, F. The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 2016, 67, 6215–6252. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Van den Ende, W. Cold tolerance triggered by soluble sugars: A multifaceted countermeasure. Front. Plant. Sci. 2015, 6, 203. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, D.Z.; Li, Q.; Ma, Y.Q.; Yao, J.W.; Huang, X.; Xu, Z.Q. Metabolomic analysis reveals the relationship between AZI1 and sugar signaling in systemic acquired resistance of Arabidopsis. Plant. Physiol. Biochem. 2016, 107, 273–287. [Google Scholar] [CrossRef]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Yang, J.; Eom, J.S.; Prior, M.; Sosso, D.; Hartwig, T.; Szurek, B.; Oliva, R.; Vera-Cruz, C.; White, F.F.; et al. Sugar flux and signaling in plant–microbe interactions. Plant. J. 2018, 93, 675–685. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 2013, 64, 1439–1449. [Google Scholar] [CrossRef]
- de Azevedo Souza, C.; Li, S.; Lin, A.Z.; Boutrot, F.; Grossmann, G.; Zipfel, C.; Somerville, S.C. Cellulose-derived oligomers act as damage-associated molecular patterns and trigger defense-like responses. Plant. Physiol. 2017, 173, 2383–2398. [Google Scholar] [CrossRef] [PubMed]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant. Sci. 2013, 4, 1–11. [Google Scholar]
- Hendry, G.A.F. Evolutionary origins and natural functions of fructans—A climatological, biogeographic and mechanistic appraisal. New Phytol. 1993, 123, 3–14. [Google Scholar] [CrossRef]
- Peukert, M.; Thiel, J.; Mock, H.P.; Marko, D.; Weschke, W.; Matros, A. Spatiotemporal dynamics of oligofructan metabolism and suggested functions in developing cereal grains. Front. Plant. Sci. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amiard, V.; Morvan-Bertrand, A.; Billard, J.P.; Huault, C.; Keller, F.; Prud’homme, M.P. Fructans, but not the sucrosyl-galactosides, raffinose and loliose, are affected by drought stress in perennial ryegrass. Plant. Physiol. 2003, 132, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.; Handford, M.; Pauly, M.; Dupree, P.; Cardemil, L. Structural modifications of fructans in aloe barbadensis miller (Aloe vera) grown under water stress. PLoS ONE 2016, 11, 1–24. [Google Scholar] [CrossRef]
- Yáñez, A.; Tapia, G.; Guerra, F.; Del Pozo, A. Stem carbohydrate dynamics and expression of genes involved in fructan accumulation and remobilization during grain growth in wheat (Triticum aestivum L.) genotypes with contrasting tolerance to water stress. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; González de la Vara, L.E.; López, M.G. Fructan active enzymes (FAZY) activities and biosynthesis of fructooligosaccharides in the vacuoles of Agave tequilana Weber Blue variety plants of different age. Planta 2017, 245, 265–281. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Van de Poel, B.; Höfte, M.; Van den Ende, W. Sweet immunity: Inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef]
- He, P.Q.; Tian, L.; Chen, K.S.; Hao, L.H.; Li, G.Y. Induction of volatile organic compounds of Lycopersicon esculentum Mill. and its resistance to Botrytis cinerea Pers. by burdock oligosaccharide. J. Integr. Plant Biol. 2006, 48, 550–557. [Google Scholar] [CrossRef]
- Wang, F.; Feng, G.; Chen, K. Burdock fructooligosaccharide induces resistance to tobacco mosaic virus in tobacco seedlings. Physiol. Mol. Plant. Pathol. 2009, 74, 34–40. [Google Scholar] [CrossRef]
- Versluys, M.; Tarkowski, Ł.P.; Van den Ende, W. Fructans as DAMPs or MAMPs: Evolutionary prospects, cross-tolerance, and multistress resistance potential. Front. Plant. Sci. 2017, 7, 2061. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, P.; Guo, M.; Yu, W.; Chen, K. Burdock fructooligosaccharide induces fungal resistance in postharvest Kyoho grapes by activating the salicylic acid-dependent pathway and inhibiting browning. Food Chem. 2013, 138, 539–546. [Google Scholar] [CrossRef]
- Holb, I.J.; Kunz, S. Integrated control of apple scab and powdery mildew in an organic apple orchard by combining potassium carbonates with wettable sulfur, pruning, and cultivar susceptibility. Plant. Dis. 2016, 100, 1894–1905. [Google Scholar] [CrossRef]
- Felipini, R.B.; Boneti, J.I.; Katsurayama, Y.; Neto, A.C.R.; Veleirinho, B.; Maraschin, M.; Di Piero, R.M. Apple scab control and activation of plant defence responses using potassium phosphite and chitosan. Eur. J. Plant. Pathol. 2016, 145, 929–939. [Google Scholar] [CrossRef]
- Bogs, J.; Richter, K.; Kim, W.S.; Jock, S.; Geider, K. Alternative methods to describe virulence of Erwinia amylovora and host-plant resistance against fireblight. Plant. Pathol. 2004, 53, 80–89. [Google Scholar] [CrossRef]
- Jamar, L.; Cavelier, M.; Lateur, M. Primary scab control using a “during-infection” spray timing and the effect on fruit quality and yield in organic apple production. Biotechnol. Agron. Soc. Environ. 2010, 14, 423–439. [Google Scholar]
- Jiang, X.; Lin, H.; Shi, J.; Neethirajan, S.; Lin, Y.; Chen, Y.; Wang, H.; Lin, Y. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 2018, 252, 134–141. [Google Scholar] [CrossRef]
- Lawniczak, A.E.; Zbierska, J.; Nowak, B.; Achtenberg, K.; Grześkowiak, A.; Kanas, K. Impact of agriculture and land use on nitrate contamination in groundwater and running waters in central-west Poland. Environ. Monit. Assess. 2016, 188, 1–17. [Google Scholar] [CrossRef]
- Köhl, J.; Scheer, C.; Holb, I.J.; Masny, S.; Molhoek, W. Toward an integrated use of biological control by Cladosporium cladosporioides H39 in apple scab (Venturia inaequalis) management. Plant. Dis. 2015, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Burr, T.J.; Matteson, M.C.; Smith, C.A.; Corral-Garcia, M.R.; Huang, T.-C. Effectiveness of bacteria and yeasts from apple orchards as biological control agents of apple scab. Biol. Control. 1996, 6, 151–157. [Google Scholar] [CrossRef]
- Poleatewich, A.M.; Ngugi, H.K.; Backman, P.A. Assessment of application timing of Bacillus spp. suppress pre- and postharvest diseases of apple. Plant. Dis. 2012, 96, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Fothegril, P.G.; Ashcroft, R. The nutritional requirements of Venturia inaequalis. J. Gen. Microbiol. 1955, 12, 387–395. [Google Scholar] [CrossRef][Green Version]
- De Coninck, B.; Le Roy, K.; Francis, I.; Clerens, S.; Vergauwen, R.; Halliday, A.M.; Smith, S.M.; Van Laere, A.; Van den Ende, W. Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant Cell Environ. 2005, 28, 432–443. [Google Scholar] [CrossRef]
- Hunter, L.D. The influence of biochemical factors on growth in culture and pathogenicity of Venturia inaequalis. Ann. Appl. Biol. 1979, 91, 119–123. [Google Scholar] [CrossRef]
- Morkunas, I.; Narozna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-talk interactions of sucrose and Fusarium oxysporum in the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J. Plant. Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef]
- Formela-Luboinska, M.; Chadzinikolau, T.; Drzewiecka, K.; Jelen, H.; Bocianowski, J.; Kesy, J.; Labudda, M.; Jeandet, P.; Morkunas, I. The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum. Int. J. Mol. Sci. 2020, 21, 4133. [Google Scholar] [CrossRef]
- Naseem, M.; Kunz, M.; Dandekar, T. Plant–pathogen maneuvering over apoplastic sugars. Trends Plant. Sci. 2017, 22, 740–743. [Google Scholar] [CrossRef]
- Chevalier, M.; Lespinasse, Y.; Renaudin, S. A microscopic study of the different classes of symptoms coded by the Vf gene in apple for resistance to scab (Venturia inaequalis). Plant. Pathol. 1991, 40, 249–256. [Google Scholar] [CrossRef]
- Maleux, K.; Van den Ende, W. Levans in excised leaves of Dactylis glomerata: Effects of light, sugars, temperature and senescence. J. Plant. Biol. 2007, 50, 671–680. [Google Scholar] [CrossRef]
- Hias, N.; Svara, A.; Keulemans, J.W. Effect of polyploidisation on the response of apple (Malus × domestica Borkh.) to Venturia inaequalis infection. Eur. J. Plant. Pathol. 2018, 151, 515–526. [Google Scholar] [CrossRef]
- Daniëls, B.; de Landtsheer, A.; Dreesen, R.; Davey, M.W.; Keulemans, J. Real-time PCR as a promising tool to monitor growth of Venturia spp. in scab-susceptible and -resistant apple leaves. Eur. J. Plant. Pathol. 2012, 134, 821–833. [Google Scholar] [CrossRef]
- Croxall, H.E.; Gwynne, D.C.; Jenkins, J.E.E. The rapid assessment of apple scab on fruit. Plant. Pathol. 1952, 1, 89–92. [Google Scholar] [CrossRef]
- Didelot, F.; Caffier, V.; Orain, G.; Lemarquand, A.; Parisi, L. Sustainable management of scab control through the integration of apple resistant cultivars in a low-fungicide input system. Agric. Ecosyst. Environ. 2016, 217, 41–48. [Google Scholar] [CrossRef]
- Maharjan, A.; Bhatta, B.; Acharya, R.P.; C., S.G.; Shrestha, S. Efficacy assessment of treatment methods against powdery mildew disease of pea (Pisum sativum L.) caused by Erysiphe pisi var. pisi. World J. Agric. Res. Vol. 2015, 3, 185–191. [Google Scholar] [CrossRef]
- Torfs, S.; Van Poucke, K.; Van Campenhout, J.; Ceustermans, A.; Croes, S.; Bylemans, D.; Van Hemelrijck, W.; Keulemans, W.; Heungens, K. Venturia inaequalis trapped: Molecular quantification of airborne inoculum using volumetric and rotating arm samplers. Eur. J. Plant. Pathol. 2019, 155, 1319–1332. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svara, A.; Tarkowski, Ł.P.; Janse van Rensburg, H.C.; Deleye, E.; Vaerten, J.; De Storme, N.; Keulemans, W.; Van den Ende, W. Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis. Int. J. Mol. Sci. 2020, 21, 5885. https://doi.org/10.3390/ijms21165885
Svara A, Tarkowski ŁP, Janse van Rensburg HC, Deleye E, Vaerten J, De Storme N, Keulemans W, Van den Ende W. Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis. International Journal of Molecular Sciences. 2020; 21(16):5885. https://doi.org/10.3390/ijms21165885
Chicago/Turabian StyleSvara, Anze, Łukasz Paweł Tarkowski, Henry Christopher Janse van Rensburg, Evelien Deleye, Jarl Vaerten, Nico De Storme, Wannes Keulemans, and Wim Van den Ende. 2020. "Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis" International Journal of Molecular Sciences 21, no. 16: 5885. https://doi.org/10.3390/ijms21165885
APA StyleSvara, A., Tarkowski, Ł. P., Janse van Rensburg, H. C., Deleye, E., Vaerten, J., De Storme, N., Keulemans, W., & Van den Ende, W. (2020). Sweet Immunity: The Effect of Exogenous Fructans on the Susceptibility of Apple (Malus × domestica Borkh.) to Venturia inaequalis. International Journal of Molecular Sciences, 21(16), 5885. https://doi.org/10.3390/ijms21165885





