CDK19 as a Potential HPV-Independent Biomarker for Recurrent Disease in HNSCC
Abstract
1. Introduction
2. Results
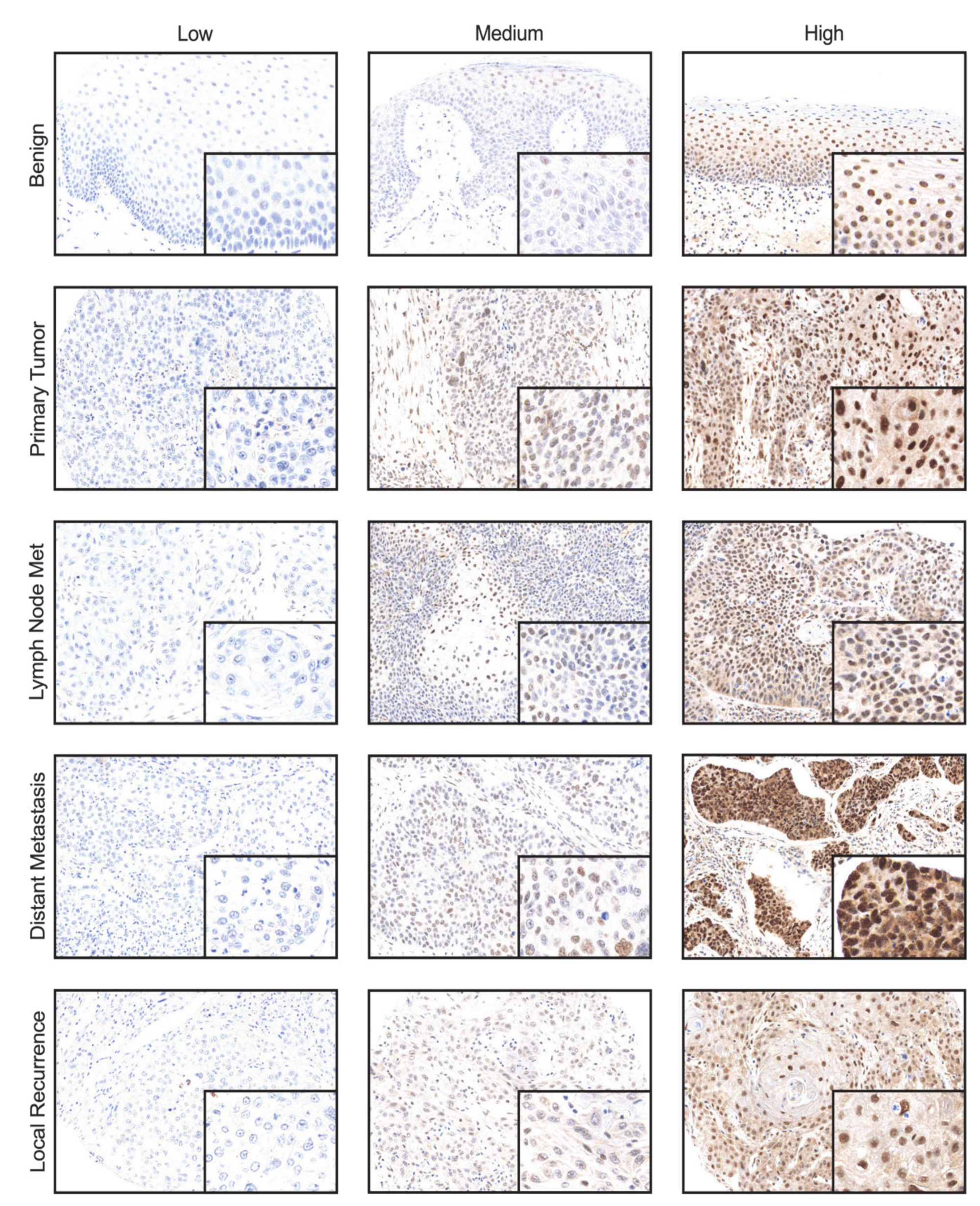
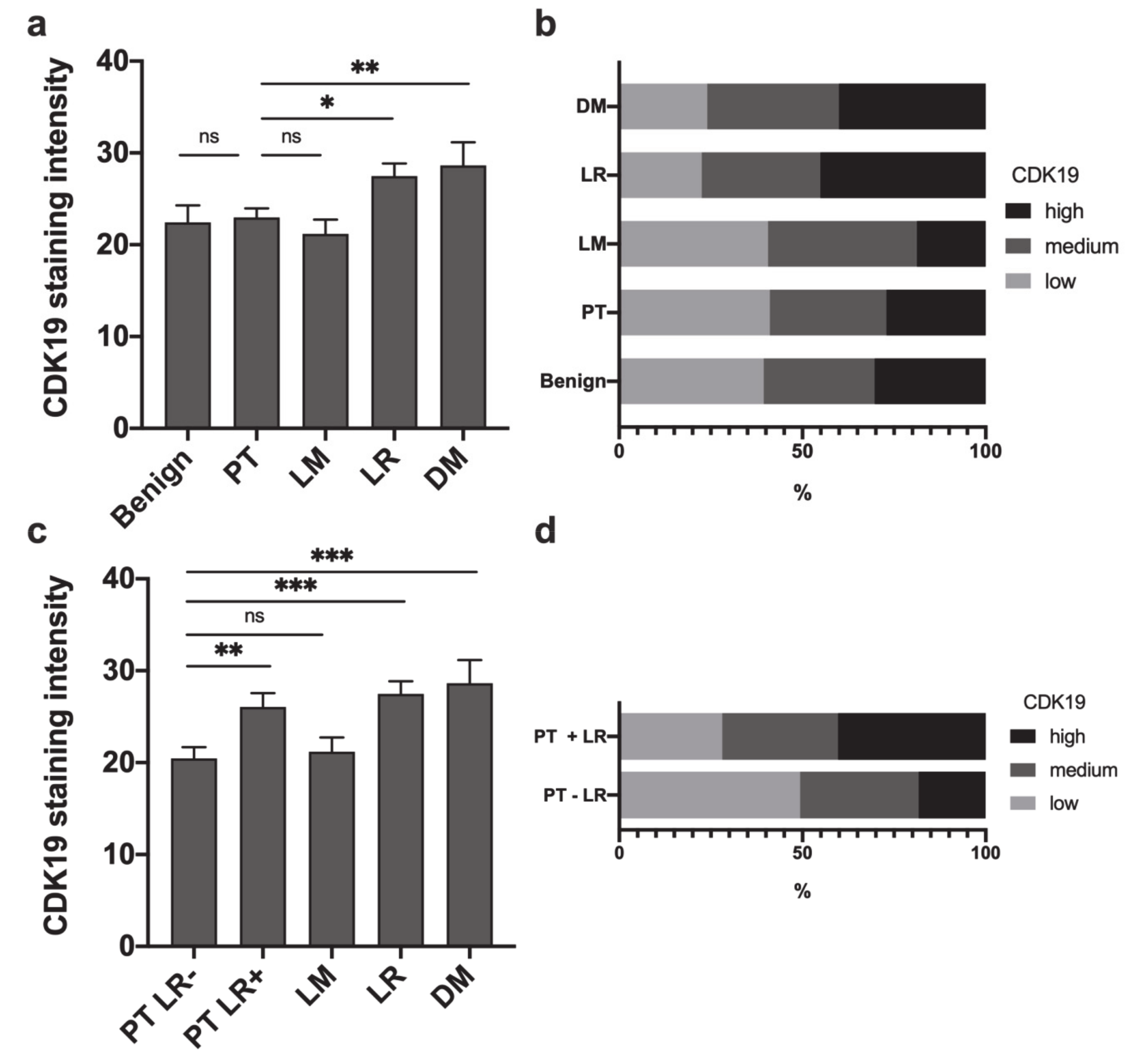
2.1. CDK19 Is Overexpressed in Local Recurrences and Distant Metastases of HNSCC
2.2. Primary HNSCC of Patients Developing Local Recurrences Have Higher CDK19 Expression
2.3. CDK19 Over-Expression in HNSCC Correlates with Reduced Disease-Free Survival
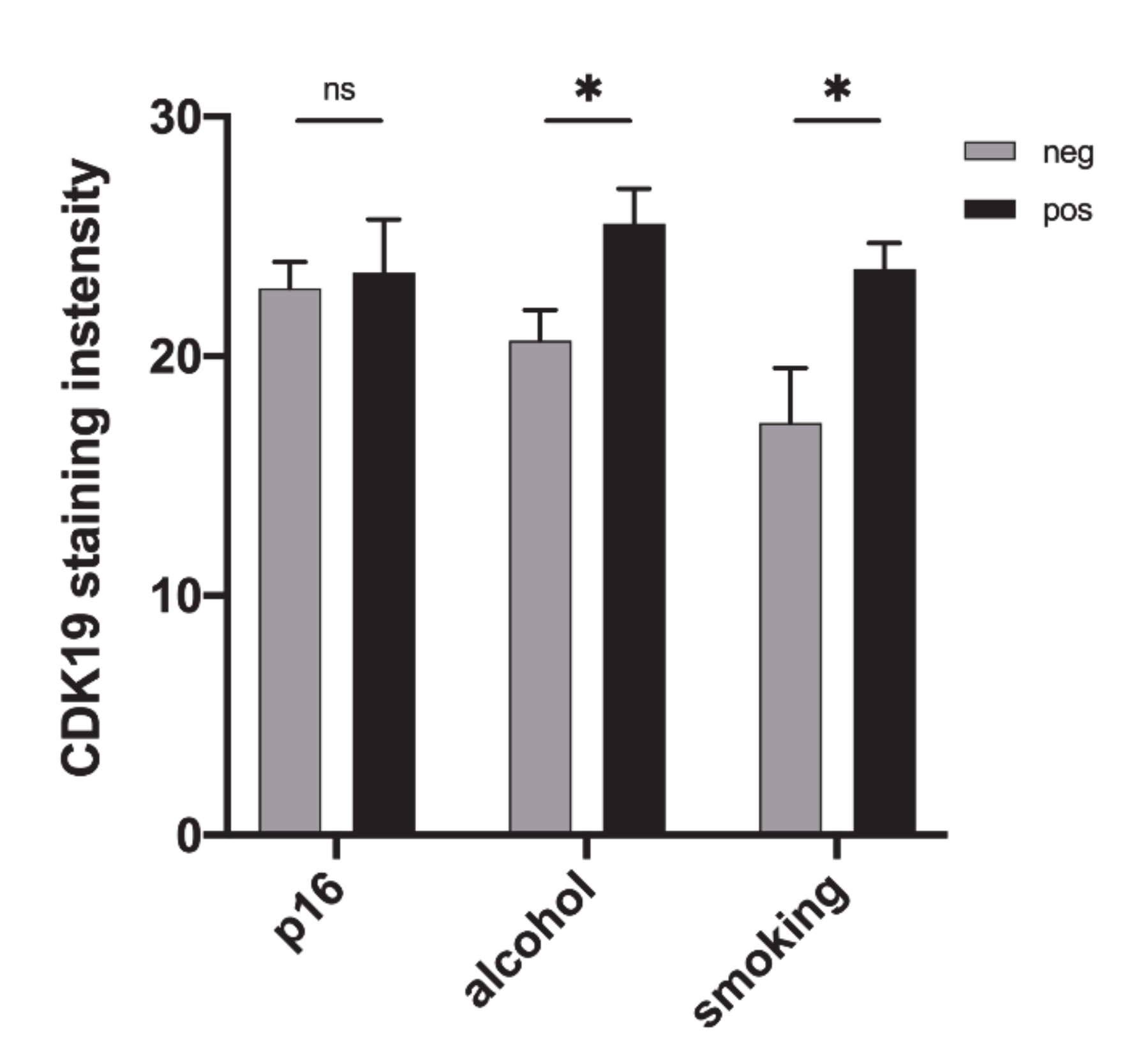
2.4. CDK19 Over-Expression in HNSCC Correlates with Smoking Status and Alcohol Consumption, But Not with p16 Status
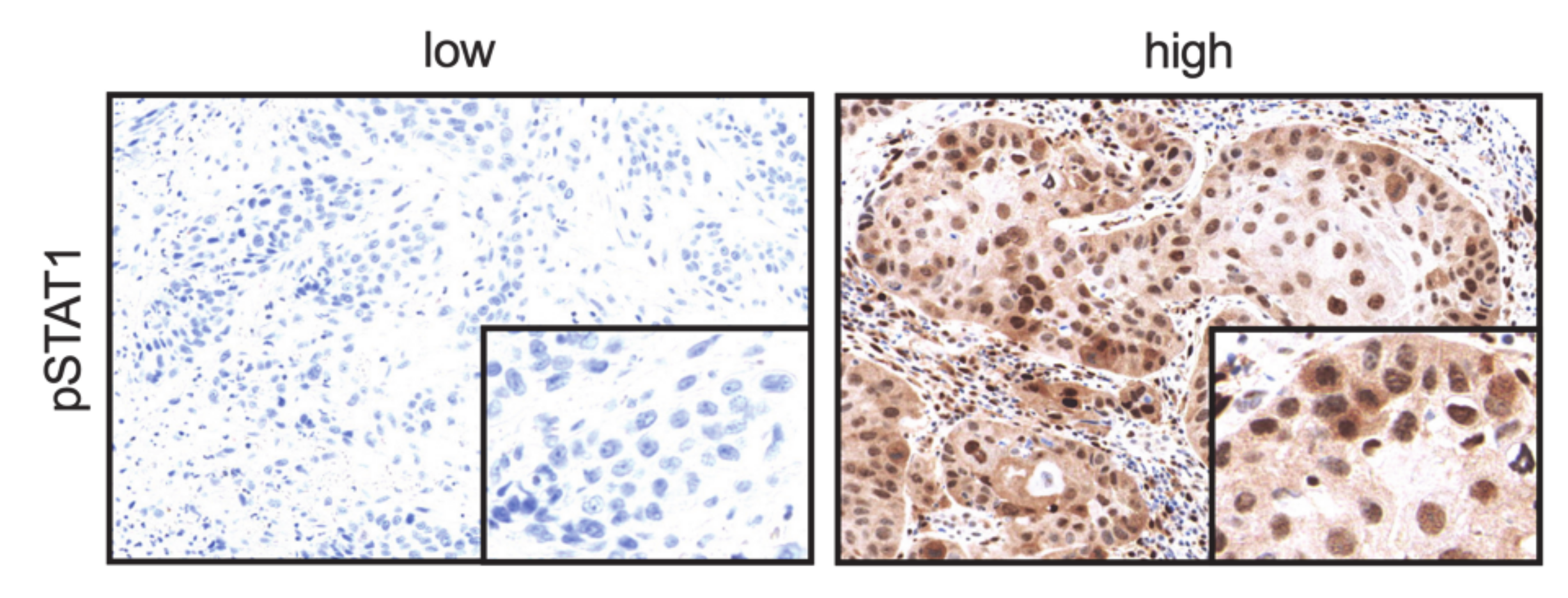
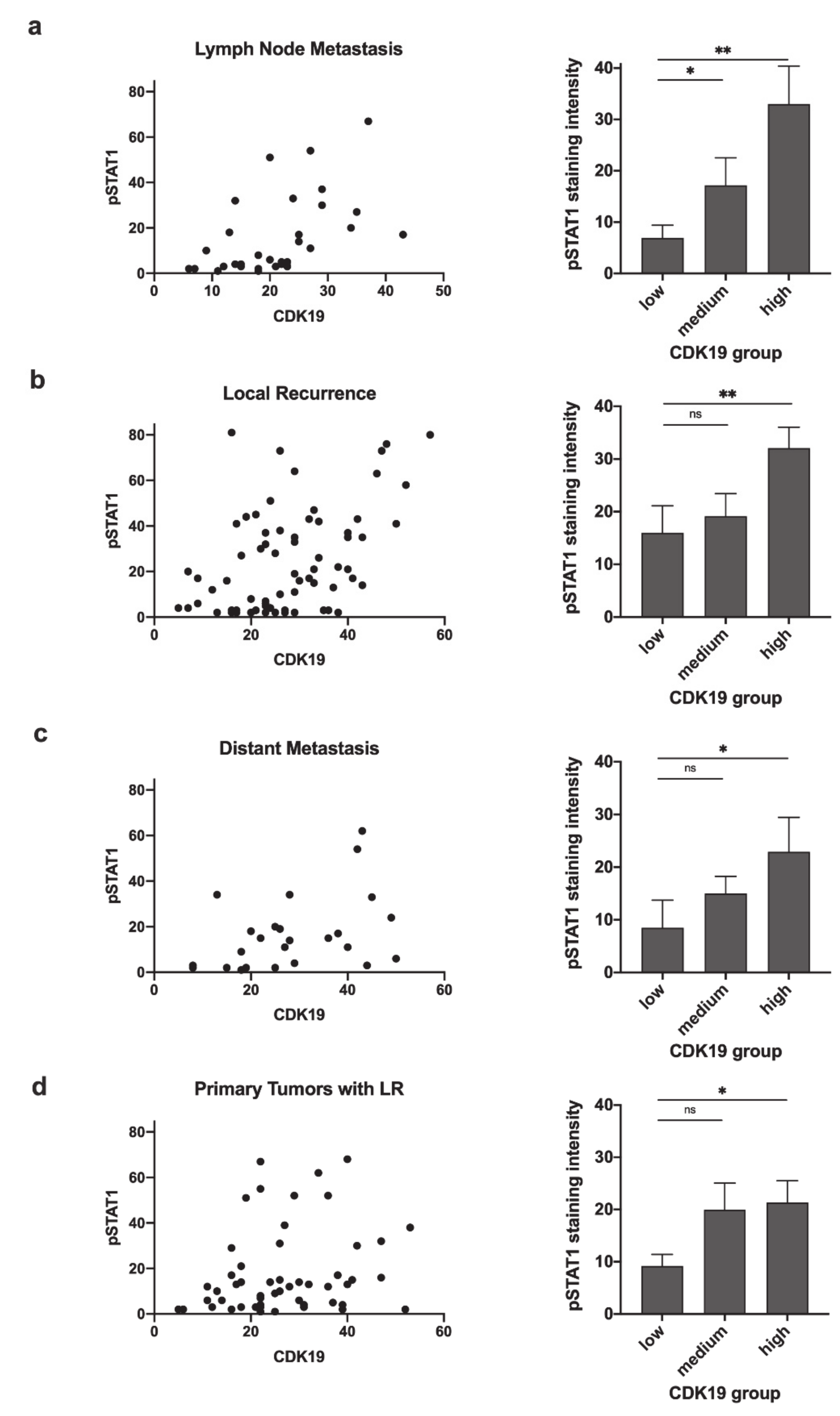
2.5. CDK19 Expression Correlates with pSTAT1 Expression in Primary Tumors Associated with Recurrent Disease, Local Recurrent Tumors, Lymph Node Metastases, and Distant Metastases
2.6. pSTAT1 Expression Correlates with PD-L1 Expression in Local Recurrences
3. Discussion
4. Materials and Methods
4.1. Patient Data and Tumor Material
4.2. Immunohistochemistry
4.3. Evaluation of Immunohistochemical Staining
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK | Cyclin-dependent kinase |
| DFS | Disease-free survival |
| DM | Distant metastasis |
| DSS | Disease-specific survival |
| FFPE | Formalin-fixed paraffin-embedded |
| HNSCC | Head and neck squamous cell carcinoma |
| IHC | Immunohistochemistry |
| JAK | Janus Kinase |
| LM | Lymph node metastasis |
| LR | Local recurrence |
| OS | Overall survival |
| PD-L1 | Programmed-Death Ligand 1 |
| PT | Primary tumor |
| ROI | Region of Interest |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| TMA | Tissue microarray |
| TPS | Tumor positivity score |
References
- Malik, S.; Roeder, R.G. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 2010, 11, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Sessa, M.; Infante, T.; Casamassimi, A. Unraveling framework of the ancestral Mediator complex in human diseases. Biochimie 2012, 94, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Casamassimi, A.; Rienzo, M.; de Nigris, F.; Sommese, L.; Napoli, C. Involvement of Mediator complex in malignancy. Biochim. Biophys. Acta 2014, 1845, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Syring, I.; Klümper, N.; Offermann, A.; Braun, M.; Deng, M.; Boehm, D.; Queisser, A.; von Mässenhausen, A.; Brägelmann, J.; Vogel, W.; et al. Comprehensive analysis of the transcriptional profile of the Mediator complex across human cancer types. Oncotarget 2016, 7, 23043–23055. [Google Scholar] [CrossRef]
- Knuesel, M.T.; Meyer, K.D.; Bernecky, C.; Taatjes, D.J. The human CDK8 subcomplex is a molecular switch that controls Mediator coactivator function. Genes Dev. 2009, 23, 439–451. [Google Scholar] [CrossRef]
- Allen, B.L.; Taatjes, D.J. The Mediator complex: A central integrator of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166. [Google Scholar] [CrossRef]
- Serrao, A.; Jenkins, L.M.; Chumanevich, A.A.; Horst, B.; Liang, J.; Gatza, M.L.; Lee, N.Y.; Roninson, I.B.; Broude, E.V.; Mythreye, K. Mediator kinase CDK8/CDK19 drives YAP1-dependent BMP4-induced EMT in cancer. Oncogene 2018, 37, 4792–4808. [Google Scholar] [CrossRef]
- Chen, M.; Liang, J.; Ji, H.; Yang, Z.; Altilia, S.; Hu, B.; Schronce, A.; McDermott, M.S.J.; Schools, G.P.; Lim, C.-U.; et al. CDK8/19 Mediator kinases potentiate induction of transcription by NFκB. Proc. Natl. Acad. Sci. USA 2017, 114, 10208–10213. [Google Scholar] [CrossRef]
- Bancerek, J.; Poss, Z.C.; Steinparzer, I.; Sedlyarov, V.; Pfaffenwimmer, T.; Mikulic, I.; Dölken, L.; Strobl, B.; Müller, M.; Taatjes, D.J.; et al. CDK8 Kinase Phosphorylates Transcription Factor STAT1 to Selectively Regulate the Interferon Response. Immunity 2013, 38, 250–262. [Google Scholar] [CrossRef]
- Alarcón, C.; Zaromytidou, A.-I.; Xi, Q.; Gao, S.; Yu, J.; Fujisawa, S.; Barlas, A.; Miller, A.N.; Manova-Todorova, K.; Macias, M.J.; et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 2009, 139, 757–769. [Google Scholar] [CrossRef]
- Poss, Z.C.; Ebmeier, C.C.; Odell, A.T.; Tangpeerachaikul, A.; Lee, T.; Pelish, H.E.; Shair, M.D.; Dowell, R.D.; Old, W.M.; Taatjes, D.J. Identification of Mediator Kinase Substrates in Human Cells using Cortistatin A and Quantitative Phosphoproteomics. Cell Rep. 2016, 15, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Firestein, R.; Bass, A.J.; Kim, S.Y.; Dunn, I.F.; Silver, S.J.; Guney, I.; Freed, E.; Ligon, A.H.; Vena, N.; Ogino, S.; et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 2008, 455, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, C.-F.; Zhang, X.; Gong, Z.; Chan, C.-H.; Lee, S.-W.; Jin, G.; Rezaeian, A.-H.; Han, F.; Wang, J.; et al. Skp2-macroH2A1-CDK8 axis orchestrates G2/M transition and tumorigenesis. Nat. Commun. 2015, 6, 6641. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.C.; Farmaki, E.; Altilia, S.; Schools, G.P.; West, D.K.; Chen, M.; Chang, B.-D.; Puzyrev, A.T.; Lim, C.; Rokow-Kittell, R.; et al. Cyclin-dependent kinase 8 mediates chemotherapy-induced tumor-promoting paracrine activities. Proc. Natl. Acad. Sci. USA 2012, 109, 13799–13804. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, X.; Ma, G.; Lin, B.; Liu, C.; Deng, Q.; Lv, W. MicroRNA-107 promotes proliferation of gastric cancer cells by targeting cyclin dependent kinase 8. Diagn. Pathol. 2014, 9, 164. [Google Scholar] [CrossRef]
- Pelish, H.E.; Liau, B.B.; Nitulescu, I.I.; Tangpeerachaikul, A.; Poss, Z.C.; Da Silva, D.H.; Caruso, B.T.; Arefolov, A.; Fadeyi, O.; Christie, A.L.; et al. Mediator kinase inhibition further activates super-enhancer-associated genes in AML. Nature 2015, 526, 273–276. [Google Scholar] [CrossRef]
- Humphreys, K.J.; Cobiac, L.; Le Leu, R.K.; Van der Hoek, M.B.; Michael, M.Z. Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol. Carcinog. 2013, 52, 459–474. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, C.; Li, D.; Yu, Z.; Li, F.; Li, F.; An, Q.; Bai, H.; Zhang, X.; Duan, Z.; et al. Cyclin-dependent kinase 11(p110) (CDK11(p110)) is crucial for human breast cancer cell proliferation and growth. Sci. Rep. 2015, 5, 10433. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Shen, J.; Yang, W.; Choy, E.; Mankin, H.; Hornicek, F.J.; Duan, Z. Cyclin-Dependent Kinase 11 (CDK11) Is Required for Ovarian Cancer Cell Growth In Vitro and In Vivo, and Its Inhibition Causes Apoptosis and Sensitizes Cells to Paclitaxel. Mol. Cancer Ther. 2016, 15, 1691–1701. [Google Scholar] [CrossRef]
- Becker, F.; Joerg, V.; Hupe, M.C.; Roth, D.; Krupar, R.; Lubczyk, V.; Kuefer, R.; Sailer, V.; Duensing, S.; Kirfel, J.; et al. Increased mediator complex subunit CDK19 expression associates with aggressive prostate cancer. Int. J. Cancer 2019, 146, 1–12. [Google Scholar] [CrossRef]
- Brägelmann, J.; Klümper, N.; Offermann, A.; Von Mässenhausen, A.; Böhm, D.; Deng, M.; Queisser, A.; Sanders, C.; Syring, I.; Merseburger, A.S.; et al. Pan-cancer analysis of the mediator complex transcriptome identifies CDK19 and CDK8 as therapeutic targets in advanced prostate cancer. Clin. Cancer Res. 2017, 23, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Audetat, K.A.; Galbraith, M.D.; Odell, A.T.; Lee, T.; Pandey, A.; Espinosa, J.M.; Dowell, R.D.; Taatjes, D.J. A Kinase-Independent Role for Cyclin-Dependent Kinase 19 in p53 Response. Mol. Cell. Biol. 2017, 37, e00626-16. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Zhao, X.D.; Liu, Y.; An, J.; Xiao, H.; Wang, C. Aberrant expression of CDK8 regulates the malignant phenotype and associated with poor prognosis in human laryngeal squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Solum, E.; Hansen, T.V.; Aesoy, R.; Herfindal, L. New CDK8 inhibitors as potential anti-leukemic agents—Design, synthesis and biological evaluation. Bioorg. Med. Chem. 2020, 28, 115461. [Google Scholar] [CrossRef]
- Dale, T.; Clarke, P.A.; Esdar, C.; Waalboer, D.; Adeniji-Popoola, O.; Ortiz-Ruiz, M.J.; Mallinger, A.; Samant, R.S.; Czodrowski, P.; Musil, D.; et al. A selective chemical probe for exploring the role of CDK8 and CDK19 in human disease. Nat. Chem. Biol. 2015, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.H.; Mani, R.; Engelhardt, H.; Impagnatiello, M.A.; Carotta, S.; Kerenyi, M.; Lorenzo-Herrero, S.; Böttcher, J.; Scharn, D.; Arnhof, H.; et al. Selective and Potent CDK8/19 Inhibitors Enhance NK-Cell Activity and Promote Tumor Surveillance. Mol. Cancer Ther. 2020, 19, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Chen, T.; Wu, C.; Gao, X.; Wu, Y.; Luo, X.; Du, K.; Yu, L.; Cai, T.; Shen, R.; et al. CDK8 as a therapeutic target for cancers and recent developments in discovery of CDK8 inhibitors. Eur. J. Med. Chem. 2019, 164, 77–91. [Google Scholar] [CrossRef]
- Meissl, K.; Macho-Maschler, S.; Müller, M.; Strobl, B. The good and the bad faces of STAT1 in solid tumours. Cytokine 2017, 89, 12–20. [Google Scholar] [CrossRef]
- Loke, P.; Allison, J.P. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5336–5341. [Google Scholar] [CrossRef]
- Liu, J.; Hamrouni, A.; Wolowiec, D.; Coiteux, V.; Kuliczkowski, K.; Hetuin, D.; Saudemont, A.; Quesnel, B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007, 110, 296–304. [Google Scholar] [CrossRef]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology 2015, 4, e1008824. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, I.I.; Meyer, S.C.; Wen, Q.J.; Crispino, J.D.; Lemieux, M.E.; Levine, R.L.; Pelish, H.E.; Shair, M.D. Mediator Kinase Phosphorylation of STAT1 S727 Promotes Growth of Neoplasms With JAK-STAT Activation. EBioMedicine 2017, 26, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Mimura, K.; Tamaki, T.; Shiraishi, K.; Kua, L.F.; Koh, V.; Ohmori, M.; Kimura, A.; Inoue, S.; Okayama, H.; et al. Phospho-STAT1 expression as a potential biomarker for anti-PD-1/anti-PD-L1 immunotherapy for breast cancer. Int. J. Oncol. 2019, 54, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Crabill, G.A.; Pritchard, T.S.; McMiller, T.L.; Wei, P.; Pardoll, D.M.; Pan, F.; Topalian, S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 2019, 7, 305–312. [Google Scholar] [CrossRef]
- Parker, S.L.; Tong, T.; Bolden, S.; Wingo, P.A. Cancer statistics, 1996. CA Cancer J. Clin. 1996, 46, 5–27. [Google Scholar] [CrossRef]
- Vokes, E.E.; Weichselbaum, R.R.; Lippman, S.M.; Hong, W.K. Head and Neck Cancer. N. Engl. J. Med. 1993, 328, 184–194. [Google Scholar] [CrossRef]
- Bernier, J. A multidisciplinary approach to squamous cell carcinomas of the head and neck: An update. Curr. Opin. Oncol. 2008, 20, 249–255. [Google Scholar] [CrossRef]
- Chin, D.; Boyle, G.M.; Porceddu, S.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Head and neck cancer: Past, present and future. Expert Rev. Anticancer. Ther. 2006, 6, 1111–1118. [Google Scholar] [CrossRef]
- Ervin, T.J.; Clark, J.R.; Weichselbaum, R.R.; Fallon, B.G.; Miller, D.; Fabian, R.L.; Posner, M.R.; Norris, C.M.; Tuttle, S.A.; Schoenfeld, D.A. An analysis of induction and adjuvant chemotherapy in the multidisciplinary treatment of squamous-cell carcinoma of the head and neck. J. Clin. Oncol. 1987, 5, 10–20. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.J.; Yan, J.; Day, A.; Sumer, B.D.; Pham, N.L.; Khan, S.; Zhu, H. Comparative effectiveness of primary radiotherapy versus surgery in elderly patients with locally advanced oropharyngeal squamous cell carcinoma. Oral Oncol. 2019, 88, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D. Standard, and novel cytotoxic and molecular-targeted, therapies for HNSCC: An evidence-based review. Curr. Opin. Oncol. 2007, 19, 216–221. [Google Scholar] [CrossRef]
- Stell, P.M.; Rawson, N. Adjuvant chemotherapy in head and neck cancer. Br. J. Cancer 1990, 61, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.C.; Bucher, J.; Tatsuoka, C.; Parikh, S.A.; Oliveira, G.H.; Gibson, M.K.; Machtay, M.; Yao, M.; Zender, C.A.; Dorth, J.A. Cardiovascular risk and prevention in patients with head and neck cancer treated with radiotherapy. Head Neck 2017, 39, 527–532. [Google Scholar] [CrossRef]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic tumor markers in head and neck squamous cell carcinoma (HNSCC) in the clinical setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef]
- Idel, C.; Ribbat-Idel, J.; Kuppler, P.; Krupar, R.; Offermann, A.; Vogel, W.; Rades, D.; Kirfel, J.; Wollenberg, B.; Perner, S. EVI1 as a marker for lymph node metastasis in HNSCC. Int. J. Mol. Sci. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Braun, M.; Goltz, D.; Shaikhibrahim, Z.; Vogel, W.; Böhm, D.; Scheble, V.; Sotlar, K.; Fend, F.; Tan, S.H.; Dobi, A.; et al. ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer—A comparative study of two monoclonal antibodies. Prostate Cancer Prostatic Dis. 2012, 15, 165–169. [Google Scholar] [CrossRef]
- Braun, M.; Kirsten, R.; Rupp, N.J.; Moch, H.; Fend, F.; Wernert, N.; Kristiansen, G.; Perner, S. Quantification of protein expression in cells and cellular subcompartments on immunohistochemical sections using a computer supported image analysis system. Histol. Histopathol. 2013, 28, 605–610. [Google Scholar]


| Status | HR | 95% CI | p-Value |
|---|---|---|---|
| CDK19 upregulated | 2.058 | 1.274–3.325 | 0.003 |
| p16 positive | 0.485 | 0.276–0.853 | 0.012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulsen, F.-O.; Idel, C.; Ribbat-Idel, J.; Kuppler, P.; Klapper, L.; Rades, D.; Bruchhage, K.-L.; Wollenberg, B.; Brägelmann, J.; Perner, S.; et al. CDK19 as a Potential HPV-Independent Biomarker for Recurrent Disease in HNSCC. Int. J. Mol. Sci. 2020, 21, 5508. https://doi.org/10.3390/ijms21155508
Paulsen F-O, Idel C, Ribbat-Idel J, Kuppler P, Klapper L, Rades D, Bruchhage K-L, Wollenberg B, Brägelmann J, Perner S, et al. CDK19 as a Potential HPV-Independent Biomarker for Recurrent Disease in HNSCC. International Journal of Molecular Sciences. 2020; 21(15):5508. https://doi.org/10.3390/ijms21155508
Chicago/Turabian StylePaulsen, Finn-Ole, Christian Idel, Julika Ribbat-Idel, Patrick Kuppler, Luise Klapper, Dirk Rades, Karl-Ludwig Bruchhage, Barbara Wollenberg, Johannes Brägelmann, Sven Perner, and et al. 2020. "CDK19 as a Potential HPV-Independent Biomarker for Recurrent Disease in HNSCC" International Journal of Molecular Sciences 21, no. 15: 5508. https://doi.org/10.3390/ijms21155508
APA StylePaulsen, F.-O., Idel, C., Ribbat-Idel, J., Kuppler, P., Klapper, L., Rades, D., Bruchhage, K.-L., Wollenberg, B., Brägelmann, J., Perner, S., & Offermann, A. (2020). CDK19 as a Potential HPV-Independent Biomarker for Recurrent Disease in HNSCC. International Journal of Molecular Sciences, 21(15), 5508. https://doi.org/10.3390/ijms21155508









