PD-L1 Expression and a High Tumor Infiltrate of CD8+ Lymphocytes Predict Outcome in Patients with Oropharyngeal Squamous Cells Carcinoma
Abstract
1. Introduction
2. Results
2.1. Patient and Tumor Characteristics
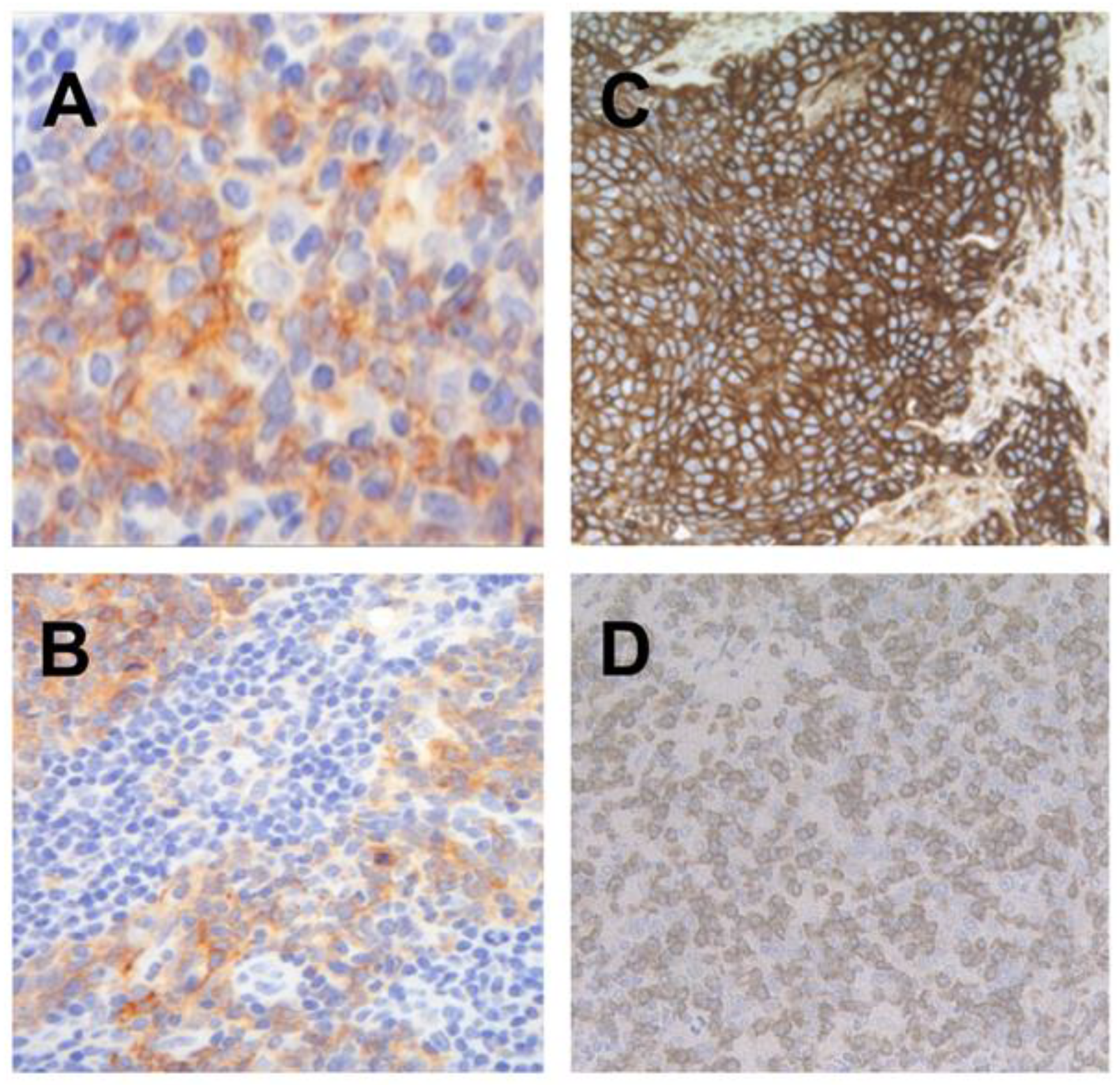
2.2. Distribution of PD-L1, MHC I, and CD8 Expression
2.3. Survival Analysis and Prognostic Significance
3. Discussion
4. Materials and Methods
4.1. Cohort
4.2. HPV-DNA Genotyping and p16INK4a Immunohistochemistry
4.3. Preparation of Tissue Microarrays
4.4. Immunohistochemistry
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Tinhofer, I.; Johrens, K.; Keilholz, U.; Kaufmann, A.; Lehmann, A.; Weichert, W.; Stenzinger, A.; Stromberger, C.; Klinghammer, K.; Becker, E.T.; et al. Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a European population with high smoking prevalence. Eur. J. Cancer 2015, 51, 514–521. [Google Scholar] [CrossRef]
- Wittekindt, C.; Wagner, S.; Bushnak, A.; Prigge, E.S.; von Knebel Doeberitz, M.; Wurdemann, N.; Bernhardt, K.; Pons-Kuhnemann, J.; Maulbecker-Armstrong, C.; Klussmann, J.P. Increasing Incidence rates of Oropharyngeal Squamous Cell Carcinoma in Germany and Significance of Disease Burden Attributed to Human Papillomavirus. Cancer Prev. Res. 2019, 12, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Castellsague, X.; Alemany, L.; Quer, M.; Halec, G.; Quiros, B.; Tous, S.; Clavero, O.; Alos, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef]
- Maxwell, J.H.; Grandis, J.R.; Ferris, R.L. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annu. Rev. Med. 2016, 67, 91–101. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. Publisher Correction: The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 662. [Google Scholar] [CrossRef]
- Ceeraz, S.; Nowak, E.C.; Noelle, R.J. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013, 34, 556–563. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Nishimura, H.; Honjo, T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001, 22, 265–268. [Google Scholar] [CrossRef]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef]
- Hersey, P.; Gallagher, S. A focus on PD-L1 in human melanoma. Clin. Cancer Res. 2013, 19, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.M.; Kwon, E.D.; Harrington, S.M.; Wampfler, J.A.; Tang, H.; Yang, P.; Aubry, M.C. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin. Lung Cancer 2013, 14, 157–163. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Letca, A.F.; Ungureanu, L.; Senila, S.C.; Grigore, L.E.; Pop, S.; Fechete, O.; Vesa, S.C.; Cosgarea, R. Regression and Sentinel Lymph Node Status in Melanoma Progression. Med. Sci. Monit. 2018, 24, 1359–1365. [Google Scholar] [CrossRef]
- Zhuang, X.; Xia, X.; Wang, C.; Gao, F.; Shan, N.; Zhang, L.; Zhang, L. A high number of CD8+ T cells infiltrated in NSCLC tissues is associated with a favorable prognosis. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 24–28. [Google Scholar] [CrossRef]
- Shimizu, S.; Hiratsuka, H.; Koike, K.; Tsuchihashi, K.; Sonoda, T.; Ogi, K.; Miyakawa, A.; Kobayashi, J.; Kaneko, T.; Igarashi, T.; et al. Tumor-infiltrating CD8(+) T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Med. 2019, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Nordfors, C.; Grun, N.; Tertipis, N.; Ahrlund-Richter, A.; Haeggblom, L.; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer 2013, 49, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, A.R.; Magalhaes, J.G.; Amigorena, S.; Marks, M.S. Presentation of phagocytosed antigens by MHC class I and II. Traffic 2013, 14, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Hunt, J.L.; Ferrone, S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: Molecular mechanisms and clinical significance. Immunol. Res. 2005, 33, 113–133. [Google Scholar] [CrossRef]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef]
- Catani, J.P.P.; Medrano, R.F.V.; Hunger, A.; Del Valle, P.; Adjemian, S.; Zanatta, D.B.; Kroemer, G.; Costanzi-Strauss, E.; Strauss, B.E. Intratumoral Immunization by p19Arf and Interferon-beta Gene Transfer in a Heterotopic Mouse Model of Lung Carcinoma. Transl. Oncol. 2016, 9, 565–574. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Tian, H.; Du, W.; Zhao, L.; Feng, J.; Yuan, D.; Li, Z. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol. Med. Rep. 2017, 15, 1063–1070. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Deron, P.; Duprez, F.; Laukens, D.; Van Dorpe, J.; Ferdinande, L.; Rottey, S. Tumor PD-L1 status and CD8(+) tumor-infiltrating T cells: Markers of improved prognosis in oropharyngeal cancer. Oncotarget 2017, 8, 80443–80452. [Google Scholar] [CrossRef]
- Hong, A.M.; Ferguson, P.; Dodds, T.; Jones, D.; Li, M.; Yang, J.; Scolyer, R.A. Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer. Oral Oncol. 2019, 92, 33–39. [Google Scholar] [CrossRef]
- Lilja-Fischer, J.K.; Eriksen, J.G.; Georgsen, J.B.; Vo, T.T.; Larsen, S.R.; Cheng, J.; Busch-Sorensen, M.; Aurora-Garg, D.; Steiniche, T.; Overgaard, J. Prognostic impact of PD-L1 in oropharyngeal cancer after primary curative radiotherapy and relation to HPV and tobacco smoking. Acta Oncol. 2020, 59, 666–672. [Google Scholar] [CrossRef]
- Steuer, C.E.; Griffith, C.C.; Nannapaneni, S.; Patel, M.R.; Liu, Y.; Magliocca, K.R.; El-Deiry, M.W.; Cohen, C.; Owonikoko, T.K.; Shin, D.M.; et al. A Correlative Analysis of PD-L1, PD-1, PD-L2, EGFR, HER2, and HER3 Expression in Oropharyngeal Squamous Cell Carcinoma. Mol. Cancer Ther. 2018, 17, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Ono, T.; Kawahara, A.; Kawaguchi, T.; Tanaka, H.; Shimamatsu, K.; Kakuma, T.; Akiba, J.; Umeno, H.; Yano, H. Prognostic impact of p16 and PD-L1 expression in patients with oropharyngeal squamous cell carcinoma receiving a definitive treatment. J. Clin. Pathol. 2019, 72, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Tsakiroglou, A.M.; Fergie, M.; Oguejiofor, K.; Linton, K.; Thomson, D.; Stern, P.L.; Astley, S.; Byers, R.; West, C.M.L. Spatial proximity between T and PD-L1 expressing cells as a prognostic biomarker for oropharyngeal squamous cell carcinoma. Br. J. Cancer 2020, 122, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.Y.; Lim, S.H.; Park, K.; Sun, J.M.; Ko, Y.H.; Baek, C.H.; Son, Y.I.; Jeong, H.S.; Ahn, Y.C.; et al. Association Between PD-L1 and HPV Status and the Prognostic Value of PD-L1 in Oropharyngeal Squamous Cell Carcinoma. Cancer Res. Treat. 2016, 48, 527–536. [Google Scholar] [CrossRef]
- Ukpo, O.C.; Thorstad, W.L.; Lewis, J.S., Jr. B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2013, 7, 113–121. [Google Scholar] [CrossRef]
- Schneider, S.; Kadletz, L.; Wiebringhaus, R.; Kenner, L.; Selzer, E.; Fureder, T.; Rajky, O.; Berghoff, A.S.; Preusser, M.; Heiduschka, G. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis-impact on clinical outcome. Histopathology 2018, 73, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Galletta-Williams, H.; Dovedi, S.J.; Roberts, D.L.; Stern, P.L.; West, C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017, 8, 14416–14427. [Google Scholar] [CrossRef]
- Lin, Y.M.; Sung, W.W.; Hsieh, M.J.; Tsai, S.C.; Lai, H.W.; Yang, S.M.; Shen, K.H.; Chen, M.K.; Lee, H.; Yeh, K.T.; et al. High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0142656. [Google Scholar] [CrossRef]
- Muller, T.; Braun, M.; Dietrich, D.; Aktekin, S.; Hoft, S.; Kristiansen, G.; Goke, F.; Schrock, A.; Bragelmann, J.; Held, S.A.E.; et al. PD-L1: A novel prognostic biomarker in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 52889–52900. [Google Scholar] [CrossRef]
- Straub, M.; Drecoll, E.; Pfarr, N.; Weichert, W.; Langer, R.; Hapfelmeier, A.; Gotz, C.; Wolff, K.D.; Kolk, A.; Specht, K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget 2016, 7, 12024–12034. [Google Scholar] [CrossRef]
- Troiano, G.; Caponio, V.C.A.; Zhurakivska, K.; Arena, C.; Pannone, G.; Mascitti, M.; Santarelli, A.; Lo Muzio, L. High PD-L1 expression in the tumour cells did not correlate with poor prognosis of patients suffering for oral squamous cells carcinoma: A meta-analysis of the literature. Cell Prolif. 2019, 52, e12537. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.; Young, R.J.; Bressel, M.; Urban, D.; Hendry, S.; Thai, A.; Angel, C.; Haddad, A.; Kowanetz, M.; Fua, T.; et al. Prognostic Significance of PD-L1(+) and CD8(+) Immune Cells in HPV(+) Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013, 73, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Tegeder, A.; Willmes, C.; Iyer, J.G.; Afanasiev, O.K.; Schrama, D.; Koba, S.; Thibodeau, R.; Nagase, K.; Simonson, W.T.; et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol. Res. 2014, 2, 1071–1079. [Google Scholar] [CrossRef]
- Cabrera, T.; Angustias Fernandez, M.; Sierra, A.; Garrido, A.; Herruzo, A.; Escobedo, A.; Fabra, A.; Garrido, F. High frequency of altered HLA class I phenotypes in invasive breast carcinomas. Hum. Immunol. 1996, 50, 127–134. [Google Scholar] [CrossRef]
- Menon, A.G.; Morreau, H.; Tollenaar, R.A.; Alphenaar, E.; Van Puijenbroek, M.; Putter, H.; Janssen-Van Rhijn, C.M.; Van De Velde, C.J.; Fleuren, G.J.; Kuppen, P.J. Down-regulation of HLA-A expression correlates with a better prognosis in colorectal cancer patients. Lab. Investig. 2002, 82, 1725–1733. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, E.M.; Lee, E.H.; Ji, K.Y.; Yi, J.; Park, M.; Kim, K.D.; Cho, Y.Y.; Kang, H.S. Human papillomavirus 16E6 suppresses major histocompatibility complex class I by upregulating lymphotoxin expression in human cervical cancer cells. Biochem. Biophys. Res. Commun. 2011, 409, 792–798. [Google Scholar] [CrossRef]
- Bottley, G.; Watherston, O.G.; Hiew, Y.L.; Norrild, B.; Cook, G.P.; Blair, G.E. High-risk human papillomavirus E7 expression reduces cell-surface MHC class I molecules and increases susceptibility to natural killer cells. Oncogene 2008, 27, 1794–1799. [Google Scholar] [CrossRef]
- Gruener, M.; Bravo, I.G.; Momburg, F.; Alonso, A.; Tomakidi, P. The E5 protein of the human papillomavirus type 16 down-regulates HLA-I surface expression in calnexin-expressing but not in calnexin-deficient cells. Virol. J. 2007, 4, 116. [Google Scholar] [CrossRef]
- Campo, M.S.; Graham, S.V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.H.; Haghshenas, M.; Marchetti, B.; Campo, M.S. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 2006, 119, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Keam, B.; Ock, C.Y.; Kim, S.; Han, B.; Kim, J.W.; Lee, K.W.; Jeon, Y.K.; Jung, K.C.; Chung, E.J.; et al. Prognostic value of the association between MHC class I downregulation and PD-L1 upregulation in head and neck squamous cell carcinoma patients. Sci. Rep. 2019, 9, 7680. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Wittekindt, C.; Reuschenbach, M.; Hennig, B.; Thevarajah, M.; Wurdemann, N.; Prigge, E.S.; von Knebel Doeberitz, M.; Dreyer, T.; Gattenlohner, S.; et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer 2016, 138, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Sobin, L.; Wittekind, C. International Union against Cancer (2002) TNM: Classification of Malignant Tumours, 6th ed.; Wiley-Liss: New York, NY, USA, 2002. [Google Scholar]
- Schmitt, M.; Bravo, I.G.; Snijders, P.J.; Gissmann, L.; Pawlita, M.; Waterboer, T. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 2006, 44, 504–512. [Google Scholar] [CrossRef]
- Prigge, E.S.; Toth, C.; Dyckhoff, G.; Wagner, S.; Muller, F.; Wittekindt, C.; Freier, K.; Plinkert, P.; Hoffmann, J.; Vinokurova, S.; et al. p16(INK4a) /Ki-67 co-expression specifically identifies transformed cells in the head and neck region. Int. J. Cancer 2015, 136, 1589–1599. [Google Scholar] [CrossRef]
- Klussmann, J.P.; Gultekin, E.; Weissenborn, S.J.; Wieland, U.; Dries, V.; Dienes, H.P.; Eckel, H.E.; Pfister, H.J.; Fuchs, P.G. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am. J. Pathol. 2003, 162, 747–753. [Google Scholar] [CrossRef]
- Pindborg, J.; Reichart, P.; Smith, C.; van der Waal, I. Histological Typing of Cancer and Precancer of the Oral Mucosa, 2nd ed.; Springer: Berlin, Germany, 1997. [Google Scholar]

| Risk Factors | All | HPV-Related | HPV-Negative | p | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 171) | 100% | (n = 32) | 19% | (n = 138) | 81% | |||
| Nicotine | never | 27 | 16% | 13 | 42% | 13 | 9% | <0.001 |
| former/current | 142 | 84% | 18 | 58% | 124 | 91% | ||
| Alcohol | ≤ 2 drinks/day | 75 | 56% | 27 | 96% | 47 | 45% | <0.001 a |
| > 2 drinks/day | 58 | 44% | 1 | 4% | 57 | 55% | ||
| Age | young (< 60 years) | 92 | 54% | 18 | 56% | 73 | 53% | 0.732 |
| old (≥ 60 years) | 79 | 46% | 14 | 44% | 65 | 47% | ||
| Gender | male | 139 | 81% | 26 | 81% | 113 | 82% | 0.933 |
| female | 32 | 19% | 6 | 19% | 25 | 18% | ||
| ECOG | healthy (0-1) | 118 | 69% | 23 | 72% | 94 | 69% | 0.719 |
| sick (2-4) | 52 | 31% | 9 | 28% | 43 | 31% | ||
| Tumor characteristics | ||||||||
| Localization | tonsil | 90 | 53% | 23 | 72% | 66 | 48% | 0.014 |
| other than tonsil | 81 | 47% | 9 | 28% | 72 | 52% | ||
| Grading | low (G1-2) | 66 | 48% | 11 | 42% | 55 | 50% | 0.506 |
| high (G3-4) | 71 | 52% | 15 | 58% | 56 | 50% | ||
| UICC stages | I - III | 64 | 38% | 12 | 38% | 52 | 38% | 0.939 |
| > III | 105 | 62% | 20 | 62% | 84 | 62% | ||
| T-stage | T1-3 | 129 | 76% | 26 | 81% | 102 | 75% | 0.455 |
| T> 3 | 40 | 24% | 6 | 19% | 34 | 25% | ||
| N-stage | N0 | 52 | 31% | 7 | 22% | 45 | 33% | 0.208 |
| N+ | 116 | 69% | 25 | 78% | 90 | 67% | ||
| M-stage | M0 | 157 | 95% | 29 | 97% | 127 | 95% | 0.664 a |
| M+ | 8 | 5% | 1 | 3% | 7 | 5% | ||
| Recurrence | no | 142 | 83% | 31 | 97% | 111 | 80% | 0.031 a |
| yes | 29 | 17% | 1 | 3% | 27 | 20% | ||
| PD-L1 Expression (Tumor Cells) | PD-L1 Expression (Macrophages) | MHC I Expression | CD8 Expression | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| yes | no | p | yes | no | p | yes | no | p | yes | no | p | ||||||||||
| All | 64 | 41% | 93 | 59% | 29 | 19% | 130 | 81% | 99 | 67% | 49 | 33% | 42 | 73% | 113 | 27% | |||||
| PD-L1 Expression (tumor cells) | yes | 14 | 22% | 49 | 78% | 0.370 | 44 | 75% | 15 | 25% | 0.064 | 28 | 46% | 33 | 54% | <0.001 | |||||
| no | 15 | 16% | 76 | 84% | 47 | 59% | 32 | 41% | 9 | 11% | 73 | 89% | |||||||||
| PD-L1 Expression (macrophages) | yes | 17 | 68% | 8 | 32% | 0.898 | 13 | 48% | 14 | 52% | 0.005 | ||||||||||
| no | 39% | 61% | 76 | 67% | 38 | 33% | 26 | 22% | 93 | 78% | |||||||||||
| MHC I Expression | yes | 25 | 26% | 70 | 74% | 0.718 | |||||||||||||||
| no | 14 | 29% | 34 | 71% | |||||||||||||||||
| HPV-association | yes | 22 | 73% | 8 | 27% | <0.001 | 8 | 27% | 22 | 73% | 0.191 | 15 | 50% | 15 | 50% | 0.028 | 19 | 61% | 12 | 39% | <0.001 |
| no | 42 | 33% | 84 | 67% | 21 | 16% | 107 | 84% | 84 | 71% | 34 | 29% | 23 | 19% | 101 | 81% | |||||
| pb | pb | pb | |||||||||||||||||||
| HPV-related OPSCC | |||||||||||||||||||||
| PD-L1 Expression (tumor cells) | yes | 6 | 27% | 16 | 73% | 1.000 | 12 | 60% | 8 | 40% | 0.209 | 16 | 76% | 5 | 24% | 0.028 | |||||
| no | 2 | 25% | 6 | 75% | 2 | 25% | 6 | 75% | 2 | 25% | 6 | 75% | |||||||||
| PD-L1 Expression (macrophages) | yes | 3 | 43% | 4 | 57% | 1.000 | 7 | 88% | 1 | 12% | 0.110 | ||||||||||
| no | 11 | 52% | 10 | 48% | 11 | 52% | 10 | 48% | |||||||||||||
| MHC I Expression | yes | 12 | 80% | 3 | 20% | 0.060 | |||||||||||||||
| no | 6 | 40% | 9 | 60% | |||||||||||||||||
| p | p | p | |||||||||||||||||||
| HPV-negative OPSCC | |||||||||||||||||||||
| PD-L1 Expression (tumor cells) | yes | 8 | 20% | 33 | 80% | 0.611 | 8 | 20% | 33 | 80% | 0.041 | 12 | 30% | 28 | 70% | 0.005 | |||||
| no | 13 | 16% | 69 | 84% | 13 | 16% | 69 | 84% | 7 | 9% | 67 | 91% | |||||||||
| PD-L1 Expression (macrophages) | yes | 14 | 18% | 65 | 82% | 0.582b | 6 | 29% | 15 | 71% | 0.091 | ||||||||||
| no | 4 | 12% | 28 | 88% | 13 | 14% | 83 | 86% | |||||||||||||
| MHC I Expression | yes | 13 | 16% | 67 | 84% | 0.321 | |||||||||||||||
| no | 8 | 24% | 25 | 76% | |||||||||||||||||
| Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | CI | pa | HR | CI | pb | |||||
| Risk factors/tumor characteristics | Lower | Upper | Lower | Upper | |||||||
| PD-L1 Expression (tumor cells) | no | 93 | 1 | <0.001 | n.s. | ||||||
| yes | 64 | 0.409 | 0.255 | 0.657 | |||||||
| PD-L1 Expression (macrophages) | no | 130 | 1 | 0.532 | |||||||
| yes | 29 | 0.837 | 0.480 | 1.461 | |||||||
| CD8 Expression | no | 113 | 1 | <0.001 | 3.539 | 1.803 | 6.945 | <0.001 | |||
| yes | 42 | 0.295 | 0.156 | 0.559 | |||||||
| MHC I Expression | no | 49 | 1 | 0.930 | |||||||
| yes | 99 | 0.979 | 0.604 | 1.585 | |||||||
| HPV | HPV-negative | 138 | 1 | 0.001 | n.s. | ||||||
| HPV-related | 32 | 0.270 | 0.125 | 0.585 | |||||||
| Age | young (<60 years) | 92 | 1 | 0.016 | n.s. | ||||||
| old (≥60 years) | 79 | 1.654 | 1.099 | 2.487 | |||||||
| ECOG | healthy (0–1) | 118 | 1 | <0.001 | 0.231 | 0.144 | 0.372 | <0.001 | |||
| sick (2–4) | 52 | 3.386 | 2.233 | 5.134 | |||||||
| UICC7 stages | I-III | 64 | 1 | 0.015 | n.s. | ||||||
| >III | 105 | 0.572 | 0.364 | 0.897 | |||||||
| HPV-related OPSCC | |||||||||||
| PD-L1 Expression (tumor cells) | no | 8 | 1 | 0.739 | n.s. | ||||||
| yes | 22 | 1.246 | 0.242 | 6.340 | |||||||
| PD-L1 Expression (macrophages) | no | 22 | 1 | 0.306 | |||||||
| yes | 8 | 35.342 | 0.038 | 32725.7 | |||||||
| CD8 Expression | no | 12 | 1 | 0.047 | 15.099 | 2.231 | 102.2 | 0.005 | |||
| yes | 19 | 5.298 | 1.022 | 27.46 | |||||||
| MHC I Expression | no | 15 | 1 | 0.553 | |||||||
| yes | 15 | 1.573 | 0.352 | 7.042 | |||||||
| Age | young (<60 years) | 18 | 1 | 0.117 | |||||||
| old (≥60 years) | 14 | 3.718 | 0.720 | 19.187 | |||||||
| ECOG | healthy (0-1) | 23 | 1 | 0.321 | n.s. | ||||||
| sick (2-4) | 9 | 0.469 | 0.105 | 2.096 | |||||||
| UICC7 stages | I-III | 12 | 1 | 0.213 | n.s. | ||||||
| >III | 20 | 0.260 | 0.031 | 2.162 | |||||||
| HPV-negative OPSCC | |||||||||||
| PD-L1 Expression (tumor cells) | no | 84 | 1 | 0.004 | 1.803 | 1.024 | 3.175 | 0.041 | |||
| yes | 42 | 2.139 | 1.247 | 3.593 | |||||||
| PD-L1 Expression (macrophages) | no | 107 | 1 | 0.566 | |||||||
| yes | 21 | 0.848 | 0.483 | 1.489 | |||||||
| CD8 Expression | no | 101 | 1 | 0.021 | n.s. | ||||||
| yes | 23 | 2.268 | 1.135 | 4.603 | |||||||
| MHC I Expression | no | 34 | 1 | 0.673 | |||||||
| yes | 84 | 1.118 | 0.666 | 1.879 | |||||||
| Age | young (<60 years) | 73 | 1 | 0.052 | |||||||
| old (≥60 years) | 65 | 1.526 | 0.996 | 2.337 | |||||||
| ECOG | healthy (0-1) | 94 | 1 | <0.001 | 0.231 | 0.137 | 0.338 | <0.001 | |||
| sick (2-4) | 43 | 0.245 | 0.157 | 0.380 | |||||||
| UICC7 stages | I-III | 52 | 1 | 0.023 | n.s. | ||||||
| >III | 84 | 0.583 | 0.336 | 0.927 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wuerdemann, N.; Gültekin, S.E.; Pütz, K.; Wittekindt, C.; Huebbers, C.U.; Sharma, S.J.; Eckel, H.; Schubotz, A.B.; Gattenlöhner, S.; Büttner, R.; et al. PD-L1 Expression and a High Tumor Infiltrate of CD8+ Lymphocytes Predict Outcome in Patients with Oropharyngeal Squamous Cells Carcinoma. Int. J. Mol. Sci. 2020, 21, 5228. https://doi.org/10.3390/ijms21155228
Wuerdemann N, Gültekin SE, Pütz K, Wittekindt C, Huebbers CU, Sharma SJ, Eckel H, Schubotz AB, Gattenlöhner S, Büttner R, et al. PD-L1 Expression and a High Tumor Infiltrate of CD8+ Lymphocytes Predict Outcome in Patients with Oropharyngeal Squamous Cells Carcinoma. International Journal of Molecular Sciences. 2020; 21(15):5228. https://doi.org/10.3390/ijms21155228
Chicago/Turabian StyleWuerdemann, Nora, Sibel E. Gültekin, Katharina Pütz, Claus Wittekindt, Christian U. Huebbers, Shachi J. Sharma, Hans Eckel, Anna B. Schubotz, Stefan Gattenlöhner, Reinhard Büttner, and et al. 2020. "PD-L1 Expression and a High Tumor Infiltrate of CD8+ Lymphocytes Predict Outcome in Patients with Oropharyngeal Squamous Cells Carcinoma" International Journal of Molecular Sciences 21, no. 15: 5228. https://doi.org/10.3390/ijms21155228
APA StyleWuerdemann, N., Gültekin, S. E., Pütz, K., Wittekindt, C., Huebbers, C. U., Sharma, S. J., Eckel, H., Schubotz, A. B., Gattenlöhner, S., Büttner, R., Speel, E.-J., Klussmann, J. P., Wagner, S., & Quaas, A. (2020). PD-L1 Expression and a High Tumor Infiltrate of CD8+ Lymphocytes Predict Outcome in Patients with Oropharyngeal Squamous Cells Carcinoma. International Journal of Molecular Sciences, 21(15), 5228. https://doi.org/10.3390/ijms21155228







