3-Hydroxyolean-12-en-27-oic Acids Inhibit RANKL-Induced Osteoclastogenesis in Vitro and Inflammation-Induced Bone Loss in Vivo
Abstract
1. Introduction
2. Results
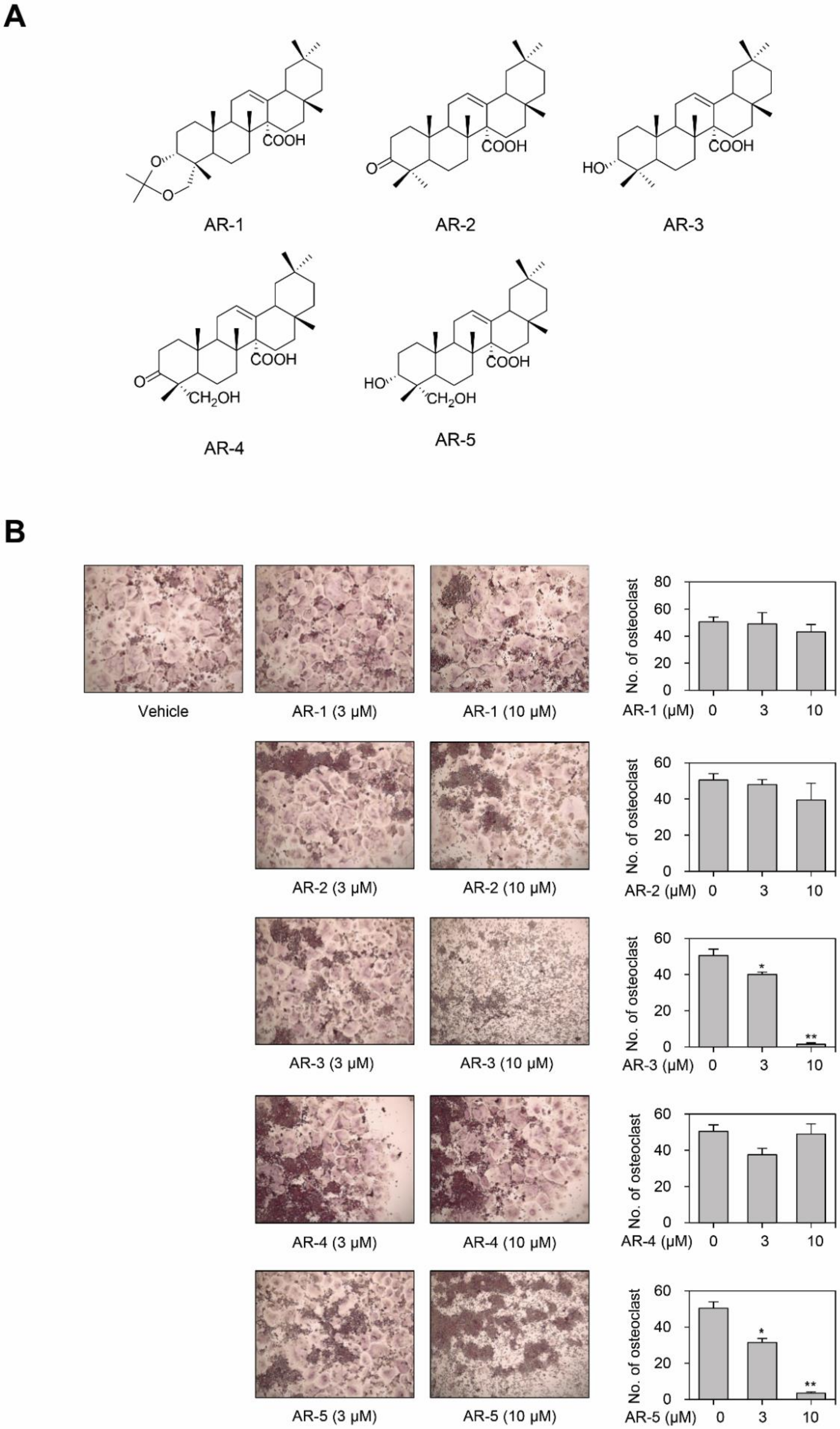
2.1. AR-3 and AR-5 Inhibits RANKL-Induced Osteoclast Formation in RAW264.7 Cells
2.2. AR-3 and AR-5 Inhibit RANKL-Induced Osteoclast Formation in Mouse BMMs
2.3. AR-3 and AR-5 Suppress RANKL-Induced Formation of Mature Osteoclasts in Mouse BMMs
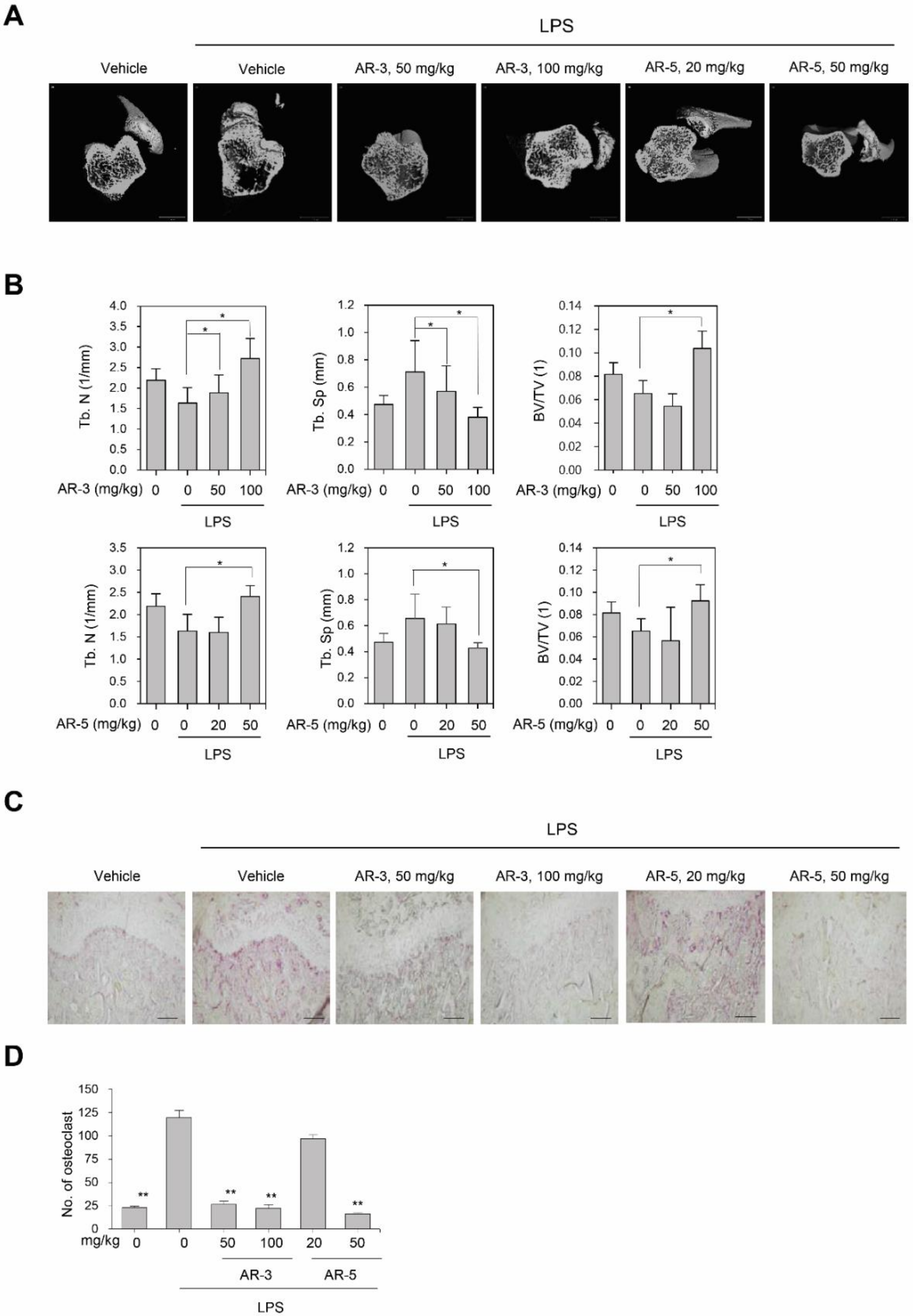
2.4. AR-3 and AR-5 Suppress LPS-Induced Bone Loss in Vivo
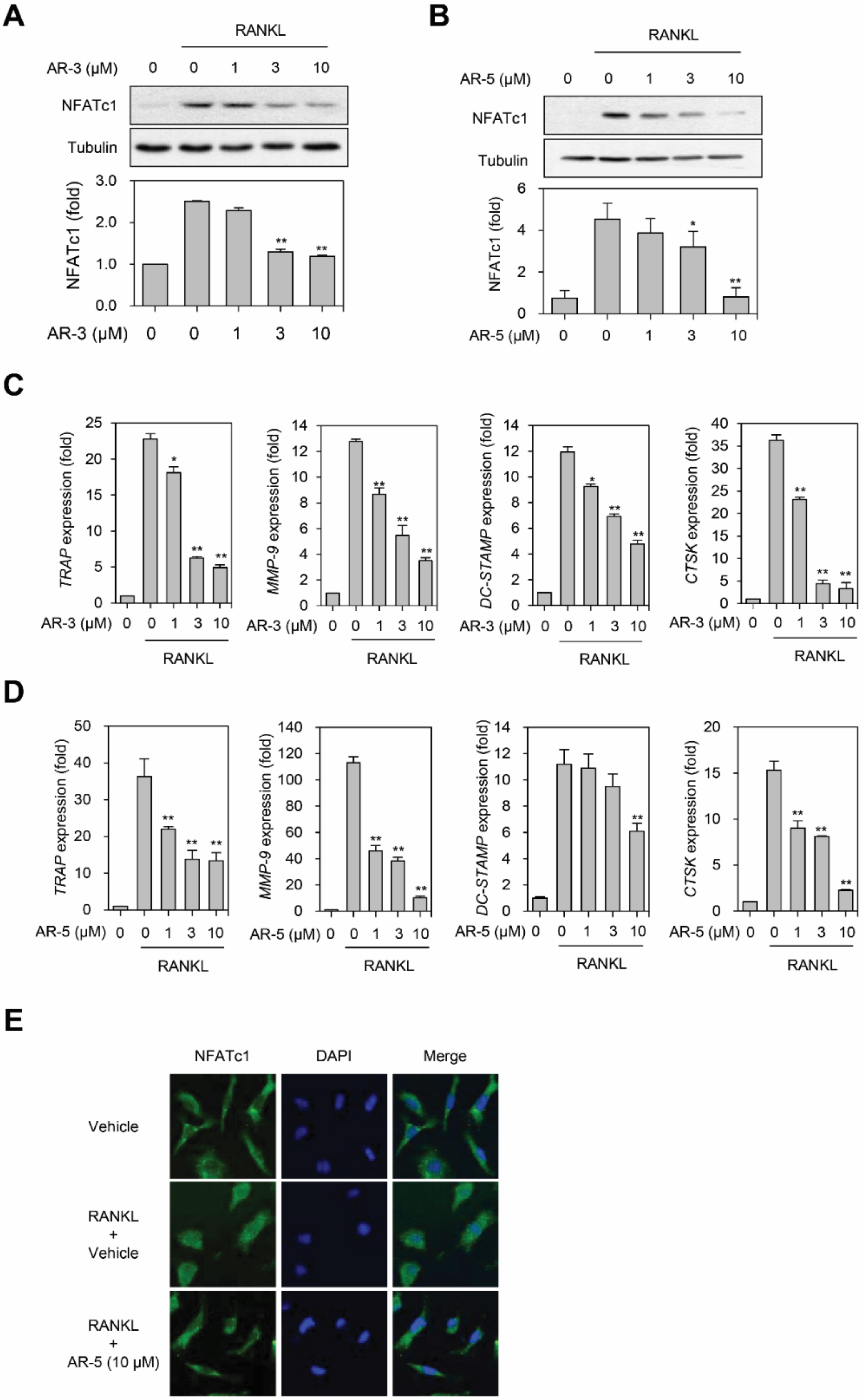
2.5. AR-3 and AR-5 Suppress RANKL-Induced NFATc1 Activation
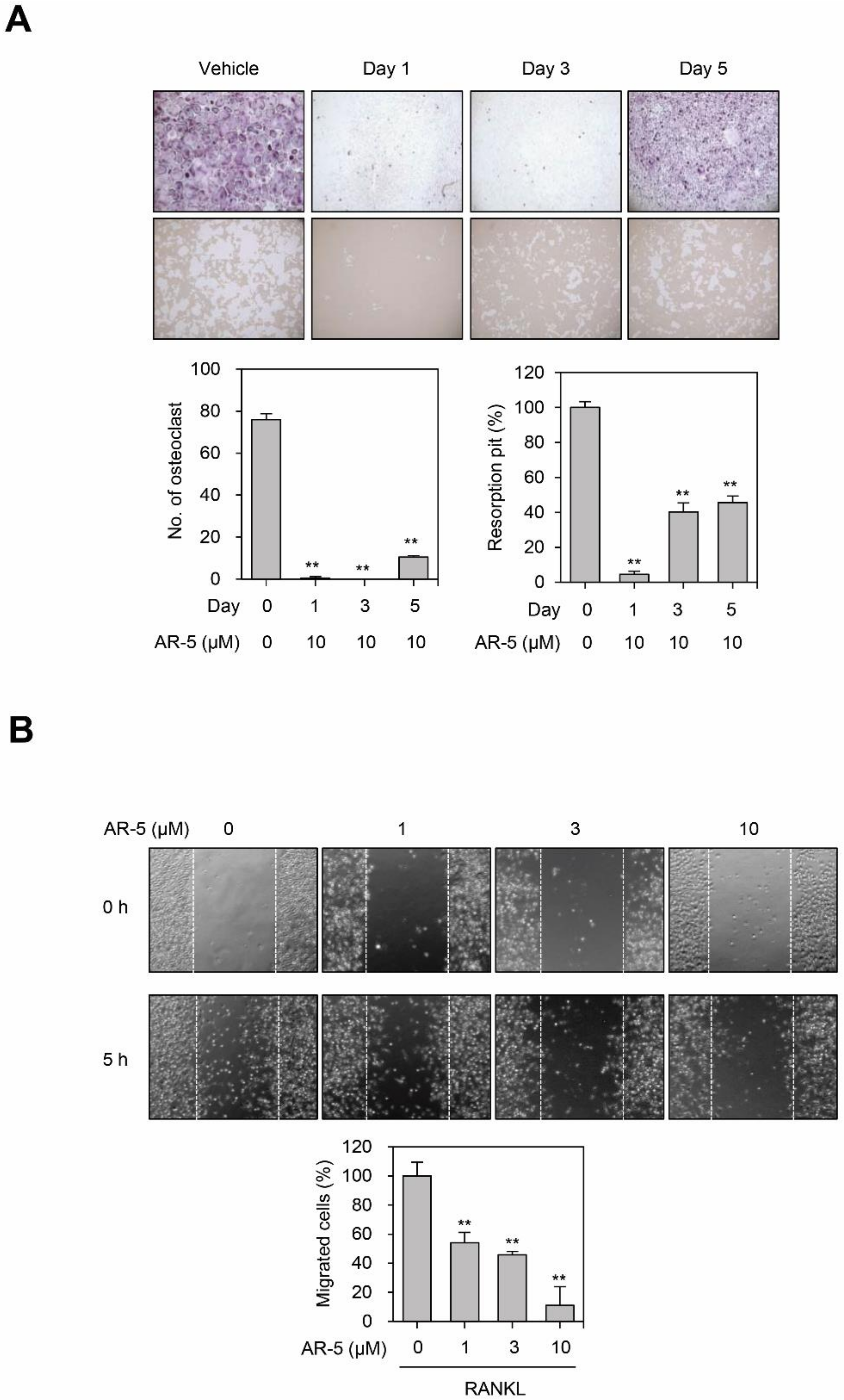
2.6. AR-5 Inhibits RANKL-Induced Osteoclast Formation at Early and Late Stage of Differentiation, and Migration
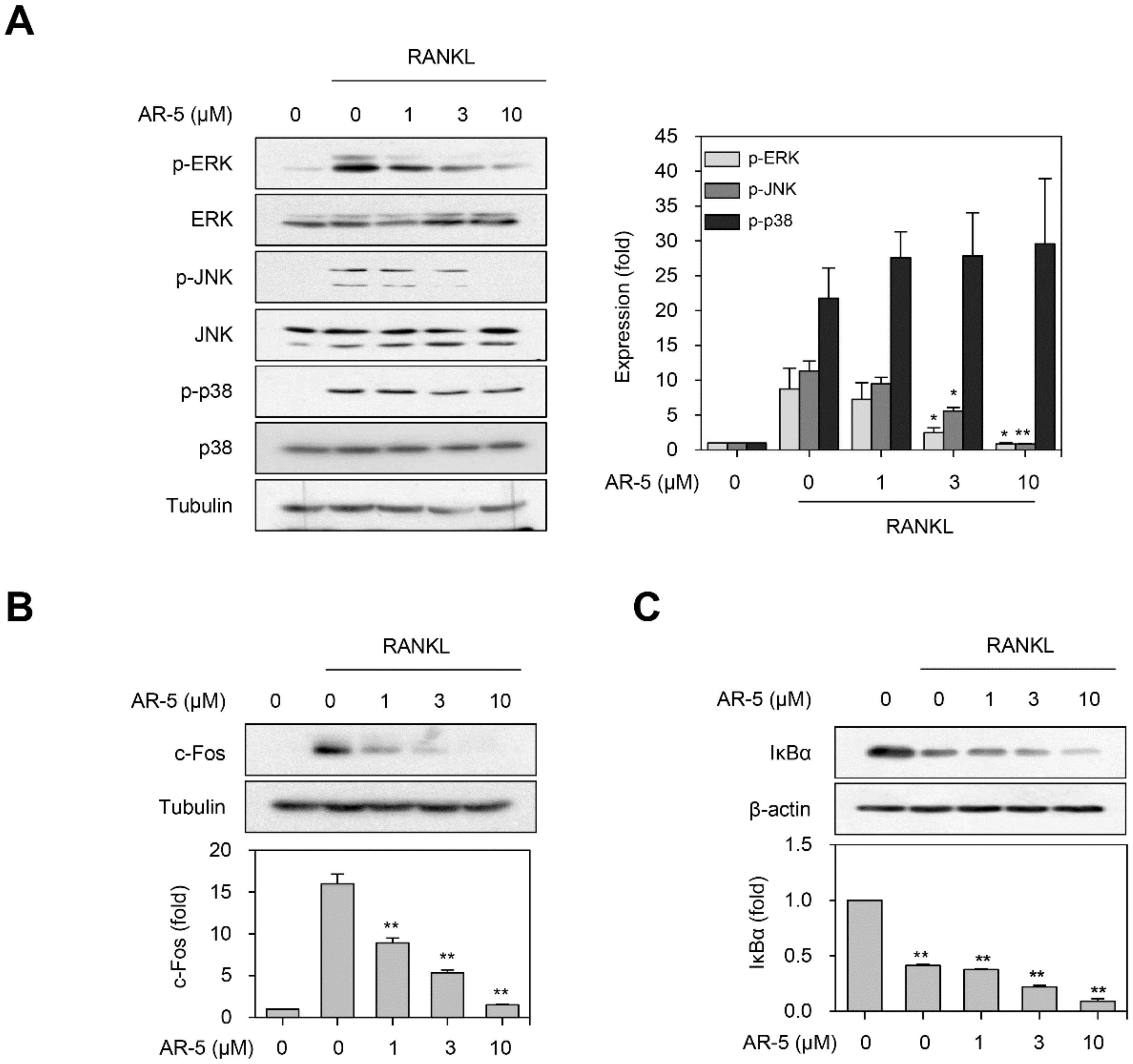
2.7. AR-5 Inhibits RANKL-Induced Activation of ERK and JNK MAPKs
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Antibodies
4.2. Cell Culture
4.3. In Vitro Osteoclastogenesis Assay
4.4. Measurement of Cell Viability
4.5. Bone Resorption Assay
4.6. Actin-Ring Formation Assay
4.7. Migration Assay
4.8. Western Blot Analysis
4.9. Immunofluorescence and Confocal Microscopy
4.10. Reverse Transcription qPCR Analysis
4.11. Animal Experiments
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AR-1 | 3α,23-isopropylidenedioxyolean-12-en-27-oic acid |
| AR-2 | 3-oxoolean-12-en-27-oic acid |
| AR-3 | 3α-hydroxyolean-12-en-27-oic acid |
| AR-4 | 23-hydroxy-3-oxoolean-12-en-27-oic acid |
| AR-5 | aceriphyllic acid A |
| BMM | bone marrow-derived macrophage |
| BS/BV | bone surface/bone volume |
| CT | Computed Tomography |
| CtsK | cathepsin K |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DC-STAMP | dendritic cell–specific transmembrane protein |
| ERK | extracellular signal-regulated kinase |
| FITC | fluorescein isothiocyanate |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| M-CSF | macrophage colony-stimulating factor |
| MMP-9 | matrix metallopeptidase 9 |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NFATc1 | nuclear factor of activated T cells cytoplasmic 1 |
| NF-κB | nuclear factor-κB |
| OSCAR | osteoclast-associated receptor |
| qPCR | quantitative polymerase chain reaction |
| RANK | receptor activator of nuclear factor-κB |
| RANKL | receptor activator of nuclear factor-κB ligand |
| Tb.N | trabecular number |
| Tb.Sp | trabecular separation |
| TARF6 | TNF-receptor-associated factor 6 |
| TRAP | tartarate-resistant acid phosphatase |
References
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Soysa, N.S.; Alles, N. Osteoclast function and bone-resorbing activity: An overview. Biochem. Biophys. Res. Commun. 2016, 476, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, Z. The Osteoclast: A Multinucleated, Hematopoietic-Origin, Bone-Resorbing Osteoimmune Cell. J. Cell. Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Jilka, R.L. Bone marrow, cytokines, and bone remodeling, Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Xiong, Y.; Pixley, F.J.; Yeung, Y.G.; Stanley, E.R. Macrophage proliferation is regulated through CSF-1 receptor tyrosines 544, 559, and 807. J. Biol. Chem. 2012, 287, 13694–13704. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Faccio, R.; Chappel, J.; Zheng, I.; Feng, X.; Weber, J.D.; Teitelbaum, S.L.; Ross, F.P. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J. Biol. Chem. 2007, 282, 18980–18990. [Google Scholar] [CrossRef]
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999, 13, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.; Azuma, S.; Tanaka, S.; Miyazaki, T.; Takaki, S.; Takatsu, K.; Nakao, K.; Nakamura, K.; Katsuki, M.; Yamamoto, T.; et al. Severe osteopetrosis, defective interleukin-1 signaling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 1999, 4, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.R.; Besser, D.; Kim, N.; Arron, J.R.; Vologodskaia, M.; Hanafusa, H.; Choi, Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell 1999, 4, 1041–1049. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of sweet cherry polyphenols on enhanced osteoclastogenesis associated with childhood obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef]
- Muffler, K.; Leipold, D.; Scheller, M.C.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Mirata, M.A.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Process Biochem. 2011, 46, 1–15. [Google Scholar] [CrossRef]
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543–593. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cheon, Y.H.; Oh, H.M.; Rho, M.C.; Erkhembaatar, M.; Kim, M.S.; Lee, C.H.; Kim, J.J.; Choi, M.K.; Yoon, K.H.; et al. Oleanolic acid acetate inhibits osteoclast differentiation by downregulating PLCγ2-Ca2+-NFATc1 signaling, and suppresses bone loss in mice. Bone 2014, 60, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Z.; Li, Z.; Ma, Y.; Zhang, L.; Zheng, C.; Qiu, W.; Wu, X.; Wang, X.; Li, H.; et al. Maslinic acid suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by regulating RANKL-mediated NF-κB and MAPK signaling pathways. J. Bone Miner. Res. 2011, 26, 644–656. [Google Scholar] [CrossRef]
- Wattel, A.; Kamel, S.; Mentaverri, R.; Lorget, F.; Prouillet, C.; Petit, J.P.; Fardelonne, P.; Brazier, M. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem. Pharmacol. 2003, 65, 35–42. [Google Scholar] [CrossRef]
- Wilson, S.R.; Peters, C.; Saftig, P.; Bromme, D. Cathepsin K. Activity dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef]
- Kikuta, J.; Ishii, M. Osteoclast migration, differentiation and function: Novel therapeutic targets for rheumatic diseases. Rheumatology 2013, 52, 226–234. [Google Scholar] [CrossRef]
- Xuan, W.; Feng, X.; Qian, C.; Peng, L.; Shi, Y.; Xu, L.; Wang, F.; Tan, W. Osteoclast differentiation gene expression profiling reveals chemokine CCL4 mediates RANKL-induced osteoclast migration and invasion via PI3K pathway. Cell Biochem. Funct. 2017, 35, 171–177. [Google Scholar] [CrossRef]
- An, J.; Hao, D.; Zhang, Q.; Chen, B.; Zhang, R.; Wang, Y.; Yang, H. Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int. Immunopharmacol. 2016, 36, 118–131. [Google Scholar] [CrossRef]
- Sethi, G.; Aggarwal, B.B. Mending the bones with natural products. Chem. Biol. 2007, 14, 738–740. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; Zhao, Y.; Qiao, P.; Tang, D.; Chen, Y.; Xue, C.; Li, C.; Liu, S.; Wang, J.; et al. Oleanolic acid exerts bone protective effects in ovariectomized mice by inhibiting osteoclastogenesis. J. Pharmacol. Sci. 2018, 137, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Van le, T.K.; Hung, T.M.; Thuong, P.T.; Ngoc, T.M.; Kim, J.C.; Jang, H.S.; Cai, X.F.; Oh, S.R.; Min, B.S.; Woo, M.H.; et al. Oleanane-type triterpenoids from Aceriphyllum rossii and their cytotoxic activity. J. Nat. Prod. 2009, 72, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Lee, I.; Chang, M.J.; Yoo, J.K.; Na, M.; Hung, T.M.; Thuong, P.T.; Lee, J.; Kim, J.H.; Kim, J.C.; et al. Anticomplementary activity of triterpenoids from the whole plant of Aceriphyllum rossii against the classical pathway. Planta Med. 2008, 74, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, M.O.; Han, A.R.; Kwon, E.B.; Kang, M.J.; Cho, S.; Moon, D.O.; Noh, J.R.; Lee, C.H.; Kim, Y.S.; et al. Oleanane-type triterpenoids of Aceriphyllum rossii and their diacylglycerol acyltransferase-inhibitory activity. Planta Med. 2015, 81, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Oh, H.W.; Kim, W.G. Potent anticariogenic activity of Aceriphyllum rossii and its components, aceriphyllic acid A and 3-oxoolean-12-en-27-oic acid. J. Food Sci. 2010, 75, M78–M82. [Google Scholar] [CrossRef]
- Han, J.T.; Kim, H.Y.; Park, Y.D.; Lee, Y.H.; Lee, K.R.; Kwon, B.M.; Baek, N.I. Aceriphyllic acid A, a new ACAT inhibitory triterpenoid, from Aceriphyllum rossii. Planta Med. 2002, 68, 558–561. [Google Scholar] [CrossRef]
- Zheng, C.J.; Sohn, M.J.; Kim, K.Y.; Yu, H.E.; Kim, W.G. Olean-27-carboxylic acid-type triterpenes with potent antibacterial activity from Aceriphyllum rossii. J. Agric. Food Chem. 2008, 56, 11752–11756. [Google Scholar] [CrossRef]
- Grigoriadis, A.E.; Wang, Z.Q.; Cecchini, M.G.; Hofstetter, W.; Felix, R.; Fleisch, H.A.; Wagner, E.F. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994, 266, 443–448. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef]
- Matsushita, T.; Chan, Y.Y.; Kawanami, A.; Balmes, G.; Landreth, G.F.; Murakami, S. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol. Cell. Biol. 2009, 29, 5843–5857. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Sabapathy, K.; Hoffmann, O.; Idarraga, M.H.; Wagner, E.F. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J. Cell Sci. 2002, 115, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Investig. 2004, 114, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Franzoso, G.; Carlson, L.; Xing, L.; Poljak, L.; Shores, E.W.; Brown, K.D.; Leonardi, A.; Tran, T.; Boyce, B.F.; Siebenlist, U. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 2007, 11, 3482–3496. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Lee, I.; Yoo, J.K.; Na, M.; Min, B.S.; Lee, J.; Yun, B.S.; Jin, W.; Kim, H.; Youn, U.; Chen, Q.C.; et al. Cytotoxicity of triterpenes isolated from Aceriphyllum rossii. Chem. Pharm. Bull. 2007, 55, 1376–1378. [Google Scholar] [CrossRef][Green Version]
- Trang, T.T.; Cuong, T.D.; Hung, T.M.; Kim, J.A.; Lee, J.H.; Woo, M.H.; Choi, J.S.; Lee, H.K.; Min, B.S. Anti-inflammatory compounds from the aerial parts of Aceriphyllum rossii. Chem. Pharm. Bull. 2014, 62, 185–190. [Google Scholar] [CrossRef]
- Tran, P.T.; Dat, N.T.; Dang, N.H.; Van Cuong, P.; Lee, S.; Hwangbo, C.; Van Minh, C.; Lee, J.H. Ganomycin I from Ganoderma lucidum attenuates RANKL-mediated osteoclastogenesis by inhibiting MAPKs and NFATc1. Phytomedicine 2019, 55, 1–8. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, W.; Lee, S.; Tran, P.T.; Ngo, T.Q.-M.; Kim, O.; Le, T.H.; Dang, N.H.; Hwangbo, C.; Min, B.S.; Lee, J.-H. 3-Hydroxyolean-12-en-27-oic Acids Inhibit RANKL-Induced Osteoclastogenesis in Vitro and Inflammation-Induced Bone Loss in Vivo. Int. J. Mol. Sci. 2020, 21, 5240. https://doi.org/10.3390/ijms21155240
Seo W, Lee S, Tran PT, Ngo TQ-M, Kim O, Le TH, Dang NH, Hwangbo C, Min BS, Lee J-H. 3-Hydroxyolean-12-en-27-oic Acids Inhibit RANKL-Induced Osteoclastogenesis in Vitro and Inflammation-Induced Bone Loss in Vivo. International Journal of Molecular Sciences. 2020; 21(15):5240. https://doi.org/10.3390/ijms21155240
Chicago/Turabian StyleSeo, Wonyoung, Suhyun Lee, Phuong Thao Tran, Thi Quynh-Mai Ngo, Okwha Kim, Thanh Huong Le, Nguyen Hai Dang, Cheol Hwangbo, Byung Sun Min, and Jeong-Hyung Lee. 2020. "3-Hydroxyolean-12-en-27-oic Acids Inhibit RANKL-Induced Osteoclastogenesis in Vitro and Inflammation-Induced Bone Loss in Vivo" International Journal of Molecular Sciences 21, no. 15: 5240. https://doi.org/10.3390/ijms21155240
APA StyleSeo, W., Lee, S., Tran, P. T., Ngo, T. Q.-M., Kim, O., Le, T. H., Dang, N. H., Hwangbo, C., Min, B. S., & Lee, J.-H. (2020). 3-Hydroxyolean-12-en-27-oic Acids Inhibit RANKL-Induced Osteoclastogenesis in Vitro and Inflammation-Induced Bone Loss in Vivo. International Journal of Molecular Sciences, 21(15), 5240. https://doi.org/10.3390/ijms21155240





