Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives
Abstract
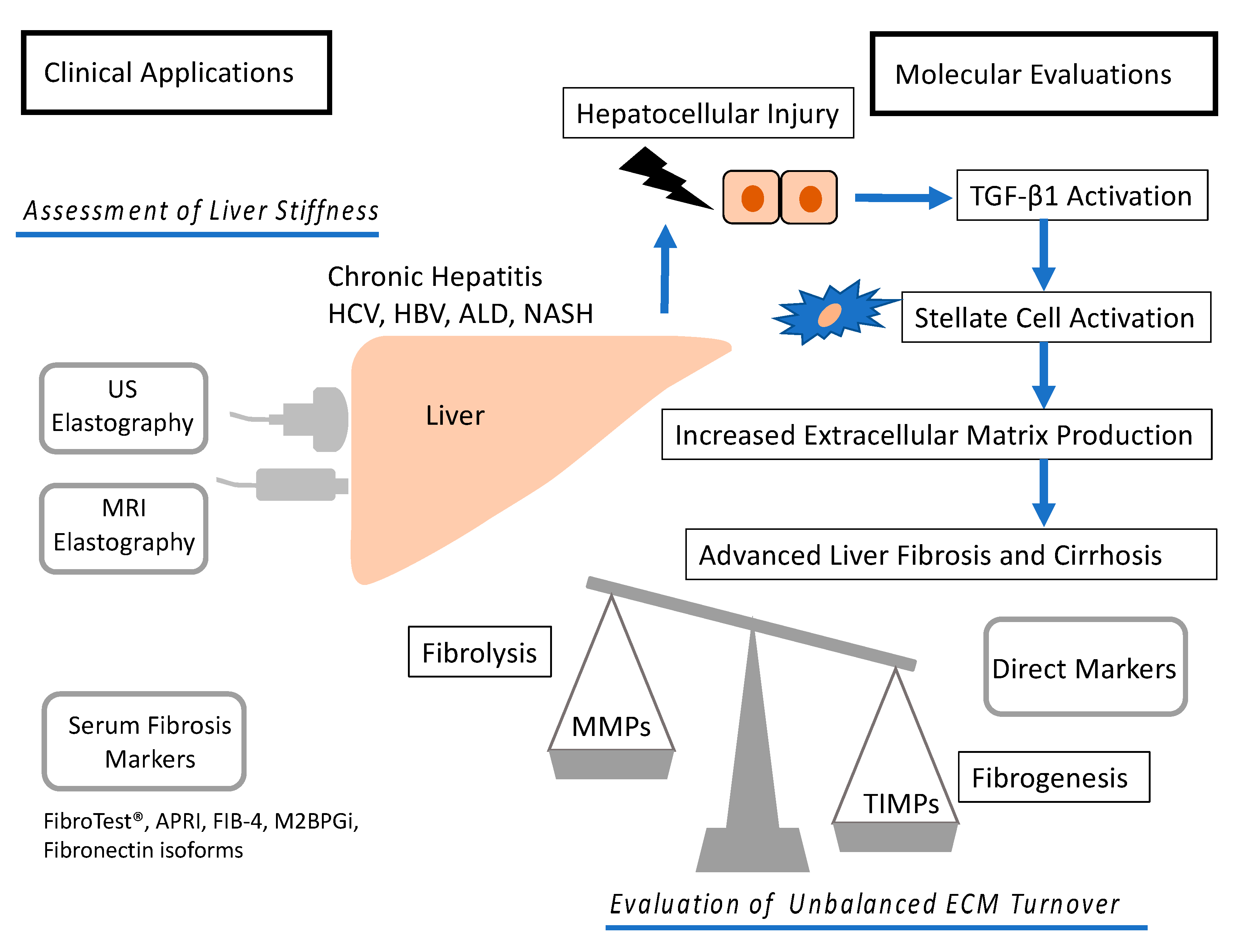
1. Introduction
2. Noninvasive Imaging Techniques
2.1. US Elastography
2.1.1. Static Strain Imaging
2.1.2. One-Dimensional Transient Elastography
2.1.3. Point SWE
2.1.4. Two-Dimensional Shear Wave Elastography
2.2. Magnetic Resonance Imaging (MRI) Elastography
2.3. Noninvasive Biomarkers and Their Combinations
2.3.1. FibroTest®
2.3.2. APRI
2.3.3. FIB-4 Index
3. Molecular Mechanism of Fibrosis
3.1. Mechanism of Fibrosis
3.1.1. Collagenase Subgroup
3.1.2. Gelatinase Subgroup
3.1.3. Stromelysin Subgroup
3.1.4. Matrilysin Subgroup
3.1.5. Membrane-Type MMP Subgroup
3.1.6. Other MMPs Subgroup
3.1.7. TIMPs
3.1.8. Fibronectin Isoforms
3.1.9. Mac-2 Binding Protein Glycan Isomer (M2BPGi)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| ALD | Alcoholic liver disease |
| NASH | Nonalcoholic liver disease |
| ECM | Extracellular matrix |
| HSC | Hepatic stellate cell |
| TGF-β1 | Transforming growth factor beta 1 |
| MMP | Matrix metalloproteinase |
| TIMP | Tissue inhibitor of metalloproteinase |
| SWE | Shear wave elastography |
| AUROC | Area under the receiver operating characteristics |
| ARFI | Acoustic radiation force impulse |
| ROI | Region of interest |
| 2D | Two dimensional |
| MRE | Magnetic resonance elastography |
| NAFLD | Nonalcoholic fatty liver disease |
| FT | FibroTest |
| GGT | Gamma-glutamyl transferase |
| AST | Aspartate aminotransferase |
| APRI | Aspartate aminotransferase to platelet ratio index |
| AST | Aspartate aminotransferase |
| ULN | Upper limit of normal |
| APRICOT | Acquired immune deficiency syndrome Pegasys Ribabirin International Coinfection Trial |
| HIV | Human immunodeficiency virus |
| ALT | Alanine aminotransferase |
| M2MPGi | Mac-2 binding protein glycosylation isomer |
| TGF | Transforming growth factor |
| MT-MMP | Membranous type-matrix metalloproteinase |
| HBXIP | Hepatitis B virus X-interacting protein |
| SNPs | Single nucleotide polymorphisms |
| TNF | Tissue necrosis factor |
| IGF-1 | Insulin-like growth factor-1 |
| EMT | Epithelial to mesenchymal transition |
| ADAM17 | A disintegrin and metalloproteinase |
References
- Masuzaki, R.; Yoshida, H.; Tateishi, R.; Shiina, S.; Omata, M. Hepatocellular carcinoma in viral hepatitis: Improving standard therapy. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 1137–1151. [Google Scholar] [CrossRef]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Masuzaki, R.; Moriyama, M.; Omata, M. Molecular mechanisms: Connections between nonalcoholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int. J. Mol. Sci. 2020, 21, 1525. [Google Scholar] [CrossRef]
- Yoshida, H.; Shiratori, Y.; Moriyama, M.; Arakawa, Y.; Ide, T.; Sata, M.; Inoue, O.; Yano, M.; Tanaka, M.; Fujiyama, S.; et al. Interferon therapy reduces the risk for hepatocellular carcinoma: National surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann. Intern. Med. 1999, 131, 174–181. [Google Scholar] [CrossRef]
- Dienstag, J.L. The role of liver biopsy in chronic hepatitis C. Hepatology 2002, 36, S152–S160. [Google Scholar]
- Masuzaki, R.; Zhao, S.R.; Csizmadia, E.; Yannas, I.; Karp, S.J. Scar formation and lack of regeneration in adult and neonatal liver after stromal injury. Wound Repair Regen. 2013, 21, 122–130. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Ong, M.F.; Martens, S.; Sarrazin, C.; Bojunga, J.; Zeuzem, S.; Herrmann, E. Performance of Transient Elastography for the Staging of Liver Fibrosis: A Meta-Analysis. Gastroenterology 2008, 134, 960–974. [Google Scholar] [CrossRef]
- Chon, Y.E.; Choi, E.H.; Song, K.J.; Park, J.Y.; Kim, D.Y.; Han, K.H.; Chon, C.Y.; Ahn, S.H.; Kim, S.U. Performance of Transient Elastography for the Staging of Liver Fibrosis in Patients with Chronic Hepatitis B: A Meta-Analysis. PLoS ONE 2012, 7, e44930. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghina, A.M.; Peck-Radosavljevic, M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, H.; Jin, C.; Wang, H.; Jiang, B. The diagnostic accuracy of liver fibrosis in non-viral liver diseases using acoustic radiation force impulse elastography: A systematic review and meta-analysis. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Herrmann, E.; de Lédinghen, V.; Cassinotto, C.; Chu, W.C.-W.; Leung, V.Y.-F.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Venkatesh, S.K.; Wang, Z.; Miller, F.H.; Motosugi, U.; Low, R.N.; Hassanein, T.; Asbach, P.; Godfrey, E.M.; Yin, M.; et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: A systematic review and meta-analysis of individual participant data. Clin. Gastroenterol. Hepatol. 2015, 13, 440–451.e6. [Google Scholar] [CrossRef] [PubMed]
- Lyshchik, A.; Higashi, T.; Asato, R.; Tanaka, S.; Ito, J.; Hiraoka, M.; Insana, M.F.; Brill, A.B.; Saga, T.; Togashi, K. Cervical lymph node metastases: Diagnosis at sonoelastography—Initial experience. Radiology 2007, 243, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Kim, E.K.; Sung, J.M.; Moon, H.J.; Kwak, J.Y. Diagnostic performance of ultrasound and ultrasound elastography with respect to physician experience. Ultrasound Med. Biol. 2014, 40, 854–863. [Google Scholar] [CrossRef]
- Masuzaki, R.; Tateishi, R.; Yoshida, H.; Sato, T.; Ohki, T.; Goto, T.; Yoshida, H.; Sato, S.; Sugioka, Y.; Ikeda, H.; et al. Assessing liver tumor stiffness by transient elastography. Hepatol. Int. 2007, 1, 394–397. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; De Lédinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- De Lédinghen, V.; Hiriart, J.B.; Vergniol, J.; Merrouche, W.; Bedossa, P.; Paradis, V. Controlled Attenuation Parameter (CAP) with the XL Probe of the Fibroscan®: A Comparative Study with the M Probe and Liver Biopsy. Dig. Dis. Sci. 2017, 62, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, R.; Tateishi, R.; Yoshida, H.; Goto, E.; Sato, T.; Ohki, T.; Imamura, J.; Goto, T.; Kanai, F.; Kato, N.; et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009, 49, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.; Lai, C.L.; Seto, W.K.; Wong, D.K.H.; Yuen, M.F. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J. Viral Hepat. 2011, 18, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.S.; Kim, S.U.; Ahn, S.H.; Park, Y.N.; Kim, D.Y.; Park, J.Y.; Chon, C.Y.; Choi, E.H.; Han, K.H. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2011, 53, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Robic, M.A.; Procopet, B.; Métivier, S.; Péron, J.M.; Selves, J.; Vinel, J.P.; Bureau, C. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: A prospective study. J. Hepatol. 2011, 55, 1017–1024. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Yin, M.; Ehman, R.L. Magnetic resonance elastography of liver: Technique, analysis, and clinical applications. J. Magn. Reson. Imaging 2013, 37, 544–555. [Google Scholar] [CrossRef]
- Babu, A.S.; Wells, M.L.; Teytelboym, O.M.; Mackey, J.E.; Miller, F.H.; Yeh, B.M.; Ehman, R.L.; Venkatesh, S.K. Elastography in chronic liver disease: Modalities, techniques, limitations, and future directions. Radiographics 2016, 36, 1987–2006. [Google Scholar] [CrossRef]
- Imbert-Bismut, F.; Ratziu, V.; Pieroni, L.; Charlotte, F.; Benhamou, Y.; Poynard, T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: A prospective study. Lancet 2001, 357, 1069–1075. [Google Scholar] [CrossRef]
- Sporea, I.; Gilja, O.H.; Bota, S.; Sirli, R.; Popescu, A. Liver elastography—An update. Med. Ultrason. 2013, 15, 304–314. [Google Scholar] [CrossRef]
- Poynard, T.; Morra, R.; Halfon, P.; Castera, L.; Ratziu, V.; Imbert-Bismut, F.; Naveau, S.; Thabut, D.; Lebrec, D.; Zoulim, F.; et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007, 7, 40. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Yang, J.; Yan, L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: A systemic review and meta-analysis. Hepatology 2015, 61, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Zhao, Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: A meta-analysis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Ito, K.; Murotani, K.; Nakade, Y.; Inoue, T.; Nakao, H.; Sumida, Y.; Kamada, Y.; Yoneda, M. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein levels and liver fibrosis: A meta-analysis. J. Gastroenterol. Hepatol. 2017, 32, 1922–1930. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef]
- Shiratori, Y.; Imazeki, F.; Moriyama, M.; Yano, M.; Arakawa, Y.; Yokosuka, O.; Kuroki, T.; Nishiguchi, S.; Sata, M.; Yamada, G.; et al. Histologic Improvement of Fibrosis in Patients with Hepatitis C Who Have Sustained Response to Interferon Therapy. Ann. Intern. Med. 2000, 132, 517. [Google Scholar] [CrossRef]
- Minola, E.; Prati, D.; Suter, F.; Maggiolo, F.; Caprioli, F.; Sonzogni, A.; Fraquelli, M.; Paggi, S.; Conte, D. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood 2002, 99, 4588–4591. [Google Scholar] [CrossRef]
- Poynard, T.; Bedossa, P.; Opolon, P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997, 349, 825–832. [Google Scholar] [CrossRef]
- Matsumura, H.; Moriyama, M.; Goto, I.; Tanaka, N.; Okubo, H.; Arakawa, Y. Natural course of progression of liver fibrosis in Japanese patients with chronic liver disease type C—A study of 527 patients at one establishment. J. Viral Hepat. 2000, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, R.; Tateishi, R.; Yoshida, H.; Arano, T.; Uchino, K.; Enooku, K.; Goto, E.; Nakagawa, H.; Asaoka, Y.; Kondo, Y.; et al. Assessment of disease progression in patients with transfusion-associated chronic hepatitis C using transient elastography. World J. Gastroenterol. 2012, 18, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Somerville, R.P.T.; Oblander, S.A.; Apte, S.S. Matrix metalloproteinases: Old dogs with new tricks. Genome Biol. 2003, 4, 216. [Google Scholar] [CrossRef][Green Version]
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1β or TNF-α Release from Human Hepatic Stellate Cells. PLoS ONE 2016, 11, e0153118. [Google Scholar] [CrossRef]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis—A systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Kawelke, N.; Vasel, M.; Sens, C.; von Au, A.; Dooley, S.; Nakchbandi, I.A. Fibronectin protects from excessive liver fibrosis by modulating the availability of and responsiveness of stellate cells to active TGF-β. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Shi, F.; Harman, J.; Fujiwara, K.; Sottile, J. Collagen I matrix turnover is regulated by fibronectin polymerization. Am. J. Physiol. Cell Physiol. 2010, 298, C1265–C1275. [Google Scholar] [CrossRef]
- Altrock, E.; Sens, C.; Wuerfel, C.; Vasel, M.; Kawelke, N.; Dooley, S.; Sottile, J.; Nakchbandi, I.A. Inhibition of fibronectin deposition improves experimental liver fibrosis. J. Hepatol. 2015, 62, 625–633. [Google Scholar] [CrossRef]
- Arthur, M.J.P. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279. [Google Scholar] [CrossRef]
- Iredale, J.P.; Thompson, A.; Henderson, N.C. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim. Biophys. Acta 2013, 1832, 876–883. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Naim, A.; Pan, Q.; Baig, M.S. Matrix Metalloproteinases (MMPs) in Liver Diseases. J. Clin. Exp. Hepatol. 2017, 7, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Lichtinghagen, R.; Bahr, M.J.; Wehmeier, M.; Michels, D.; Haberkorn, C.I.; Arndt, B.; Flemming, P.; Manns, M.P.; Boeker, K.H.W. Expression and coordinated regulation of matrix metalloproteinases in chronic hepatitis C and hepatitis C virus-induced liver cirrhosis. Clin. Sci. (Lond.) 2003, 105, 373–382. [Google Scholar] [CrossRef]
- Iimuro, Y.; Nishio, T.; Morimoto, T.; Nitta, T.; Stefanovic, B.; Choi, S.K.; Brenner, D.A.; Yamaoka, Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology 2003, 124, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Harty, M.W.; Huddleston, H.M.; Papa, E.F.; Puthawala, T.; Tracy, A.P.; Ramm, G.A.; Gehring, S.; Gregory, S.H.; Tracy, T.F. Repair after cholestatic liver injury correlates with neutrophil infiltration and matrix metalloproteinase 8 activity. Surgery 2005, 138, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Siller-López, F.; Sandoval, A.; Salgado, S.; Salazar, A.; Bueno, M.; Garcia, J.; Vera, J.; Gálvez, J.; Hernández, I.; Ramos, M.; et al. Treatment with Human Metalloproteinase-8 Gene Delivery Ameliorates Experimental Rat Liver Cirrhosis. Gastroenterology 2004, 126, 1122–1133. [Google Scholar] [CrossRef]
- Prystupa, A.; Boguszewska-Czubara, A.; Bojarska-Junak, A.; Toruń-Jurkowska, A.; Roliński, J.; Załuska, W. Activity of MMP-2, MMP-8 and MMP-9 in serum as a marker of progression of alcoholic liver disease in people from Lublin Region, eastern Poland. Ann. Agric. Environ. Med. 2015, 22, 325–328. [Google Scholar] [CrossRef]
- Hattori, N.; Mochizuki, S.; Kishi, K.; Nakajima, T.; Takaishi, H.; D’Armiento, J.; Okada, Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 2009, 175, 533–546. [Google Scholar] [CrossRef]
- Prystupa, A.; Szpetnar, M.; Boguszewska-Czubara, A.; Grzybowski, A.; Grzybowski, A.; Sak, J.; Sak, J.; Załuska, W. Activity of MMP1 and MMP13 and amino acid metabolism in patients with alcoholic liver cirrhosis. Med. Sci. Monit. 2015, 21, 1008–1014. [Google Scholar]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4 and 1/4 -length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.L.; Atkinson, S.J.; Knäuper, V.; Murphy, G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001, 503, 158–162. [Google Scholar] [CrossRef]
- Radbill, B.D.; Gupta, R.; Ramirez, M.C.M.; DiFeo, A.; Martignetti, J.A.; Alvarez, C.E.; Friedman, S.L.; Narla, G.; Vrabie, R.; Bowles, R.; et al. Loss of matrix metalloproteinase-2 amplifies murine toxin-induced liver fibrosis by upregulating collagen I expression. Dig. Dis. Sci. 2011, 56, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, I.; Kakinuma, S.; Kamiya, A.; Miyoshi, M.; Sakamoto, N.; Kiyohashi, K.; Watanabe, T.; Funaoka, Y.; Ueyama, M.; Nakagawa, M.; et al. Cholestatic liver fibrosis and toxin-induced fibrosis are exacerbated in matrix metalloproteinase-2 deficient mice. Biochem. Biophys. Res. Commun. 2011, 406, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Murphy, F.R.; Gehdu, N.; Zhang, J.; Iredale, J.P.; Benyon, R.C. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J. Biol. Chem. 2004, 279, 23996–24006. [Google Scholar] [CrossRef] [PubMed]
- Roderfeld, M.; Rath, T.; Lammert, F.; Dierkes, C.; Graf, J.; Roeb, E. Innovative immunohistochemistry identifies MMP-9 expressing macrophages at the invasive front of murine HCC. World J. Hepatol. 2010, 2, 175–179. [Google Scholar] [CrossRef]
- Nart, D.; Yaman, B.; Yilmaz, F.; Zeytunlu, M.; Karasu, Z.; Kiliç, M. Expression of matrix metalloproteinase-9 in predicting prognosis of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2010, 16, 621–630. [Google Scholar] [CrossRef]
- Hori, T.; Uemoto, S.; Walden, L.B.; Chen, F.; Baine, A.-M.T.; Hata, T.; Kogure, T.; Nguyen, J.H. Matrix metalloproteinase-9 as a therapeutic target for the progression of fulminant liver failure with hepatic encephalopathy: A pilot study in mice. Hepatol. Res. 2014, 44, 651–662. [Google Scholar] [CrossRef]
- Suzuki, K.; Morodomi, T.; Nagase, H.; Enghild, J.J.; Salvesen, G. Mechanisms of Activation of Tissue Procollagenase by Matrix Metalloproteinase 3 (Stromelysin). Biochemistry 1990, 29, 10261–10270. [Google Scholar] [CrossRef]
- Bodey, B.; Bodey, J.B.; Siegel, S.E.; Kaiser, H.E. Immunocytochemical detection of MMP-3 and -10 expression in hepatocellular carcinomas. Anticancer Res. 2000, 20, 4585–4590. [Google Scholar]
- Murawaki, Y.; Ikuta, Y.; Okamoto, K.; Koda, M.; Kawasaki, H. Serum matrix metalloproteinase-3 (stromelysin-1) concentration in patients with chronic liver disease. J. Hepatol. 1999, 31, 474–481. [Google Scholar] [CrossRef]
- Lefebvre, O.; Régnier, C.; Chenard, M.P.; Wendling, C.; Chambon, P.; Basset, P.; Rio, M.C. Developmental expression of mouse stromelysin-3 mRNA. Development 1995, 121, 947–955. [Google Scholar] [PubMed]
- Rouyer, N.; Wolf, C.; Chenard, M.P.; Rio, M.C.; Chambon, P.; Bellocq, J.P.; Basset, P. Stromelysin-3 gene expression in human cancer: An overview. Invasion Metastasis 1994, 14, 269–275. [Google Scholar] [PubMed]
- Wang, B.; Hsu, C.J.; Lee, H.L.; Chou, C.H.; Su, C.M.; Yang, S.F.; Tang, C.H. Impact of matrix metalloproteinase-11 gene polymorphisms upon the development and progression of hepatocellular carcinoma. Int. J. Med. Sci. 2018, 15, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Uría, J.A.; López-Otín, C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000, 60, 4745–4751. [Google Scholar] [PubMed]
- Park, H.I.; Ni, J.; Gerkema, F.E.; Liu, D.; Belozerov, V.E.; Sang, Q.X. Identification and characterization of human endometase (Matrix metalloproteinase-26) from endometrial tumor. J. Biol. Chem. 2000, 275, 20540–20544. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chuang, J.-H.; Chou, M.-H.; Wu, C.-L.; Chen, C.-M.; Wang, C.-C.; Chen, Y.-S.; Chen, C.-L.; Tai, M.-H. Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod. Pathol. 2005, 18, 941–950. [Google Scholar] [CrossRef]
- Zeng, Z.-S.; Shu, W.-P.; Cohen, A.M.; Guillem, J.G. Matrix metalloproteinase-7 expression in colorectal cancer liver metastases: Evidence for involvement of MMP-7 activation in human cancer metastases. Clin. Cancer Res. 2002, 8, 144–148. [Google Scholar]
- Zhao, Y.-G.; Xiao, A.-Z.; Newcomer, R.G.; Park, H.I.; Kang, T.; Chung, L.W.; Swanson, M.G.; Zhau, H.E.; Kurhanewicz, J.; Sang, Q.-X.A. Activation of pro-gelatinase B by endometase/matrilysin-2 promotes invasion of human prostate cancer cells. J. Biol. Chem. 2003, 278, 15056–15064. [Google Scholar] [CrossRef]
- Ohuchi, E.; Imai, K.; Fujii, Y.; Sato, H.; Seiki, M.; Okada, Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997, 272, 2446–2451. [Google Scholar] [CrossRef]
- Harada, T.; Arii, S.; Mise, M.; Imamura, T.; Higashitsuji, H.; Furutani, M.; Niwano, M.; Ishigami, S.I.; Fukumoto, M.; Seiki, M.; et al. Membrane-type matrix metalloproteinase-1(MT1-MMP) gene is overexpressed in highly invasive hepatocellular carcinomas. J. Hepatol. 1998, 28, 231–239. [Google Scholar] [CrossRef]
- Pepper, M.S. Extracellular proteolysis and angiogenesis. Thromb. Haemost. 2001, 86, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wu, H.; Wang, F.; Lv, J.; Lu, J.; Fang, Q.; Wang, F.; Lu, Y.; Zhang, S.; Xu, Y.; et al. The oncoprotein HBXIP facilitates metastasis of hepatocellular carcinoma cells by activation of MMP15 expression. Cancer Manag. Res. 2019, 11, 4529–4540. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal. Cell. Pathol. 2019, 2019. [Google Scholar] [CrossRef]
- Truong, A.; Yip, C.; Paye, A.; Blacher, S.; Munaut, C.; Deroanne, C.; Noel, A.; Sounni, N.E. Dynamics of internalization and recycling of the prometastatic membrane type 4 matrix metalloproteinase (MT4-MMP) in breast cancer cells. FEBS J. 2016, 283, 704–722. [Google Scholar] [CrossRef]
- Sekine-Aizawa, Y.; Hama, E.; Watanabe, K.; Tsubuki, S.; Kanai-Azuma, M.; Kanai, Y.; Arai, H.; Aizawa, H.; Iwata, N.; Takaomi, C.S. Matrix metalloproteinase (MMP) system in brain: Identification and characterization of brain-specific MMP highly expressed in cerebellum. Eur. J. Neurosci. 2001, 13, 935–948. [Google Scholar] [CrossRef]
- Pei, D. Leukolysin/MMP25/MT6-MMP: A novel matrix metalloproteinase specifically expressed in the leukocyte lineage. Cell Res. 1999, 9, 291–303. [Google Scholar] [CrossRef]
- Velasco, G.; Cal, S.; Merlos-Suárez, A.; Ferrando, A.A.; Alvarez, S.; Nakano, A.; Arribas, J.; López-Otín, C. Human MT6-matrix metalloproteinase: Identification, progelatinase A activation, and expression in brain tumors. Cancer Res. 2000, 60, 877–882. [Google Scholar]
- Shapiro, S.D.; Kobayashi, D.K.; Ley, T.J. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J. Biol. Chem. 1993, 268, 23824–23829. [Google Scholar]
- Shipley, J.M.; Wesselschmidt, R.L.; Kobayashi, D.K.; Ley, T.J.; Shapiro, S.D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 3942–3946. [Google Scholar] [CrossRef]
- Pendás, A.M.; Knäuper, V.; Puente, X.S.; Llano, E.; Mattei, M.G.; Apte, S.; Murphy, G.; López-Otín, C. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J. Biol. Chem. 1997, 272, 4281–4286. [Google Scholar] [CrossRef]
- Kolb, C.; Mauch, S.; Peter, H.H.; Krawinkel, U.; Sedlacek, R. The matrix metalloproteinase RASI-1 is expressed in synovial blood vessels of a rheumatoid arthritis patient. Immunol. Lett. 1997, 57, 83–88. [Google Scholar] [CrossRef]
- Jirouskova, M.; Zbodakova, O.; Gregor, M.; Chalupsky, K.; Sarnova, L. Hepatoprotective Effect of MMP-19 Deficiency in a Mouse Model of Chronic Liver Fibrosis. PLoS ONE 2012, 7, 46271. [Google Scholar] [CrossRef]
- Li, W.; Gibson, C.W.; Abrams, W.R.; Andrews, D.W.; DenBesten, P.K. Reduced hydrolysis of amelogenin may result in X-linked amelogenesis imperfecta. Matrix Biol. 2001, 19, 755–760. [Google Scholar] [CrossRef]
- Yang, M.; Kurkinen, M. Cloning and characterization of a novel matrix metalloproteinase (MMP), CMMP, from chicken embryo fibroblasts. CMMP, Xenopus XMMP, and human MMP19 have a conserved unique cysteine in the catalytic domain. J. Biol. Chem. 1998, 273, 17893–17900. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Pendás, A.M.; Fueyo, A.; Knäuper, V.; Murphy, G.; López-Otín, C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J. Biol. Chem. 1999, 274, 4570–4576. [Google Scholar] [CrossRef]
- Pei, D.; Kang, T.; Qi, H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J. Biol. Chem. 2000, 275, 33988–33997. [Google Scholar] [CrossRef] [PubMed]
- Lohi, J.; Wilson, C.L.; Roby, J.D.; Parks, W.C. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J. Biol. Chem. 2001, 276, 10134–10144. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, G.N.; Strongin, A.Y. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene 2001, 265, 87–93. [Google Scholar] [CrossRef]
- Saarialho-Kere, U.; Kerkelä, E.; Jahkola, T.; Suomela, S.; Keski-Oja, J.; Lohi, J. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J. Investig. Dermatol. 2002, 119, 14–21. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, X.; Feng, M.; Mo, Z.; Shan, Y.; Wang, Y.; Jin, J. Upregulated MMP28 in Hepatocellular Carcinoma Promotes Metastasis via Notch3 Signaling and Predicts Unfavorable Prognosis. Int. J. Biol. Sci 2019, 15, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 3–19. [Google Scholar] [CrossRef]
- Allan, J.A.; Docherty, A.J.P.; Barker, P.J.; Huskisson, N.S.; Reynolds, J.J.; Murphy, G. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem. J. 1995, 309, 299–306. [Google Scholar] [CrossRef] [PubMed]
- El-Gindy, I.; El Rahman, A.T.A.; El-Alim, M.A.; Zaki, S.S.A. Diagnostic potential of serum matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 as non-invasive markers of hepatic fibrosis in patients with HCV related chronic liver disease. Egypt. J. Immunol. 2003, 10, 27–35. [Google Scholar]
- Yata, Y.; Takahara, T.; Furui, K.; Zhang, L.P.; Jin, B.; Watanabe, A. Spatial distribution of tissue inhibitor of metalloproteinase-1 mRNA in chronic liver disease. J. Hepatol. 1999, 30, 425–432. [Google Scholar] [CrossRef]
- Ruiz, V.; Ordóñez, R.M.; Berumen, J.; Ramírez, R.; Uhal, B.; Becerril, C.; Pardo, A.; Selman, M. Unbalanced collagenases/TIMP-1 expression and epithelial apoptosis in experimental lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L1026–L1036. [Google Scholar] [CrossRef]
- Selman, M.; Ruiz, V.; Cabrera, S.; Segura, L.; Ramírez, R.; Barrios, R.; Pardo, A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279. [Google Scholar] [CrossRef]
- Johnson, T.S.; Haylor, J.L.; Thomas, G.L.; Fisher, M.; El Nahas, A.M. Matrix metalloproteinases and their inhibitions in experimental renal scarring. Exp. Nephrol. 2002, 10, 182–195. [Google Scholar] [CrossRef]
- Hörstrup, J.H.; Gehrmann, M.; Schneider, B.; Plöger, A.; Froese, P.; Schirop, T.; Kampf, D.; Frei, U.; Neumann, R.; Eckardt, K.-U. Elevation of serum and urine levels of TIMP-1 and tenascin in patients with renal disease. Nephrol. Dial. Transpl. 2002, 17, 1005–1013. [Google Scholar] [CrossRef]
- Phillips, P.A.; McCarroll, J.A.; Park, S.; Wu, M.J.; Pirola, R.; Korsten, M.; Wilson, J.S.; Apte, M.V. Rat pancreatic stellate cells secrete matrix metalloproteinases: Implications for extracellular matrix turnover. Gut 2003, 52, 275–282. [Google Scholar] [CrossRef]
- Ishihara, T.; Hayasaka, A.; Yamaguchi, T.; Kondo, F.; Saisho, H. Immunohistochemical study of transforming growth factor-beta 1, matrix metalloproteinase-2,9, tissue inhibitors of metalloproteinase-1,2, and basement membrane components at pancreatic ducts in chronic pancreatitis. Pancreas 1998, 17, 412–418. [Google Scholar] [CrossRef]
- Böker, K.H.; Pehle, B.; Steinmetz, C.; Breitenstein, K.; Bahr, M.; Lichtinghagen, R. Tissue inhibitors of metalloproteinases in liver and serum/plasma in chronic active hepatitis C and HCV-induced cirrhosis. Hepatogastroenterology 2000, 47, 812–819. [Google Scholar]
- Lichtinghagen, R.; Michels, D.; Haberkorn, C.I.; Arndt, B.; Bahr, M.; Flemming, P.; Manns, M.P.; Boeker, K.H.W. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J. Hepatol. 2001, 34, 239–247. [Google Scholar] [CrossRef]
- Kossakowska, A.E.; Edwards, D.R.; Lee, S.S.; Urbanski, L.S.; Stabbler, A.L.; Zhang, C.L.; Phillips, B.W.; Zhang, Y.; Urbanski, S.J. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am. J. Pathol. 1998, 153, 1895–1902. [Google Scholar] [CrossRef]
- Wang, Z.; Juttermann, R.; Soloway, P.D. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. 2000, 275, 26411–26415. [Google Scholar] [CrossRef]
- Kandalam, V.; Basu, R.; Abraham, T.; Wang, X.; Soloway, P.D.; Jaworski, D.M.; Oudit, G.Y.; Kassiri, Z. TIMP2 Deficiency Accelerates Adverse Post–Myocardial Infarction Remodeling Because of Enhanced MT1-MMP Activity Despite Lack of MMP2 Activation. Circ. Res. 2010, 106, 796–808. [Google Scholar] [CrossRef]
- Mohammed, F.F.; Smookler, D.S.; Taylor, S.E.M.; Fingleton, B.; Kassiri, Z.; Sanchez, O.H.; English, J.L.; Matrisian, L.M.; Au, B.; Yeh, W.C.; et al. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat. Genet. 2004, 36, 969–977. [Google Scholar] [CrossRef]
- Takawale, A.; Fan, D.; Basu, R.; Shen, M.; Parajuli, N.; Wang, W.; Wang, X.; Oudit, G.Y.; Kassiri, Z. Myocardial recovery from ischemia-reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circ. Heart Fail. 2014, 7, 652–662. [Google Scholar] [CrossRef]
- Nakchbandi, I.A.; van der Merwe, S.W. Current understanding of osteoporosis associated with liver disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 660–670. [Google Scholar] [CrossRef]
- Kawelke, N.; Bentmann, A.; Hackl, N.; Hager, H.D.; Feick, P.; Geursen, A.; Singer, M.V.; Nakchbandi, I.A. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J. Bone Miner. Res. 2008, 23, 1278–1286. [Google Scholar] [CrossRef]
- Hackl, N.J.; Bersch, C.; Feick, P.; Antoni, C.; Franke, A.; Singer, M.V.; Nakchbandi, I.A. Circulating fibronectin isoforms predict the degree of fibrosis in chronic hepatitis C. Scand. J. Gastroenterol. 2010, 45, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Ikehara, Y.; Tanaka, Y.; Ito, K.; Matsuda, A.; Sekiya, S.; Hige, S.; Sakamoto, M.; Kage, M.; Mizokami, M.; et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013, 3, 1065. [Google Scholar] [CrossRef]
- Narimatsu, H. Development of M2BPGi: A novel fibrosis serum glyco-biomarker for chronic hepatitis/cirrhosis diagnostics. Expert Rev. Proteom. 2015, 12, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Baudi, I.; Inoue, T.; Tanaka, Y. Novel biomarkers of hepatitis B and hepatocellular carcinoma: Clinical significance of HBcrAg and M2BPGI. Int. J. Mol. Sci. 2020, 21, 949. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; Yoh, K.; et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein for patients with chronic hepatitis B and C: A comparative study. J. Viral Hepat. 2016, 23, 977–984. [Google Scholar] [CrossRef] [PubMed]

| Elastography | Etiology | F2 | F3 | F4 | Reference | |
|---|---|---|---|---|---|---|
| One-dimensional Ultrasound (Transient elastography) | Various etiologies | Cutoff (kPa) | 7.65 | N/A | 13.01 | [10] |
| Sensitivity | N/A | N/A | N/A | |||
| Specificity | N/A | N/A | N/A | |||
| AUROC | 0.84 | 0.89 | 0.94 | |||
| HBV | Cutoff (kPa) | 7.9 | 8.8 | 11.7 | [11] | |
| Sensitivity | 74.3 | 74.0 | 84.6 | |||
| Specificity | 78.3 | 63.8 | 81.5 | |||
| AUROC | 0.859 | 0.887 | 0.929 | |||
| Point shear wave Ultrasound | Various etiologies | Cutoff (m/s) | 1.31 | N/A | 1.80 | [12] |
| Sensitivity | 74 | N/A | 87 | |||
| Specificity | 83 | N/A | 87 | |||
| AUROC | 0.85 | N/A | 0.93 | |||
| Nonviral | Cutoff (m/s) | N/A | N/A | N/A | [13] | |
| Sensitivity | 79 | 92 | 89 | |||
| Specificity | 81 | 85 | 89 | |||
| AUROC | 0.87 | 0.94 | ||||
| Two dimensional Ultrasound | HCV | Cutoff (kPa) | 7.1 | 9.2 | 13.0 | [14] |
| Sensitivity | 94.7 | 90.3 | 85.8 | |||
| Specificity | 52.0 | 76.8 | 87.8 | |||
| AUROC | 0.863 | 0.915 | 0.929 | |||
| HBV | Cutoff (kPa) | 7.1 | 8.1 | 11.5 | ||
| Sensitivity | 87.6 | 94.9 | 79.9 | |||
| Specificity | 73.6 | 73.1 | 93.3 | |||
| AUROC | 0.906 | 0.931 | 0.955 | |||
| NAFLD | Cutoff (kPa) | 7.1 | 9.2 | 13.0 | ||
| Sensitivity | 93.8 | 93.1 | 75.3 | |||
| Specificity | 52.0 | 80.9 | 87.8 | |||
| AUROC | 0.855 | 0.928 | 0.917 | |||
| Others | Cutoff (kPa) | 7.1 | 9.2 | 13.0 | ||
| Sensitivity | 94.8 | 95.1 | 79.4 | |||
| Specificity | 39.9 | 86.6 | 83.6 | |||
| AUROC | N/A | N/A | N/A | |||
| MRI elastography | Various etiologies | Cutoff (kPa) | 3.66 | 4.11 | 4.71 | [15] |
| Sensitivity | 79 | 85 | 91 | |||
| Specificity | 81 | 85 | 81 | |||
| AUROC | 0.88 | 0.93 | 0.92 |
| Elastography | Technique | Advantages | Disadvantages | References |
|---|---|---|---|---|
| US elastography | Static strain imaging | Real-time imaging with elastogram which can distinguish a tumor from background tissue. | Variability due to inconsistent compression (heartbeat). Semi-quantification | [16,17] |
| 1D transient elastography | The most widely used and validated. | Needs special equipment. Lacking B-mode | [10,11,18,19,20,21,22,23,24,25] | |
| Point shear wave elastography | Controllable ROI | Small ROI. Needs high-end US apparatus | [12,13] | |
| 2D shear wave elastography | Controllable ROI. Real-time imaging | Needs high-end US apparatus | [14] | |
| MRI elastography | Assessment of whole liver | Needs special equipment Not indicated to patients with claustrophobia | [26,27,28] |
| Factors | Etiology | F2 | F3 | F4 | Reference | ||
|---|---|---|---|---|---|---|---|
| FibroTest | α2-macroglobulin, haptoglobin, GGT, γ-globulin, total bilirubin, and apolipoprotein A1 | HCV | AUROC | 0.66 | 0.66 | 0.66 | [30] |
| HBV | AUROC | 0.63 | 0.78 | 0.54 | |||
| ALD | AUROC | 0.65 | 0.66 | 0.82 | |||
| NAFLD | AUROC | 0.69 | 0.69 | 0.71 | |||
| APRI | AST, platelet count | HCV | Cutoff | 0.7 | 1.0 | 2.0 | [31] |
| Sensitivity | 77 | 61 | 46 | ||||
| Specificity | 72 | 64 | 91 | ||||
| AUROC | 0.77 | 0.80 | 0.83 | ||||
| HBV | Cutoff | 0.5 | 1.0 | 1.5 | [32] | ||
| Sensitivity | 70 | 50 | 36.9 | ||||
| Specificity | 60 | 83 | 92.5 | ||||
| AUROC | 0.72 | 0.76 | 0.72 | ||||
| FIB-4 Index | Age, AST, ALT, platelet count | HCV | Cutoff | 3.25 | [33] (single study) | ||
| Sensitivity | 23 | ||||||
| Specificity | 97 | ||||||
| AUROC | 0.737 | ||||||
| HBV | Cutoff | 0.8–1.085 | 1.45–1.65 | 2.9–3.6 | [34] | ||
| Sensitivity | 73 | 68 | 42 | ||||
| Specificity | 62 | 75 | 96 | ||||
| AUROC | 0.73 | 0.77 | 0.96 | ||||
| M2BPGi | Various etiology | Cutoff | 0.90–1.42 | 0.94–3.70 | 1.26–4.62 | [35] | |
| Sensitivity | 69 | 76 | 82 | ||||
| Specificity | 78 | 76 | 84 | ||||
| AUROC | N/A | N/A | N/A |
| MMP Classification | Type | Aliases | Pathology | References |
|---|---|---|---|---|
| Collagenases | MMP-1 | Interstitial collagenase | ECM degradation | [53,54,55] |
| MMP-8 | Neutrophil collagenase | Fibrosis attenuation | [56,57,58] | |
| MMP-13 | Collagenase 3 | Promote TGF-β1 activation | [59,60] | |
| Gelatinases | MMP-2 | Gelatinase A | Suppress collagen I expression | [61,62,63,64] |
| MMP-9 | Gelatinase B | Promote apoptosis of HSCs | [65,66,67,68] | |
| Stromelysins | MMP-3 | Stromelysin-1 | ECM degradation. Activate pro-MMPs | [69,70,71] |
| MMP-10 | Stromelysin-2, Transin-2 | Found in HCC | [72,73] | |
| MMP-11 | Stomelysin-3 | Tumor migration, invasion, metastasis | [74] | |
| Matrilysins | MMP-7 | Matrilysin-1, Pump-1, Uterine metalloproteinase | Activated in biliary atresia related liver fibrosis | [75,76,77] |
| MMP-26 | Matrilysin-2, Endometase | ECM degradation and activates MMP-9 | [75,78,79] | |
| Membranous Type | MMP-14 | MT1-MMP | Angiogenesis and activates MMP-2 | [80,81,82] |
| MMP-15 | MT2-MMP | Cell migration and invasion | [83] | |
| MMP-16 | MT3-MMP | Cell invasion and metastases | [84] | |
| MMP-17 | MT4-MMP | Expressed in breast cancer cells | [85] | |
| MMP-24 | MT5-MMP | Brain specific | [86] | |
| MMP-25 | MT6-MMP | Expressed in peripheral blood leukocytes | [87,88] | |
| Others | MMP-12 | Macrophage elastase | Macrophage migration | [89,90] |
| MMP-19 | RASI-1 | Destruction and development of hepatic basement membrane | [91,92,93] | |
| MMP-20 | Enamelysin | Degrades amelogenin | [94] | |
| MMP-22 | N/A | Cloned from chicken fibroblast | [95] | |
| MMP-23 | Femalysin | Expressed in reproductive tissues | [96,97] | |
| MMP-28 | Epilysin | Degrades casein. Promotes EMT, migration and invasion of HCC cells. | [98,99,100,101] |
| TIMP Classification | Pathology | References |
|---|---|---|
| TIMP1 | Inhibition of collagenase Inhibition of activation of pro-MMPs Inhibition of programmed cell death of HSCs | [104,105,106,107,108,109,110,111] |
| TIMP2 | Inhibition of MT1-MMP, MMP-2 Activation of pro-MMP2 | [112,113,114,115,116] |
| TIMP3 | Promotion of apoptosis Regulation of inflammation through inhibition of ADAM17 | [117] |
| TIMP4 | Inhibition of MT1-MMP | [118] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuzaki, R.; Kanda, T.; Sasaki, R.; Matsumoto, N.; Ogawa, M.; Matsuoka, S.; Karp, S.J.; Moriyama, M. Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives. Int. J. Mol. Sci. 2020, 21, 4906. https://doi.org/10.3390/ijms21144906
Masuzaki R, Kanda T, Sasaki R, Matsumoto N, Ogawa M, Matsuoka S, Karp SJ, Moriyama M. Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives. International Journal of Molecular Sciences. 2020; 21(14):4906. https://doi.org/10.3390/ijms21144906
Chicago/Turabian StyleMasuzaki, Ryota, Tatsuo Kanda, Reina Sasaki, Naoki Matsumoto, Masahiro Ogawa, Shunichi Matsuoka, Seth J. Karp, and Mitsuhiko Moriyama. 2020. "Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives" International Journal of Molecular Sciences 21, no. 14: 4906. https://doi.org/10.3390/ijms21144906
APA StyleMasuzaki, R., Kanda, T., Sasaki, R., Matsumoto, N., Ogawa, M., Matsuoka, S., Karp, S. J., & Moriyama, M. (2020). Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives. International Journal of Molecular Sciences, 21(14), 4906. https://doi.org/10.3390/ijms21144906







