Effect of Heat Stress on Seed Protein Composition and Ultrastructure of Protein Storage Vacuoles in the Cotyledonary Parenchyma Cells of Soybean Genotypes That Are Either Tolerant or Sensitive to Elevated Temperatures
Abstract
1. Introduction
2. Results
2.1. Accumulation of Lipoxygenase, the β-Subunit of β-Conglycinin and Bowman-Birk Protease Inhibitor in Soybean Seeds Is Negatively Impacted by Heat Stress
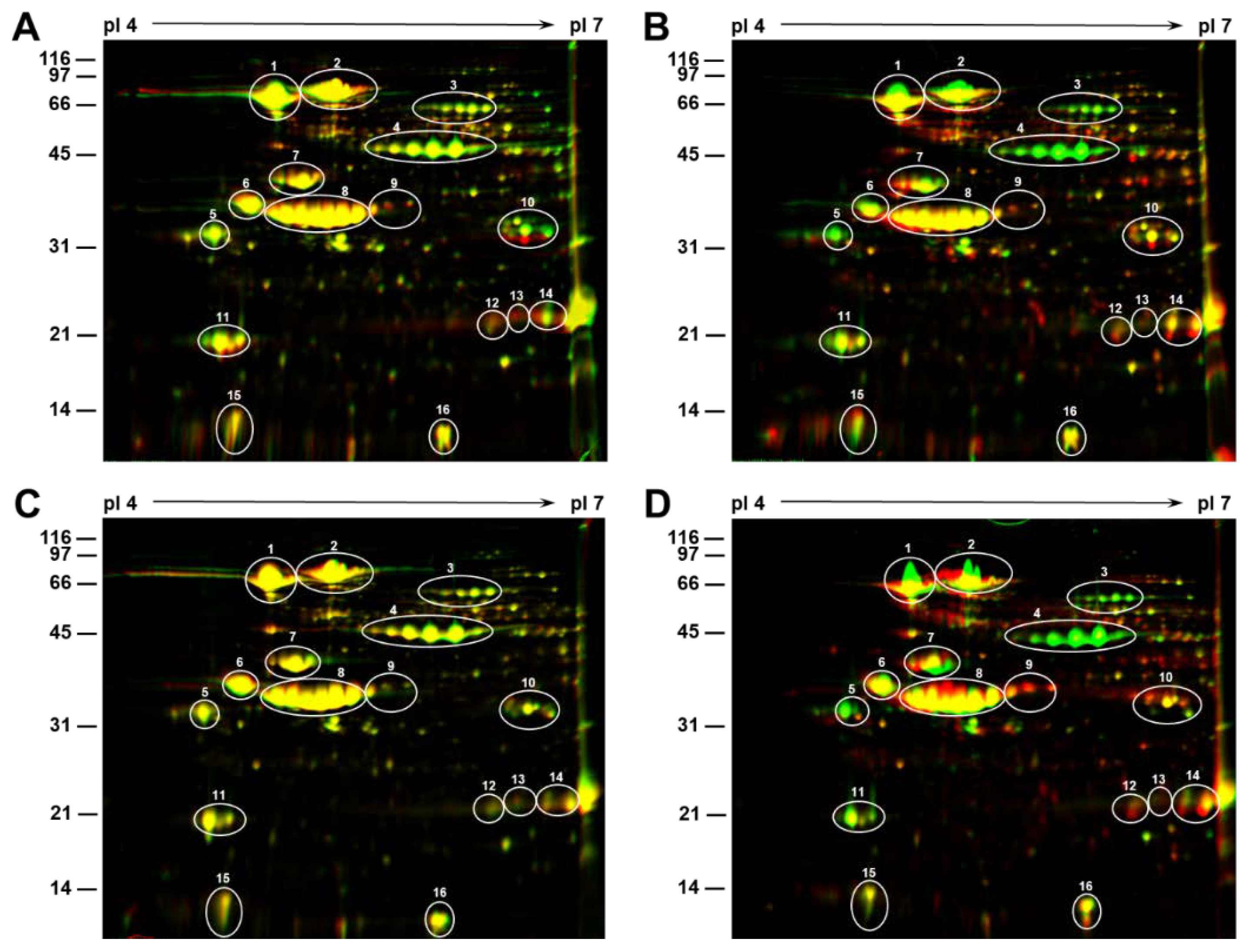
2.2. 2D-Gel Electrophoresis Reveals the Negative Effect of Heat Stress on Seed Storage Protein Accumulation
2.3. Effect of Heat Stress on the Accumulation of HSP70, HSP17.6 and BiP in Seeds of Heat-Tolerant and Heat-Sensitive Genotypes
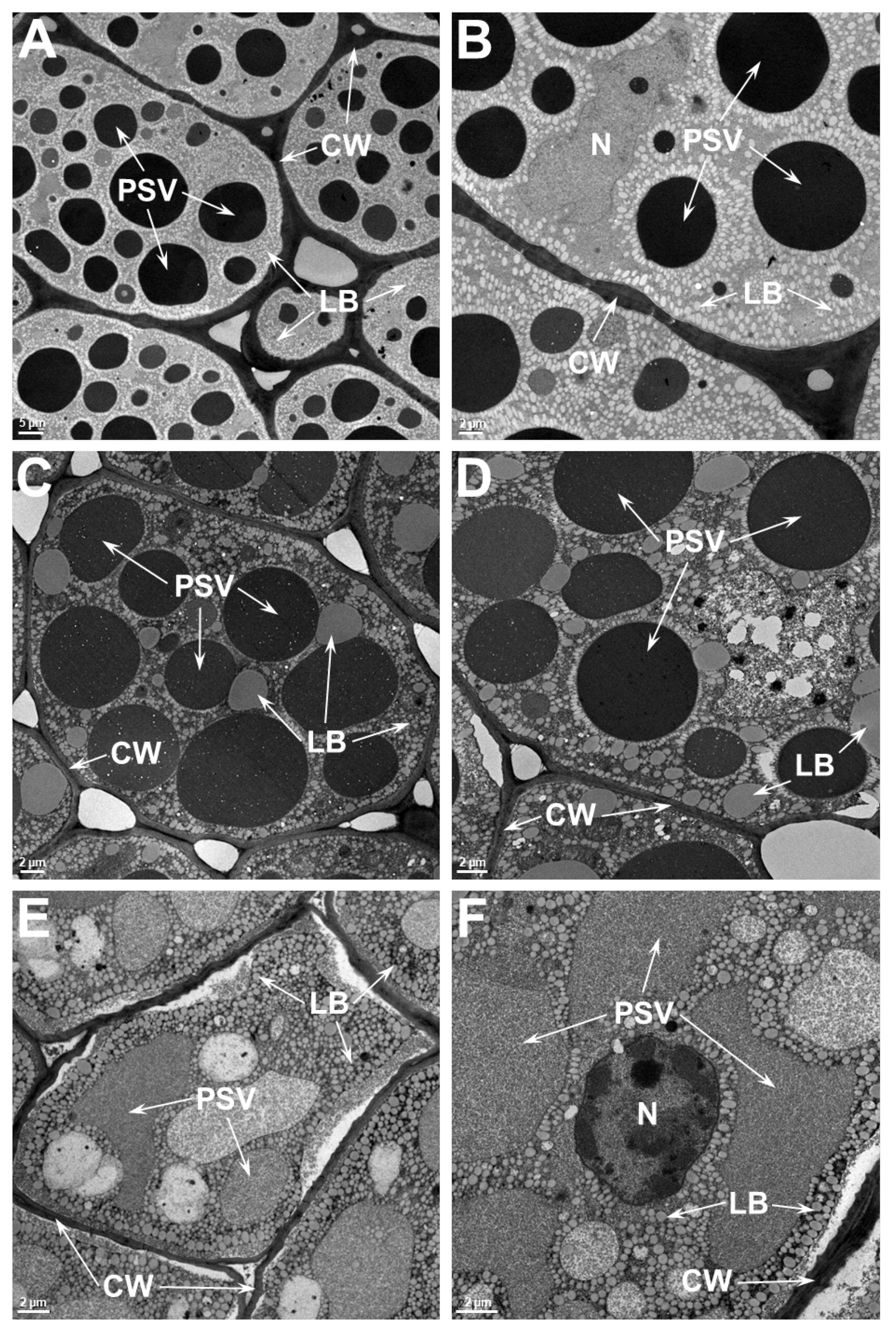
2.4. Ultrastructure Examination of Soybean Cotyledonary Cells Show That Heat-Tolerant Genotype Maintains Cellular Integrity Superior to the Heat-Sensitive Genotype at Elevated Temperatures
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. 1D and 2D Electrophoresis
4.3. Immunoblot Analysis
4.4. Transmission Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HSP | Heat shock protein |
| PSV | Protein storage vacuoles |
| TG | Soybean genotype DS25-1, tolerant to elevated temperatures during seed fill |
| SG | Soybean genotype DT97-4290, sensitive to elevated temperatures during seed fill |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| LOX | Lipoxygenase |
| KTI | Kunitz Trypsin inhibitor |
| BBI | Bowman-Birk inhibitor |
References
- Hymowitz, T.; Harlan, J.R. Introduction of soybean to North America by Samuel Bowen in 1765. Econ. Bot. 1983, 37, 371–379. [Google Scholar] [CrossRef]
- Wilson, R.F. Soybeans: Improvement, Production, and Uses, 3rd ed.; American Society of Agronomy: Madison, WI, USA, 2004; pp. 621–677, Seed Composition. [Google Scholar]
- Heatherly, L.G.; Spurlock, S.R. Yield and economics of traditional and early soybean production system (ESPS) seedings in the midsouthern United States. Field Crops Res. 1999, 63, 35–45. [Google Scholar] [CrossRef]
- Fukushima, A.; Kusano, M.; Redestig, H.; Arita, M.; Saito, K. Integrated omics approaches in plant systems biology Curr. Opin. Chem. Biol. 2009, 13, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Zhu, Y.; Jones, A.; Rose, R.J.; Song, Y. Heat Stress in Legume Seed Setting: Effects, Causes, and Future Prospects. Front. Plant Sci. 2019, 10, 938. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate Impacts on Agriculture: Implications for Crop Production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Heatherly, L.G. Yield and Germinability of Seed from Irrigated and Nonirrigated Early- and Late-Planted MG IV and V Soybean. Crop Sci. 1996, 36, 1000–1006. [Google Scholar] [CrossRef]
- Smith, J.R.; Mengistu, A.; Nelson, R.L.; Paris, R.L. Identification of Soybean Accessions with High Germinability in High-Temperature Environments. Crop Sci. 2008, 48, 2279–2288. [Google Scholar] [CrossRef]
- Gillman, J.; Biever, J.J.; Ye, S.; Spollen, W.G.; Givan, S.A.; Lyu, Z.; Joshi, T.; Smith, J.R.; Fritschi, F.B. A seed germination transcriptomic study contrasting two soybean genotypes that differ in terms of their tolerance to the deleterious impacts of elevated temperatures during seed fill. BMC Res. Notes 2019, 12, 522. [Google Scholar] [CrossRef]
- Smith, J.R.; Gillman, J.D.; Bellaloui, N.; Gillen, A.; Ray, J.D. Soybean Germplasm Line DS25-1 with Heat Tolerance and Competitive Yield under Heat Stress; USDA-ARS: Columbia, MO, USA, 2017.
- Chebrolu, K.K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 2016, 12, 28. [Google Scholar] [CrossRef]
- Narayanan, S.; Zoong-Lwe, Z.S.; Gandhi, N.; Welti, R.; Fallen, B.; Smith, J.R.; Rustgi, S. Comparative Lipidomic Analysis Reveals Heat Stress Responses of Two Soybean Genotypes Differing in Temperature Sensitivity. Plants 2020, 9, 457. [Google Scholar] [CrossRef]
- Krishnan, H.B. Preparative Procedures Markedly Influence the Appearance and Structural Integrity of Protein Storage Vacuoles in Soybean Seeds. J. Agric. Food Chem. 2008, 56, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.; Larkins, B.A. Protein Storage Bodies and Vacuoles. Plant Cell 1999, 11, 601. [Google Scholar] [CrossRef]
- Bair, C.W.; Snyder, H.E. Electron microscopy of soybean lipid bodies. J. Am. Oil Chem. Soc. 1980, 57, 279–282. [Google Scholar] [CrossRef]
- Ren, C.; Bilyeu, K.; Beuselinck, P.R. Composition, Vigor, and Proteome of Mature Soybean Seeds Developed under High Temperature. Crop Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Nakagawa, A.C.S.; Ario, N.; Tomita, Y.; Tanaka, S.; Murayama, N.; Mizuta, C.; Iwaya-Inoue, M.; Ishibashi, Y. High temperature during soybean seed development differentially alters lipid and protein metabolism. Plant Prod. Sci. 2020, 1–9. [Google Scholar] [CrossRef]
- Mooney, B.P.; Thelen, J.J. High-throughput peptide mass fingerprinting of soybean seed proteins: Automated workflow and utility of UniGene expressed sequence tag databases for protein identification. Phytochemistry 2004, 65, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Oehrle, N.W.; Natarajan, S.S. A rapid and simple procedure for the depletion of abundant storage proteins from legume seeds to advance proteome analysis: A case study using Glycine max. Proteomics 2009, 9, 3174–3188. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Nelson, R.L. Proteomic Analysis of High Protein Soybean (Glycine max) Accessions Demonstrates the Contribution of Novel Glycinin Subunits. J. Agric. Food Chem. 2011, 59, 2432–2439. [Google Scholar] [CrossRef]
- Song, K.; Yim, W.C.; Lee, B.-M. Expression of Heat Shock Proteins by Heat Stress in Soybean. Plant Breed. Biotechnol. 2017, 5, 344–353. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.-K.; Dong, Q.-L.; Zhang, Y.-Y.; Wang, Y.-M.; Li, H.-Y.; Xing, G.-J.; Li, Q.; Dong, Y. Genome-wide analysis and expression profiling under heat and drought treatments of HSP70 gene family in soybean (Glycine max L.). Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2018, 294, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.D.; Leopold, A.C. The ultrastructure of dry and imbibed cotyledons of soybean. Am. J. Bot. 1977, 64, 1286–1293. [Google Scholar] [CrossRef]
- Mengistu, A.; Heatherly, L. Planting date, irrigation, maturity group, year, and environment effects on Phomopsis longicolla, seed germination, and seed health rating of soybean in the early soybean production system of the midsouthern USA. Crop Prot. 2006, 25, 310–317. [Google Scholar] [CrossRef]
- Wolf, R.B.; Cavins, J.F.; Kleiman, R.; Black, L.T. Effect of temperature on soybean seed constituents: Oil, protein, moisture, fatty acids, amino acids and sugars. J. Am. Oil Chem. Soc. 1982, 59, 230–232. [Google Scholar] [CrossRef]
- Dornbos, D.L.; Mullen, R.E. Soybean seed protein and oil contents and fatty acid composition adjustments by drought and temperature. J. Am. Oil Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- Zarkadas, C.G.; Voldeng, H.D.; Yu, Z.R.; Choi, V.K. Assessment of the protein quality of nine northern adapted yellow and brown seed coated soybean cultivars by amino acid analysis. J. Agric. Food Chem. 1999, 47, 5009–5018. [Google Scholar] [CrossRef]
- Xu, G.; Singh, S.; Barnaby, J.; Buyer, J.; Reddy, V.; Sicher, R.C. Effects of growth temperature and carbon dioxide enrichment on soybean seed components at different stages of development. Plant Physiol. Biochem. 2016, 108, 313–322. [Google Scholar] [CrossRef]
- Deshimaru, M.; Yoshimi, S.; Shioi, S.; Terada, S. Multigene Family for Bowman–Birk Type Proteinase Inhibitors of Wild Soja and Soybean: The Presence of Two BBI-A Genes and Pseudogenes. Biosci. Biotechnol. Biochem. 2004, 68, 1279–1286. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Bennett, J.O.; Kim, W.-S.; Krishnan, A.H.; Mawhinney, T.P. Nitrogen Lowers the Sulfur Amino Acid Content of Soybean (Glycine max [L.] Merr.) by Regulating the Accumulation of Bowman−Birk Protease Inhibitor. J. Agric. Food Chem. 2005, 53, 6347–6354. [Google Scholar] [CrossRef]
- Hayward, S.; Cilliers, T.; Swart, A.C. Lipoxygenases: From Isolation to Application. Compr. Rev. Food Sci. Food Saf. 2016, 16, 199–211. [Google Scholar] [CrossRef]
- Hildebrand, D.F. Lipoxygenases. Physiol. Plant. 1989, 76, 249–253. [Google Scholar] [CrossRef]
- Croft, K.; Juttner, F.; Slusarenko, A.J. Volatile Products of the Lipoxygenase Pathway Evolved from Phaseolus vulgaris (L.) Leaves Inoculated with Pseudomonas syringae pv phaseolicola. Plant Physiol. 1993, 101, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Slusarenko, A.J.; Meier, M.M.; Croft, K.P.C.; Eiben, H.G. Lipoxygenase in plant disease. In Mechanisms of Plant Defense Responses; Fritig, B., LeGrand, M., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1993; pp. 211–220. [Google Scholar]
- Tranbarger, T.J.; Franceschi, V.R.; Hildebrand, D.F.; Grimes, H.D. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophvll cell vacuoles. Plant Cell 1991, 3, 973–987. [Google Scholar]
- Fujimaki, M.; Arai, S.; Norimasa, K.; Sakurai, Y. Studies on flavor components in soybean. Part I. Aliphatic carbonyl compounds. Agric. Biol. Chem. 1966, 30, 364–369. [Google Scholar]
- Wolf, W.J. Lipoxygenase and flavor of soybean protein products. J. Agric. Food Chem. 1975, 23, 136–141. [Google Scholar] [CrossRef]
- Linko, Y.-Y.; Javanainen, P.; Linko, S. Biotechnology of bread baking. Trends Food Sci. Technol. 1997, 8, 339–344. [Google Scholar] [CrossRef]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Wathelet, J.-P.; du Jardin, P.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Societ. Environ. 2010, 14, 451–460. [Google Scholar]
- Lee, K.J.; Hwang, J.E.; Velusamy, V.; Ha, B.-K.; Kim, J.-B.; Kim, S.H.; Ahn, J.-W.; Kang, S.-Y.; Kim, N.S. Selection and molecular characterization of a lipoxygenase-free soybean mutant line induced by gamma irradiation. Theor. Appl. Genet. 2014, 127, 2405–2413. [Google Scholar] [CrossRef]
- Trawatha, S.E.; Tekrony, D.M.; Hildebrand, D.F. Soybean Lipoxygenase Mutants and Seed Longevity. Crop Sci. 1995, 35, 862–868. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.-D.; et al. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, S.; Song, Q.; Li, K.; Tao, H.; Huang, J.; Chen, X.; Que, S.; He, H. Genome-wide identification of heat shock proteins (Hsps) and Hsp interactors in rice: Hsp70s as a case study. BMC Genom. 2014, 15, 344. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Dapena, P.; Castaño, R.; Almoguera, C.; Jordano, J. Improved Resistance to Controlled Deterioration in Transgenic Seeds1[W][OA]. Plant Physiol. 2006, 142, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Nover, L.; Scharf, K.D.; Neumann, D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell. Boil. 1983, 3, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Scharf, K.D.; Nover, L. Heat shock induced changes of plant cell ultrastructure and autoradiographic localization of heat shock proteins. Eur. J. Cell Boil. 1984, 34, 254–264. [Google Scholar]
- Vacha, F.; Adamec, F.; Valenta, J.; Vacha, M. Spatial location of photosystem pigment-protein complexes in thylakoid membranes of chloroplast of Pisum sativum studied by chlorophyll fluroscence. J. Lumin. 2007, 122, 301–303. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.; Al-Khatib, K. Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress. Environ. Exp. Bot. 2011, 71, 215–223. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.-C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Paris, R.L.; Mengistu, A.; Tyler, J.M.; Smith, J.R. Registration of soybean germplasm line DT97–4290 with moderate resistance to charcoal rot. Crop Sci. 2006, 46, 2324–2325. [Google Scholar] [CrossRef]
- Park, T.K.; Polacco, J.C. Distinct Lipoxygenase Species Appear in the Hypocotyl/Radicle of Germinating Soybean. Plant Physiol. 1989, 90, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Jang, S.; Baxter, I.; Wiebold, W.J. Growing Location has a Pronounced Effect on the Accumulation of Cancer Chemopreventive Agent Bowman-Birk Inhibitor in Soybean Seeds. Crop Sci. 2012, 52, 1786–1794. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Jiang, G.; Krishnan, A.H.; Wiebold, W.J. Seed storage protein composition of non-nodulating soybean (Glycine max (L.) Merr.) and its influence on protein quality. Plant Sci. 2000, 157, 191–199. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, H.B.; Kim, W.-S.; Oehrle, N.W.; Smith, J.R.; Gillman, J.D. Effect of Heat Stress on Seed Protein Composition and Ultrastructure of Protein Storage Vacuoles in the Cotyledonary Parenchyma Cells of Soybean Genotypes That Are Either Tolerant or Sensitive to Elevated Temperatures. Int. J. Mol. Sci. 2020, 21, 4775. https://doi.org/10.3390/ijms21134775
Krishnan HB, Kim W-S, Oehrle NW, Smith JR, Gillman JD. Effect of Heat Stress on Seed Protein Composition and Ultrastructure of Protein Storage Vacuoles in the Cotyledonary Parenchyma Cells of Soybean Genotypes That Are Either Tolerant or Sensitive to Elevated Temperatures. International Journal of Molecular Sciences. 2020; 21(13):4775. https://doi.org/10.3390/ijms21134775
Chicago/Turabian StyleKrishnan, Hari B., Won-Seok Kim, Nathan W. Oehrle, James R. Smith, and Jason D. Gillman. 2020. "Effect of Heat Stress on Seed Protein Composition and Ultrastructure of Protein Storage Vacuoles in the Cotyledonary Parenchyma Cells of Soybean Genotypes That Are Either Tolerant or Sensitive to Elevated Temperatures" International Journal of Molecular Sciences 21, no. 13: 4775. https://doi.org/10.3390/ijms21134775
APA StyleKrishnan, H. B., Kim, W.-S., Oehrle, N. W., Smith, J. R., & Gillman, J. D. (2020). Effect of Heat Stress on Seed Protein Composition and Ultrastructure of Protein Storage Vacuoles in the Cotyledonary Parenchyma Cells of Soybean Genotypes That Are Either Tolerant or Sensitive to Elevated Temperatures. International Journal of Molecular Sciences, 21(13), 4775. https://doi.org/10.3390/ijms21134775



