Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML
Abstract
1. Introduction
2. CML Therapeutic Approach
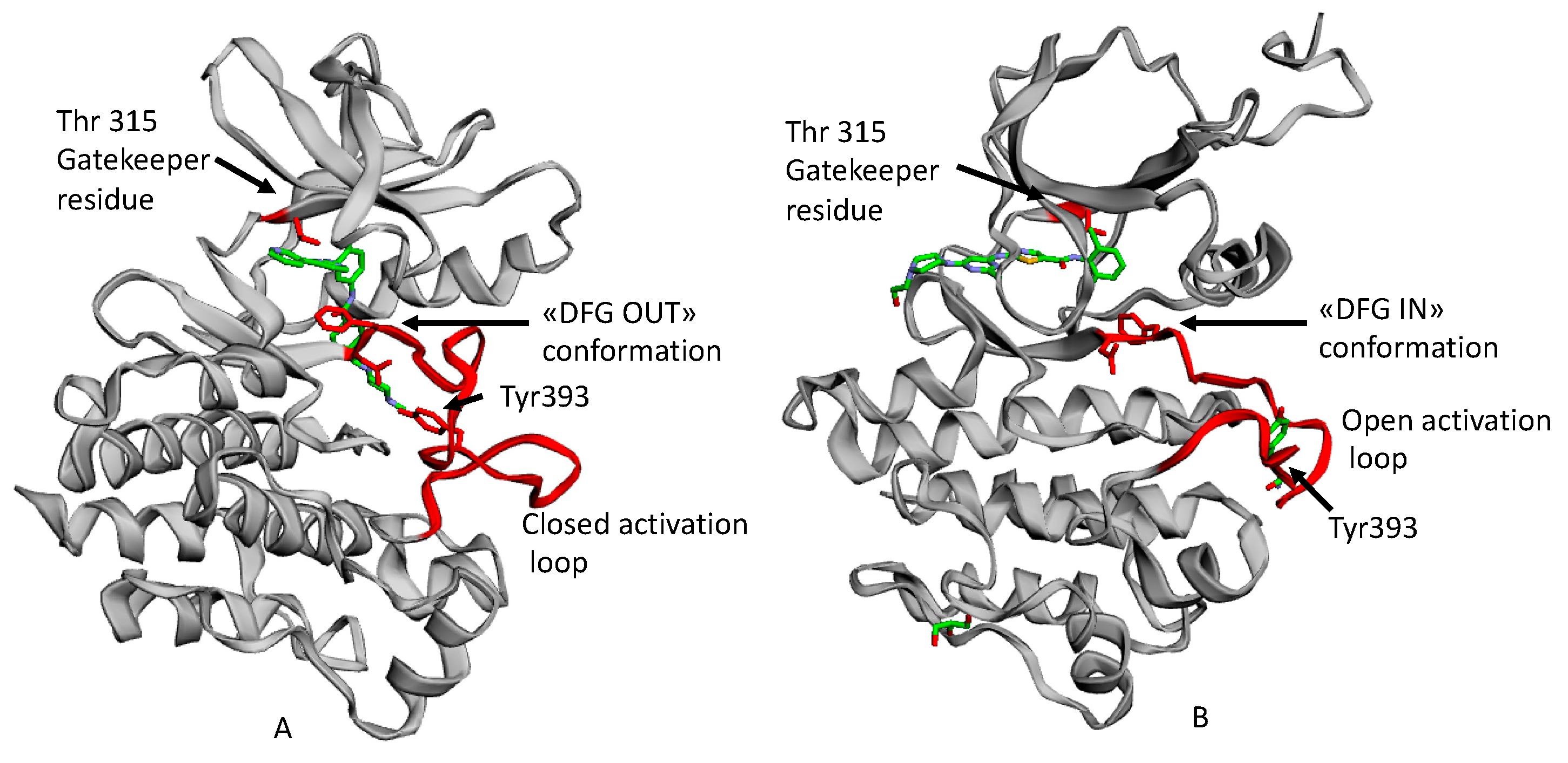
2.1. Resistance and Intolerance to Imatinib
2.2. Second Generation TKI
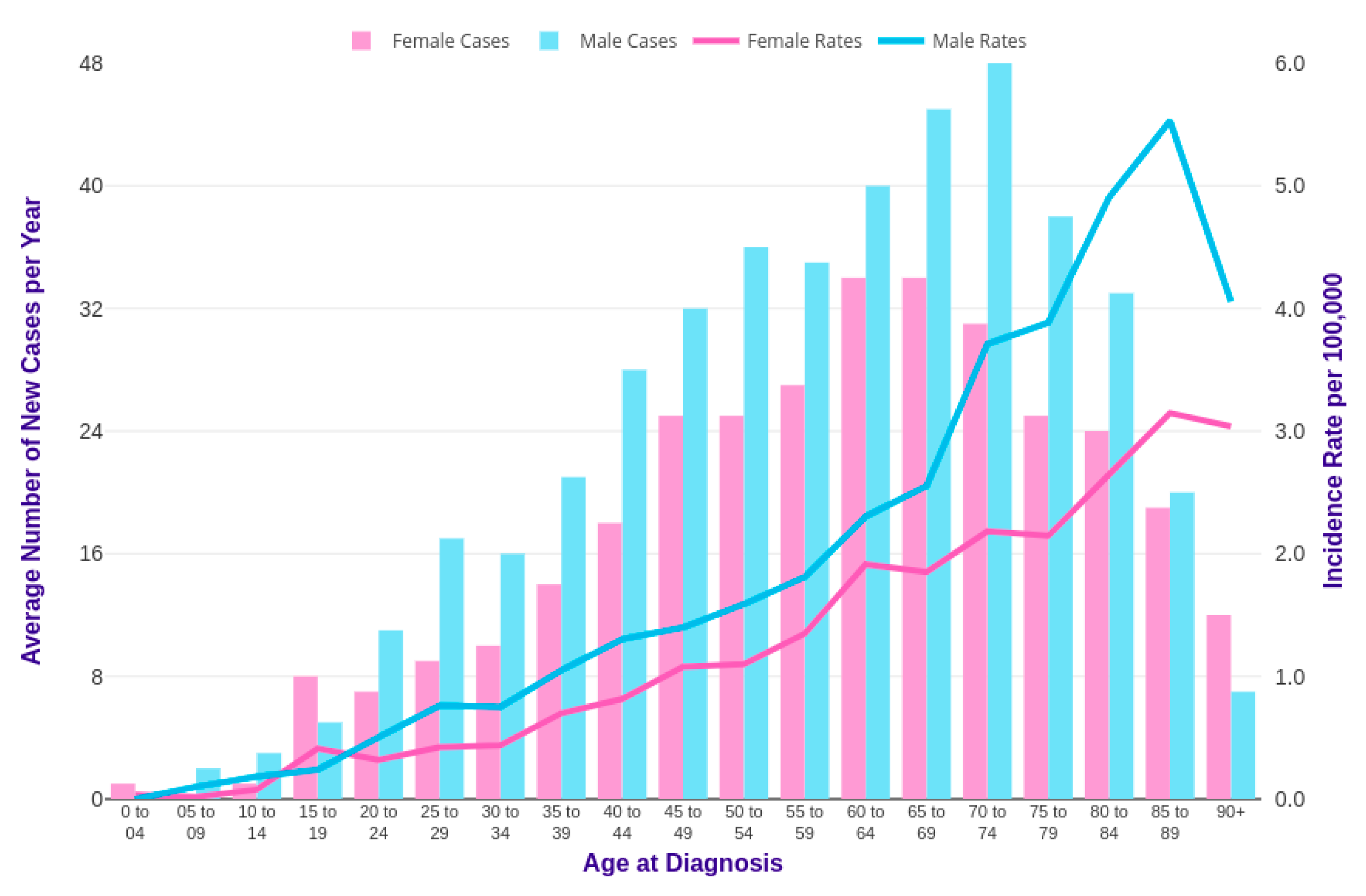
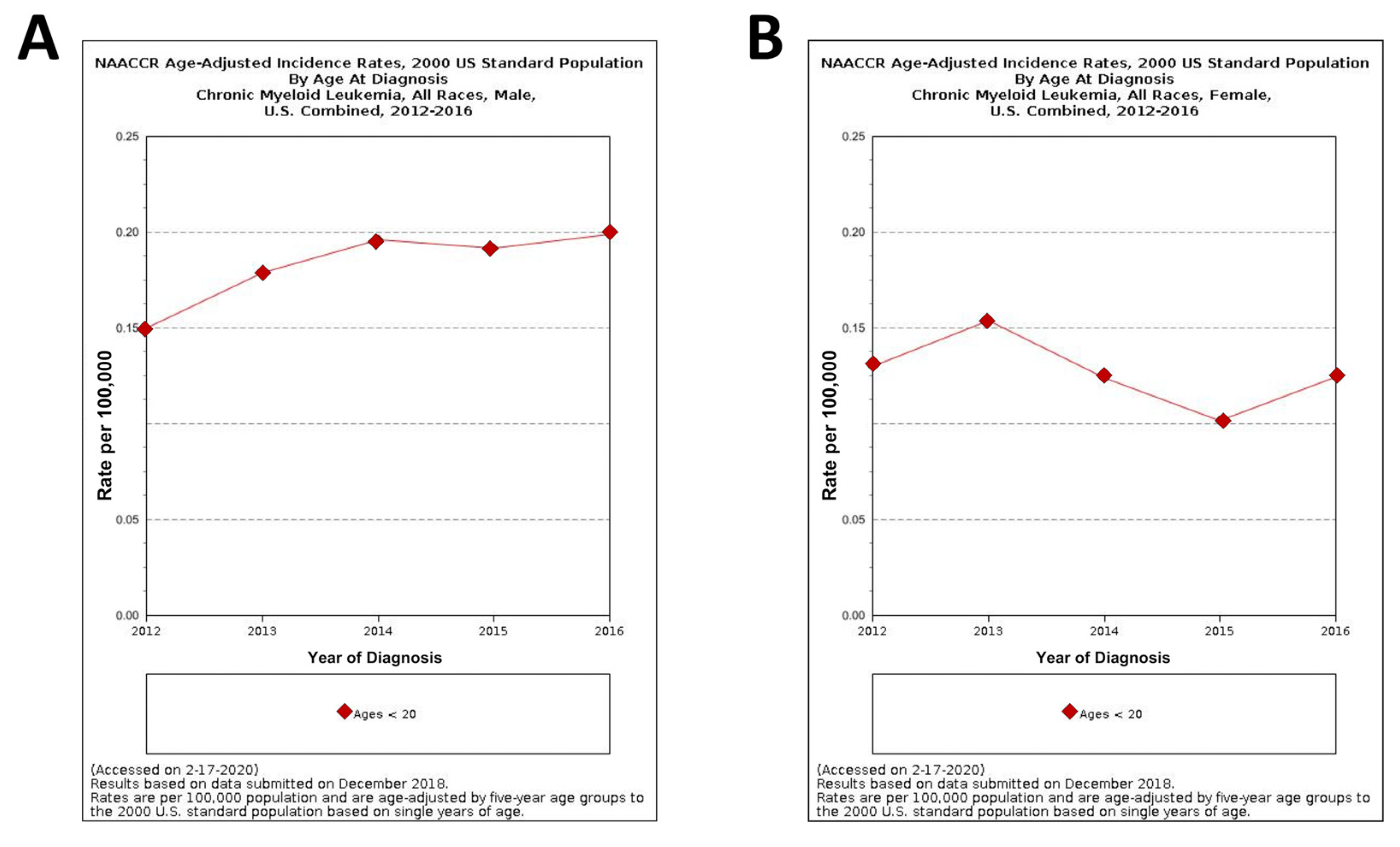
3. CML in Pediatric Age
3.1. TKI Pediatric Therapy: Issues and Concerns
3.2. HSCT vs. TKI in the Therapy of Pediatric CML
3.3. TKIs Discontinuation: Treatment-Free Remission as an Additional Goal in CMLTKI
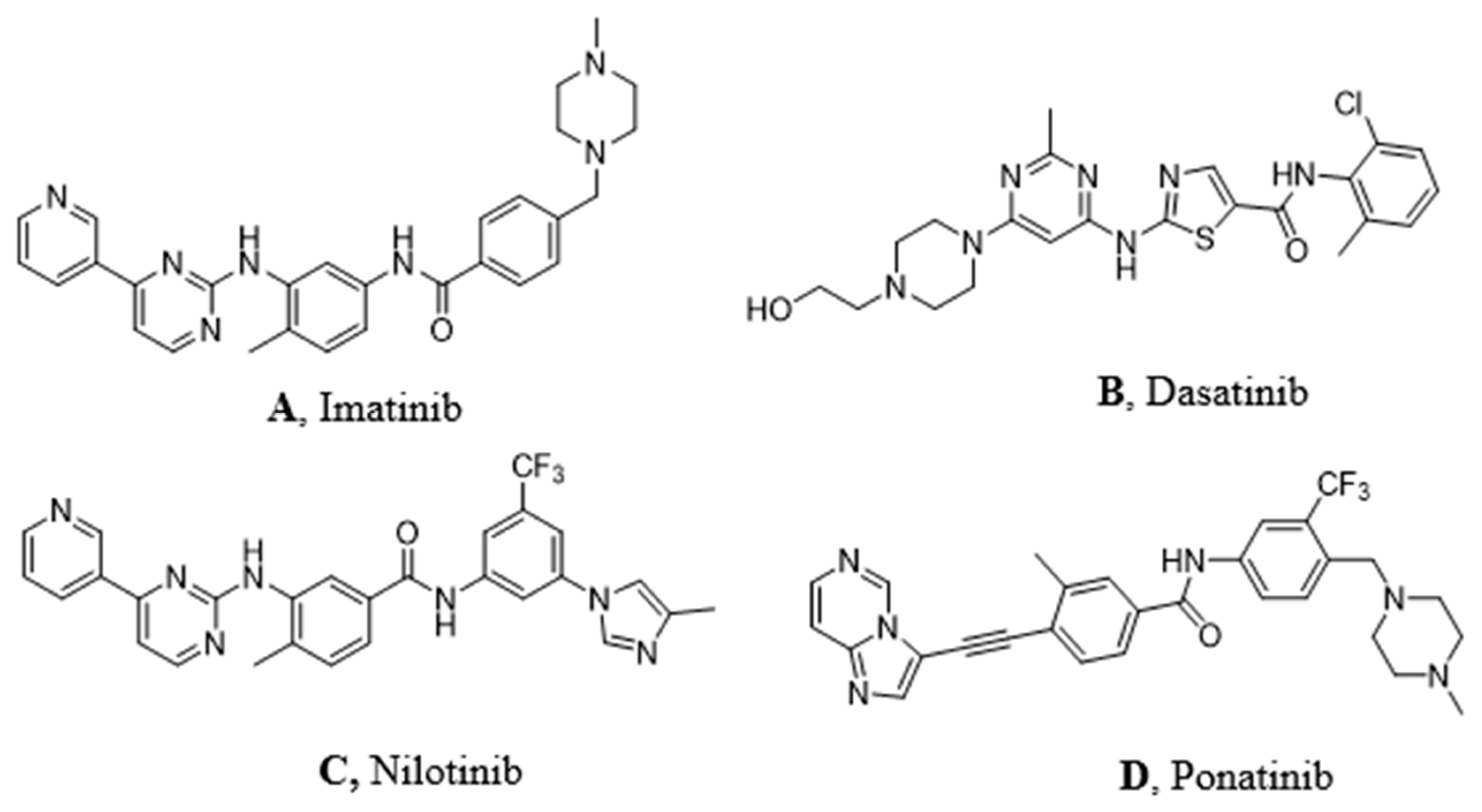
3.4. Imatinib in Pediatric CML
3.5. Dasatinib in Pediatric CML
3.6. Nilotinib in Pediatric CML
3.7. Ponatinib in Pediatric CML
4. TKI Dosage Forms for Children: Formulation Considerations
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CML | Chronic Myeloid Leukemia |
| PK | Protein Kinase |
| TK | Tyrosine Kinase |
| TKI | Tyrosine Kinase Inhibitor |
| RTK | Receptor Tyrosine Kinase |
| NRTK | Non-Receptor Tyrosine Kinase |
| VEGFR | Vascular Endothelial Growth Factor |
| PDGF | Platelet Derived Growth Factor |
| Src | Sarcoma |
| JAK | Janus Kinase |
| ABL | Abelson (gene) |
| BCR | Breakpoint Cluster Region (gene) |
| PI3K | Phosphoinositide 3-Kinase |
| STAT | Signal Transducer and Activator of Transcription (protein) |
| CP | Chronic Phase |
| AP | Accelerated Phase |
| BC | Blast Crisis |
| Asp | Aspartate |
| Phe | Phenylalanine |
| Gly | Glycine |
| ATP | Adenosine Triphosphate |
| DFG | Aspartate-Phenilalanine-Glycine |
| Thr | Threonine |
| HSCT | Hematopoietic Stem Cells Transplantation |
| HCT | Hematopoietic Cell Transplantation |
| INF | Interferon |
| CHR | Complete Hematological Response |
| MMR | Major Molecular Response |
| CCR | Complete Cytogenetic Remission |
| Ph | Philadelphia (chromosome) |
| Ph+ | Philadelphia positive |
| P-gp | Glycoprotein P |
| OCT | Organic Cation Transporter |
| PDGFR | Platelet Derived Growth Factor Receptor |
| UK | United Kingdom |
| NAACCR | North American Association of Central Cancer Registries |
| ELN | European Leukemia Net |
| NCCN | National Comprehensive Cancer Network |
| CI | Confidence Interval |
| STIM | Stop Imatinib |
| LSC | Leukemic Stem Cells |
| ALL | Acute Lymphoblastic Leukemia |
| CMR | Complete Molecular Response |
| FDA | Food and Drug Administration |
| EMA | European Medicinal Agency |
| R/I | Resistance/Intolerance |
| SAE | Severe Adverse Event |
| HLA | Human Leukocyte Antigen |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| OS | Overall Survival |
| LFS | Leukemia-Free Survival |
| MSD | Matching Sibling Donor |
| HCT | Hematopoietic Cell Transplantation |
| HRQOL | Health-Related Quality of Life |
| ISD | Identical Sibling Donor |
| TFR | Treatment-free Remission |
| QoL | Quality of Life |
| DMR | Deep Molecular Response |
| EURO-SKI | European Stop Tyrosine Kinase Inhibitor |
| ESMO | European Society for Medical Oncology |
| RQ-PCR | Real-time Quantitative Polymerase Chain Reaction |
References
- Chen, M.H.; Kerkela, R.; Force, T. Mechanisms of cardiomyopathy associated with tyrosine kinase inhibitor cancer therapeutics. Circulation 2008, 118, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Tyrosine-kinase-inhibitor. Available online: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet (accessed on 20 January 2020).
- Cortes, J.E.; Talpaz, M.; Beran, M.; O’Brien, S.M.; Rios, M.B.; Stass, S.; Kantarjian, H.M. Philadelphia chromosome-negative chronic myelogenous leukemia with rearrangement of the breakpoint cluster region. Long-term follow-up results. Cancer 1995, 75, 464–470. [Google Scholar] [CrossRef]
- Reddy, E.P.; Aggarwal, A.K. The Ins and Outs of Bcr-Abl Inhibition. Genes Cancer 2012, 3, 447–454. [Google Scholar] [CrossRef]
- Reynolds, C.R.; Islam, S.A.; Sternberg, M.J.E. EzMol: A web server wizard for the rapid visualisation and image production of protein and nucleic acid structures. J. Mol. Biol. 2018, 430, 1–5. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Rezvani, A.; Storb, R.F. Allogeneic hematopoietic cell transplantation: The state of the art. Expert Rev. Hematol. 2010, 3, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Nardi, V.; Azam, M.; Daley, G.Q. Mechanisms and implications of imatinib resistance mutations in BCR-ABL. Curr. Opin. Hematol. 2004, 11, 35–43. [Google Scholar] [CrossRef]
- Widmer, N.; Colombo, S.; Buclin, T.; Decosterd, L.A. Functional consequence of MDR1 expression on imatinib intracellular concentrations. Blood 2003, 102, 1142. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Hughes, T.; White, D. OCT1 and imatinib transport in CML: Is it clinically relevant? Leukemia 2015, 29, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Cavalluzzi, M.M.; Imbrici, P.; Gualdani, R.; Stefanachi, A.; Mangiatordi, G.F.; Lentini, G.; Nicolotti, O. Human ether-à-go-go-related potassium channel: Exploring SAR to improve drug design. Drug Discov. Today 2020, 25, 344–366. [Google Scholar] [CrossRef] [PubMed]
- Ángeles-Velázquez, J.L.; Hurtado-Monroy, R.; Vargas-Viveros, P.; Carrillo-Muñoz, S.; Candelaria-Hernández, M. Imatinib Intolerance Is Associated with Blastic Phase Development in Philadelphia Chromosome-Positive Chronic Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2016, 16, S82–S85. [Google Scholar] [CrossRef] [PubMed]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; Donato, N.; Nicoll, J.; Paquette, R.; Cortes, J.; O’Brien, S.; Nicaise, C.; Bleickardt, E.; et al. Dasatinib in Imatinib-Resistant Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2006, 354, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Tokarski, J.S.; Newitt, J.A.; Chang, C.Y.; Cheng, J.D.; Wittekind, M.; Kiefer, S.E.; Kish, K.; Lee, F.Y.; Borzillerri, R.; Lombardo, L.J.; et al. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006, 66, 5790–5797. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Park, H.; Al-Khafaji, J.; Grant, S. Strategies to circumvent the T315I gatekeeper mutation in the Bcr-Abl tyrosine kinase. Leuk. Res. Rep. 2013, 2, 18–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Redaelli, S.; Piazza, R.; Rostagno, R.; Magistroni, V.; Perini, P.; Marega, M.; Gambacorti-Passerini, C.; Boschelli, F. Activity of Bosutinib, Dasatinib, and Nilotinib Against 18 Imatinib Resistant BCR/ABL Mutants. J. Clin. Oncol. 2009, 27, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Saglio, G.; Kim, D.-W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.P.; et al. Nilotinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef]
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.-S.; Xu, Q.; et al. AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance. Cancer Cell 2009, 16, 401–412. [Google Scholar] [CrossRef]
- Azam, M.; Nardi, V.; Shakespeare, W.C.; Metcalf III, C.A.; Bohacek, R.S.; Wang, Y.; Sundaramoorthi, R.; Sliz, P.; Veach, D.R.; Bornmann, W.G.; et al. Activity of dual SRC-ABL inhibitors highlights the role of BCR/ABL kinase dynamics in drug resistance. Proc. Natl. Acad. Sci. USA 2006, 103, 9244–9249. [Google Scholar] [CrossRef]
- Horibe, K.; Tsukimoto, I.; Ohno, R. Clinicopathologic characteristics of leukemia in Japanese children and young adults. Leukemia 2001, 15, 1256–1261. [Google Scholar] [CrossRef][Green Version]
- Mattano, L.; Nachman, J.; Ross, J.; Stock, W. Leukemias. In Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000; Bleyer, A., O’Leary, M., Barr, R., Ries, L.A.G., Eds.; National Cancer Institute, NIH: Bethesda, MD, USA, 2006; pp. 39–51. [Google Scholar]
- Millot, F.; Traore, P.; Guilhot, J.; Nelken, B.; Leblanc, T.; Leverger, G.; Plantaz, D.; Bertrand, Y.; Bordigoni, P.; Guilhot, F. Clinical and biological features at diagnosis in 40 children with chronic myeloid leukemia. Pediatrics 2005, 116, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Baruchel, A.; Guilhot, J.; Petit, A.; Leblanc, T.; Bertrand, Y.; Mazingue, F.; Lutz, P.; Verite, C.; Berthou, C.; et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: Results of the French national phase IV trial. J. Clin. Oncol. 2011, 29, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Kantarjian, H.; Cortes, J. Trends in chronic myeloid leukemia incidence and survival in the United States from 1975 to 2009. Leuk. Lymph. 2013, 54, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/leukaemia-cml/incidence#heading-One (accessed on 20 January 2020).
- Gugliotta, G.; Castagnetti, F.; Apolinari, M.; Pirondi, S.; Cavo, M.; Baccarani, M.; Rosti, G. First-Line Treatment of Newly Diagnosed Elderly Patients with Chronic Myeloid Leukemia: Current and Emerging Strategies. Drugs 2014, 74, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Ries, L.A.G.; Smith, M.A.; Gurney, J.G.; Linet, M.; Tamra, T.; Young, J.L.; Bunin, G.R. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995; National Cancer Institute: Bethesda, MD, USA, 1999; NIH Pub. No. 99–4649. Available online: https://seer.cancer.gov/archive/publications/childhood/ (accessed on 22 June 2020).
- North American Association of Central Cancer Registries. NAACCR Fast Stats: An Interactive Tool for Quick Access to Key NAACCR Cancer Statistics. Available online: http://www.naaccr.org/ (accessed on 17 February 2020).
- Hijiya, N.; Schultz, K.R.; Metzler, M.; Millot, F.; Suttorp, M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood 2016, 127, 392–399. [Google Scholar] [CrossRef]
- Ernst, T.; Busch, M.; Rinke, J.; Ernst, J.; Haferlach, C.; Beck, J.F.; Hochhaus, A.; Gruhn, B. Frequent ASXL1 mutations in children and young adults with chronic myeloid leukemia. Leukemia 2018, 32, 2046–2049. [Google Scholar] [CrossRef]
- Radich, J.P.; Deininger, M.; Abboud, C.N.; Altman, J.K.; Berman, E.; Bhatia, R.; Bhatnagar, B.; Curtin, P.; DeAngelo, D.J.; Gotlib, J.; et al. Chronic Myeloid Leukemia, Version 1.2019 NCCN clinical practice guidelines in oncology. JNCCN 2018, 16, 1108–1135. [Google Scholar] [CrossRef]
- Millot, F.; Guilhot, J.; Suttorp, M.; Güneş, A.M.; Sedlacek, P.; De Bont, E.; Li, C.K.; Kalwak, K.; Lausen, B.; Culic, S.; et al. Prognostic discrimination based on the EUTOS long-term survival score within the International Registry for Chronic Myeloid Leukemia in children and adolescents. Haematologica 2017, 102, 1704–1708. [Google Scholar] [CrossRef]
- Salas, D.G.; Glauche, I.; Tauer, J.T.; Thiede, C.; Suttorp, M. Can prognostic scoring system for chronic myeloid leukemia as established in adults be applied to pediatric patients? Ann. Hematol. 2015, 94, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Shima, H.; Tokuyama, M.; Tanizawa, A.; Tono, C.; Hamamoto, K.; Muramatsu, H.; Watanabe, A.; Hotta, N.; Ito, M.; Kurosawa, H.; et al. Distinct Impact of Imatinib on Growth at Prepubertal and Pubertal Ages of Children with Chronic Myeloid Leukemia. J. Pediatr. 2011, 159, 676–681. [Google Scholar] [CrossRef]
- Jeyaraman, P.; Naithani, R. Discontinuation of Imatinib in a Child with Chronic Myeloid Leukemia. J. Pediatr. Hematol. Oncol. 2020, 42, e64–e65. [Google Scholar] [CrossRef]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Hijiya, N.; Millot, F.; Suttorp, M. Chronic myeloid leukemia in children: Clinical findings, management, and unanswered questions. Pediatr. Clin. N. Am. 2015, 62, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Suttorp, M.; Schulze, P.; Glauche, I.; Göhring, G.; von Neuhoff, N.; Metzler, M.; Sedlacek, P.; de Bont, E.S.J.M.; Balduzzi, A.; Lausen, B.; et al. Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: Results from a phase III trial. Leukemia 2018, 32, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Giona, F.; Saglio, G.; Moleti, M.L.; Piciocchi, A.; Rea, M.; Nanni, M.; Marzella, D.; Testi, A.M.; Mariani, S.; Laurino, M.; et al. Treatment-free remission after imatinib discontinuation is possible in paediatric patients with chronic myeloid leukaemia. Br. J. Haematol. 2015, 168, 305–308. [Google Scholar] [CrossRef]
- Mangiatordi, G.F.; Alberga, D.; Altomare, C.D.; Carotti, A.; Catto, M.; Cellamare, S.; Gadaleta, D.; Lattanzi, G.; Leonetti, F.; Pisani, L.; et al. Mind the gap! A journey towards computational toxicology. Mol. Inform. 2016, 35, 294–308. [Google Scholar] [CrossRef]
- Castagnetti, F.; Gugliotta, G.; Baccarani, M.; Breccia, M.; Specchia, G.; Levato, L.; Abruzzese, E.; Rossi, G.; Iurlo, A.; Martino, B.; et al. A GIMEMA CML Working Party. Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann. Oncol. 2015, 26, 185–192. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Peters, C.; Gaspar, H.B.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Falkenburg, J.H.F.; Farge-Bancel, D.; Gennery, A.; et al. Hematopoietic SCT in Europe: Data and trends in 2012 with special consideration of pediatric trans-plantation. Bone Marrow Trans. 2014, 49, 744–750. [Google Scholar] [CrossRef]
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood 2013, 122, 872–884. [Google Scholar] [CrossRef]
- Chaudhury, S.; Sparapani, R.; Hu, Z.-H.; Nishihori, T.; Abdel-Azim, H.; Malone, A.; Olsson, R.; Hamadani, M.; Daly, A.; Bacher, U.; et al. Outcomes of Allogeneic Hematopoietic Cell Transplantation in Children and Young Adults with Chronic Myeloid Leukemia: A CIBMTR Cohort Analysis. Biol. Blood Marrow Trans. 2016, 22, 1056–1064. [Google Scholar]
- Millot, F.; Suttorp, M.; Guilhot, J.; Sedlacek, P.; De Bont, E.S.; Li, C.K.; Kalwak, K.; Lausen, B.; Srdjana, C.; Dresse, M.-F.; et al. The International Registry for Chronic Myeloid Leukemia (CML) in Children and Adolescents (I- CML-Ped-Study): Objectives and preliminary results. Blood 2012, 120, 3741. [Google Scholar] [CrossRef]
- Efficace, F.; Baccarani, M.; Breccia, M.; Alimena, G.; Rosti, G.; Cottone, F. Health related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 2011, 118, 4554–4560. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Orav, E.J.; McNamara, T.K.; Tong, M.Y.; Antin, J.H. Psychosocial function after hematopoietic stem cell transplantation. Psychosomatics 2005, 46, 34–40. [Google Scholar] [CrossRef]
- Mo, X.-D.; Jiang, Q.; Xu, L.-P.; Liu, D.-H.; Liu, K.-Y.; Jiang, B.; Jiang, H.; Chen1, H.; Chen, Y.-H.; Zhang, X.-H.; et al. Health-related quality of life of patients with newly diagnosed chronic myeloid leukemia treated with allogeneic hematopoietic SCT versus Imatinib. Bone Marrow Trans. 2014, 49, 576–580. [Google Scholar] [CrossRef]
- Athale, U.; Andolina, J.R.; Redell, M.S.; Hijiya, N.; Patterson, B.C.; Bergsagel, J.; Bittencourt, H.; Schultz, K.R.; Burke, M.J.E.; Kolb, A.; et al. Management of chronic myeloid leukemia in children and adolescents: Recommendations from the Children’s Oncology Group CML Working Group. Pediatr. Blood Cancer 2019, 66, e2782. [Google Scholar]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef]
- Mahon, F.X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia, Version 1.2020. Available online: https://www.nccn.org/patients/guidelines/content/PDF/cml-patient.pdf (accessed on 22 June 2020).
- Saussele, S.; Richter, J.; Guilhot, J.G.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; Olsson-Strömberg, U.; et al. Duration of deep molecular response has most impact on the success of cessation of tyrosine kinase inhibitor treatment in chronic myeloid leukemia—results from the EURO-SKI trial. Blood 2017, 130, 313. [Google Scholar]
- Ault, P.; Kantarjian, H.; O’Brien, S.; Faderl, S.; Beran, M.; Rios, M.B.; Koller, C.; Giles, F.; Keating, M.; Talpaz, M.; et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J. Clin. Oncol. 2006, 24, 1204–1208. [Google Scholar] [CrossRef]
- Pye, S.M.; Cortes, J.; Ault, P.; Hatfield, A.; Kantarjian, H.; Pilot, R.; Rosti, G.; Apperley, J.F. The effects of imatinib on pregnancy outcome. Blood 2008, 111, 5505–5508. [Google Scholar] [CrossRef]
- Cortes, J.E.; Abruzzese, E.; Chelysheva, E.; Guha, M.; Wallis, N.; Apperley, J.F. The impact of dasatinib on pregnancy outcomes. Am. J. Hematol. 2015, 90, 1111–1115. [Google Scholar] [CrossRef]
- Hughes, T.P.; Ross, D.M. Moving treatment-free remission into mainstream clinical practice in CML. Blood 2016, 128, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Söderlund, S.; Lübking, A.; Dreimane, A.; Lotfi, K.; Markevärn, B.; Själander, A.; Saussele, S.; Olsson-Strömberg, U.; Stenke, L. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: A tyrosine kinase inhibitor withdrawal syndrome? J. Clin. Oncol. 2014, 32, 2821–2823. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Nicolini, F.E.; Tulliez, M.; Guilhot, F.; Guilhot, J.; Guerci-Bresler, A.; Gardembas, M.; Coiteux, V.; Guillerm, G.; Legros, L.; et al. France Intergroupe des Leucémies Myéloïdes Chroniques. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood 2017, 129, 846–854. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saussele, S.; Rosti, G.; Mahon, F.X.; Janssen, J.J.W.M.; Hjorth-Hansen, H.; Richter, J.; Buske, C.; ESMO Guidelines Committee. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv41–iv51. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Claviez, A.; Leverger, G.; Corbaciglu, S.; Groll, A.H.; Suttorp, M. Imatinib cessation in children and adolescents with chronic myeloid leukemia in chronic phase. Pediatr. Blood Cancer 2014, 61, 355–357. [Google Scholar] [CrossRef]
- FDA Approves Gleevec for Children with Acute Lymphoblastic Leukemia. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm336868.html (accessed on 20 January 2020).
- Suttorp, M.; Claviez, A.; Bader, P.; Peters, C.; Gadner, H.; Ebell, W.; Dilloo, D.; Kremens, B.; Kabisch, H.; Führer, M.; et al. Allogeneic stem cell transplantation for pediatric and adolescent patients with CML: Results from the prospective trial CML-paed I. Klin. Padiatr. 2009, 221, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Suttorp, M.; Millot, F. Treatment of pediatric chronic myeloid leukemia in the year 2010: Use of tyrosine kinase inhibitors and stem-cell transplantation. Hematol. Am. Soc. Hematol. Educ. Program. 2010, 2010, 368–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Champagne, M.A.; Capdeville, R.; Krailo, M.; Qu, W.; Peng, B.; Rosamilia, M.; Therrien, M.; Zoellner, U.; Blaney, S.M.; Bernsteinet, M. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: Results from a Children’s Oncology Group phase 1 study. Blood 2004, 104, 2655–2660. [Google Scholar] [CrossRef]
- Lee, J.W.; Chung, N.G. The treatment of pediatric chronic myelogenous leukemia in the imatinib era. Korean J. Pediatr. 2011, 54, 111–116. [Google Scholar] [CrossRef]
- Muramatsu, H.; Takahashi, Y.; Sakaguchi, H.; Shimada, A.; Nishio, N.; Hama, A.; Doisaki, S.; Yagasaki, H.; Matsumoto, K.; Kato, K.; et al. Excellent outcomes of children with CML treated with imatinib mesylate compared to that in pre-imatinib era. Int. J. Hematol. 2011, 93, 186–191. [Google Scholar] [CrossRef]
- Millot, F.; Guilhot, J.; Nelken, B.; Leblanc, T.; De Bont, E.S.; Be’kassy, A.N.; Gadner, H.; Sufliarska, S.; Stary, J.; Gschaidmeier, H.; et al. Imatinib mesylate is effective in children with chronic myelogenous leukemia in late chronic and advanced phase and in relapse after stem cell transplantation. Leukemia 2006, 20, 187–192. [Google Scholar]
- Miranda, M.B.; Lauseker, M.; Kraus, M.P.; Proetel, U.; Hanfstein, B.; Fabarius, A.; Baerlocher, G.M.; Heim, D.; Hossfeld, D.K.; Kolb, H.J.; et al. Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: Long-term observation in CML Study IV. Leukemia 2016, 30, 1255–1262. [Google Scholar] [PubMed]
- Kalmanti, L.; Saussele, S.; Lauseker, M.; Müller, M.C.; Dietz, C.T.; Heinrich, L.; Hanfstein, B.; Proetel, U.; Fabarius, A.; Krause, S.W.; et al. Safety and efficacy of imatinib in CML over a period of 10 years: Data from the randomized CML-study IV. Leukemia 2015, 29, 1123–1132. [Google Scholar] [CrossRef]
- Drucker, B.J.; Guilhot, F.; O’Brien, S.G.; Gathmann, I.; Kantarjian, H.; Gattermann, N.; Deininger, M.W.; Silver, R.T.; Goldman, J.M.; Stone, R.M.; et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukaemia. N. Engl. J. Med. 2006, 355, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- De Lavallade, H.; Apperley, J.F.; Khorashad, J.S.; Milojkovic, D.; Reid, A.G.; Bua, M.; Szydlo, R.; Olavarria, E.; Kaeda, J.; Goldman, J.M.; et al. Imatinib for newly diagnosed patients with chronic myeloid leukaemia: Incidence of sustained responses in an intention-to-treat analysis. J. Clin. Oncol. 2008, 26, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, B.A.; Tauer, J.T.; Ulmer, A.; Kuhlisch, E.; Roth, H.J.; Suttorp, M. Changes in bone metabolic parameters in children with chronic myeloid leukemia on imatinib treatment. Med. Sci. Monit. 2010, 18, CR721–CR728. [Google Scholar] [CrossRef]
- Giona, F.; Mariani, S.; Gnessi, L.; Moleti, M.L.; Rea, M.; De Vellis, A.; Marzella, D.; Testi, A.M.; Foà, R. Bone metabolism, growth rate and pubertal development in children with chronic myeloid leukemia treated with imatinib during puberty. Haematologica 2013, 98, e25–e27. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Guilhot, J.; Baruchel, A.; Petit, A.; Leblanc, T.; Bertrand, Y.; Mazingue, F.; Lutz, P.; Vérité, C.; Berthouh, C.; et al. Growth deceleration in children treated with imatinib for chronic myeloid leukaemia. Eur. J. Cancer 2014, 50, 3206–3211. [Google Scholar] [CrossRef]
- Suttorp, M.; Bornhäuser, M.; Metzler, M.; Millot, F.; Schleyer, E. Pharmacology and pharmacokinetics of imatinib in pediatric patients. Exp. Rev. Clin. Pharm. 2018, 11, 219–231. [Google Scholar] [CrossRef]
- Giona, F.; Putti, M.C.; Micalizzi, C.; Menna, G.; Moleti, M.L.; Santoro, N.; Iaria, G.; Ladogana, S.; Burnelli, R.; Consarino, C.; et al. Long-term results of high-dose imatinib in children and adolescents with chronic myeloid leukaemia in chronic phase: The Italian experience. Br. J. Haematol. 2015, 170, 398–407. [Google Scholar] [CrossRef]
- FDA Approved Dasatinib for Pediatric Patients. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dasatinib-pediatric-patients-cml (accessed on 20 January 2020).
- Gore, L.; Kearns, P.R.; de Martino, M.L.; Lee, C.; De Souza, A.; Bertrand, Y.; Hijiya, N.; Stork, L.C.; Chung, N.-G.; Cardenas Cardos, R.; et al. Dasatinib in Pediatric Patients with Chronic Myeloid Leukemia in Chronic Phase: Results From a Phase II Trial. J. Clin. Oncol. 2018, 36, 1330–1338. [Google Scholar] [CrossRef]
- FDA-approves-nilotinib-pediatric-patients. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nilotinib-pediatric-patients-newly-diagnosed-or-resistantintolerant-ph-cml-chronic (accessed on 20 January 2020).
- Hijiya, N.; Maschan, A.; Rizzari, C.; Shimada, H.; Dufour, C.; Goto, H.; Kang, H.J.; Guinipero, T.; Karakas, Z.; Bautista, F.; et al. Phase 2 study of nilotinib in pediatric patients with philadelphia chromosome-positive chronic myeloid leukemia. Blood 2019, 134, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef]
- Giles, F.J.; le Coutre, P.D.; Pinilla-Ibarz, J.; Larson, R.A.; Gattermann, N.; Ottmann, O.G.; Hochhaus, A.; Radich, J.P.; Saglio, G.; Hughes, T.P.; et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia 2013, 27, 107–112. [Google Scholar] [CrossRef]
- EU Nilotib Approval for Children. Available online: https://www.novartis.com/news/media-releases/novartis-drug-tasignar-nilotinib-secures-eu-approval-first-and-second-line-treatment-ph-cml-cp-children (accessed on 20 January 2020).
- Nickel, R.S.; Daves, M.; Keller, F. Treatment of an adolescent with chronic myeloid leukemia and the T315I mutation with ponatinib. Pediatr. Blood Cancer 2015, 62, 2050–2051. [Google Scholar] [CrossRef]
- Müller, M.C.; Cervantes, F.; Hjorth-Hansen, H.; Janssen, J.J.W.M.; Milojkovic, D.; Rea, D.; Rosti, G. Ponatinib in chronic myeloid leukemia (CML): Consensus on patient treatment and management from a European expert panel. Crit. Rev. Oncol. Hematol. 2017, 120, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Hochhaus, A.; Nicolini, F.E.; Gruber, F.; Lange, T.; Saglio, G.; Pane, F.; Müller, M.C.; Ernst, T.; Rosti, G.; et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Recommendations from an expert panel on behalf of European LeukemiaNet. Blood 2011, 118, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Suttorp, M.; de Bont, E.; Kalwak, K.; Nelken, B.; Ducassou, S.; Bertrand, Y.; Baruchel, A. Ponatinib In Childhood Philadelphia Positive Leukemias: The Experience of The International Registry of Childhood Chronic Myleloid Leukemia I-Cml-Ped-Study. Hemasphere 2019, 3, 161–162. [Google Scholar] [CrossRef]
- Lam, M.S. Extemporaneous compounding of oral liquid dosage formulations and alternative drug delivery methods for anticancer drugs. Pharmacotherapy 2011, 31, 164–192. [Google Scholar] [CrossRef]
- Glivec Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/glivec-epar-product-information_it.pdf (accessed on 18 August 2009).
- Sprycel Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/sprycel-epar-product-information_it.pdf (accessed on 18 August 2009).
- Iclusig Product Information. Available online: https://www.ema.europa.eu/en/documents/referral/iclusig-article-20-procedure-product-information_it.pdf (accessed on 11 July 2009).
- Tasigna Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/tasigna-epar-product-information_it.pdf (accessed on 11 July 2009).
- Andolina, J.R.; Neudorf, S.M.; Corey, S.J. How I treat childhood CML. Blood 2012, 23, 1821–1830. [Google Scholar] [CrossRef]
- De la Fuente, J.; Baruchel, A.; Biondi, A.; de Bont, E.; Dresse, M.F.; Suttorp, M.; Millot, F.; International BFM Group (iBFM) Study Group Chronic Myeloid Leukaemia Committee. Managing children with chronic myeloid leukaemia (CML): Recommendations for the management of CML in children and young people up to the age of 18 years. Br. J. Haematol. 2014, 167, 33–47. [Google Scholar] [CrossRef]
- Hochhaus, A.; Ernst, T.; Eigendorff, E.; La Rosée, P. Causes of resistance and treatment choices of second- and third-line treatment in chronic myelogenous leukemia patients. Ann. Hematol. 2015, 94, S133–S140. [Google Scholar] [CrossRef] [PubMed]
- Cutrignelli, A.; Sanarica, F.; Lopalco, A.; Lopedota, A.; Laquintana, V.; Franco, M.; Boccanegra, B.; Mantuano, P.; De Luca, A.; Denora, N. Dasatinib/HP-β-CD Inclusion Complex Based Aqueous Formulation as a Promising Tool for the Treatment of Paediatric Neuromuscular Disorders. Int. J. Mol. Sci. 2019, 30, 591. [Google Scholar] [CrossRef] [PubMed]
- Melville, N.A. Dasatinib in Children: An Effective Alternative to Imatinib. In Proceedings of the European Hematology Association (EHA) 2017 Congress, Madrid, Spain, 22–25 June 2017. [Google Scholar]
- Zwaan, C.M.; Rizzari, C.; Mechinaud, F.; Lancaster, D.I.; Lehrnbecher, T.; Van der Velden, V.H.J.; Beverloo, B.B.; den Boer, M.L.; Pieters, R.; Reinhardt, D.; et al. Dasatinib in Children and Adolescent With Relapsed or Refractory Leukemia: Results of the CA180-018 Phase I Dose-Escalation Study of the innovative Therapies for Children With Cancer Consortium. J. Clin. Oncol. 2013, 31, 2460–2469. [Google Scholar] [CrossRef]
- Allen, L.V. The Art and Science of Pharmaceutical Compounding, 2nd ed.; APhA Publications: Washington, DC, USA, 2005; p. 493. [Google Scholar]
- Nahata, M.C.; Allen, L.V., Jr. Extemporaneous drug formulations. Clin. Ther. 2008, 30, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.V. Compounding, stability and beyond-use dates. Secundum. Artem. 2003, 7. Minneapolis, MN: Paddock Laboratories. Available online: www.paddocklabs.com (accessed on 24 February 2010).
- Woods, D.J. Extemporaneous formulations: Problems and solutions. Paediatr. Perinatal. Drug Ther. 1997, 1, 25–29. [Google Scholar]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef]
- Lopalco, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Franco, M.; Robota, M.; Hauschildt, N.; Mondelli, F.; Arduino, I.; Lopedota, A. Taste masking of propranolol hydrochloride by microbeads of EUDRAGIT® E PO obtained with prilling technique for paediatric oral administration. Int. J. Pharm. 2020, 574, 118922. [Google Scholar] [CrossRef]
- Laquintana, V.; Asim, M.H.; Lopedota, A.; Cutrignelli, A.; Lopalco, A.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. Thiolated hydroxypropyl-β-cyclodextrin as mucoadhesive excipient for oral delivery of budesonide in liquid paediatric formulation. Int. J. Pharm. 2019, 572, 118820. [Google Scholar] [CrossRef]
- Lopalco, A.; Curci, A.; Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Franco, M.; Denora, N. Pharmaceutical preformulation studies and paediatric oral formulations of sodium dichloroacetate. Eur. J. Pharm. Sci. 2019, 127, 339–350. [Google Scholar] [CrossRef]
- Hijiya, N.; Suttorp, M. How I treat chronic myeloid leukemia in children and adolescents. Blood 2019, 133, 2374–2384. [Google Scholar] [CrossRef]
- Champagne, M.A.; Fu, C.H.; Chang, M.; Chen, H.; Gerbing, R.B.; Alonzo, T.A.; Cooley, L.D.; Heerema, N.A.; Oehler, V.; Wood, C.; et al. Higher dose imatinib for children with de novo chronic phase chronic myelogenous leukemia: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2011, 57, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, A. Optimal management for pediatric chronic myeloid leukemia. Ped. Int. 2016, 58, 171–179. [Google Scholar] [CrossRef] [PubMed]
| TKI | Instructions |
|---|---|
| Imatinib | Tablets may be dispersed in water or apple juice using 50 mL for 100 mg tablet or 200 mL for 400 mg tablet. The contents must be stirred until dissolved and used immediately. For children < 3 years old, it is recommended that at least 120 mL of water or food be taken to avoid esophageal irritation. |
| Dasatinib | Tablets can be allowed to dissolve over 20 min at room temperature in 30 mL of lemonade, preservative-free apple juice, or preservative-free orange juice. After ingestion, rinse the residue off glass with 15 mL of the juice and administer. |
| Nilotinib | Capsules may be dispersed in 5 mL of applesauce and ingested immediately on an empty stomach and abstain from eating for at least 1 h. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carofiglio, F.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Nicolotti, O.; Denora, N.; Stefanachi, A.; Leonetti, F. Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML. Int. J. Mol. Sci. 2020, 21, 4469. https://doi.org/10.3390/ijms21124469
Carofiglio F, Lopalco A, Lopedota A, Cutrignelli A, Nicolotti O, Denora N, Stefanachi A, Leonetti F. Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML. International Journal of Molecular Sciences. 2020; 21(12):4469. https://doi.org/10.3390/ijms21124469
Chicago/Turabian StyleCarofiglio, Francesca, Antonio Lopalco, Angela Lopedota, Annalisa Cutrignelli, Orazio Nicolotti, Nunzio Denora, Angela Stefanachi, and Francesco Leonetti. 2020. "Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML" International Journal of Molecular Sciences 21, no. 12: 4469. https://doi.org/10.3390/ijms21124469
APA StyleCarofiglio, F., Lopalco, A., Lopedota, A., Cutrignelli, A., Nicolotti, O., Denora, N., Stefanachi, A., & Leonetti, F. (2020). Bcr-Abl Tyrosine Kinase Inhibitors in the Treatment of Pediatric CML. International Journal of Molecular Sciences, 21(12), 4469. https://doi.org/10.3390/ijms21124469









