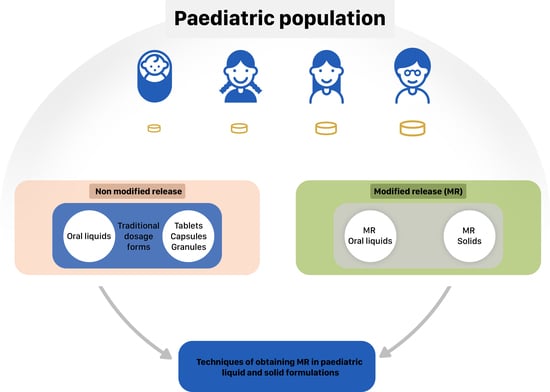
How to Modify Drug Release in Paediatric Dosage Forms? Novel Technologies and Modern Approaches with Regard to Children’s Population
Abstract
1. Introduction
2. MR Liquid Dosage Forms
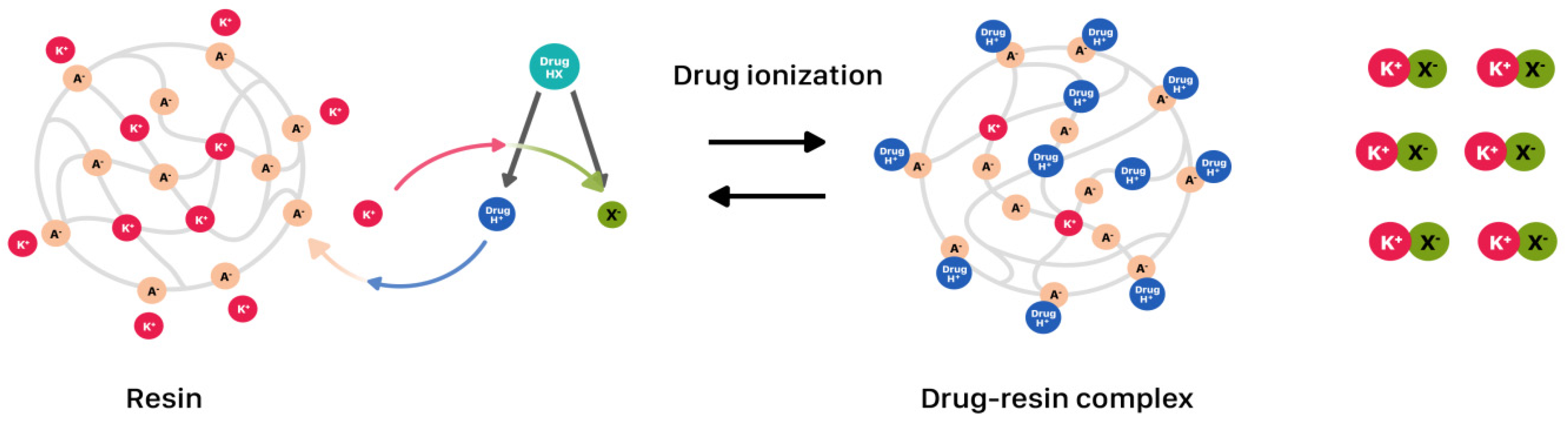
2.1. Drug-Resin Complexes
2.2. Microparticles—Spray Drying Technique
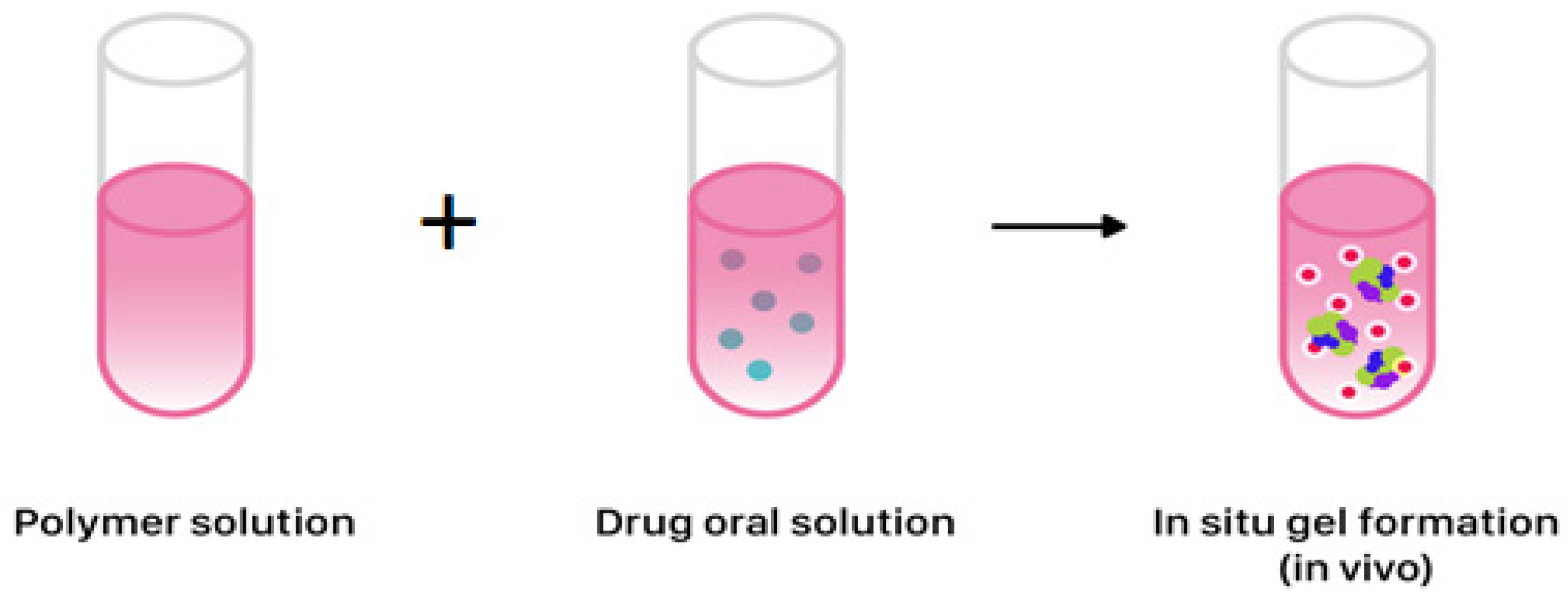
2.3. In situ Gel Formation
3. MR Solid Dosage Forms
3.1. Matrix and Coated Tablets
3.2. Multiparticulate MR Solid Dosage Forms (MultiP)
3.3. Minitablets
3.4. MR Orodispersible Formulations
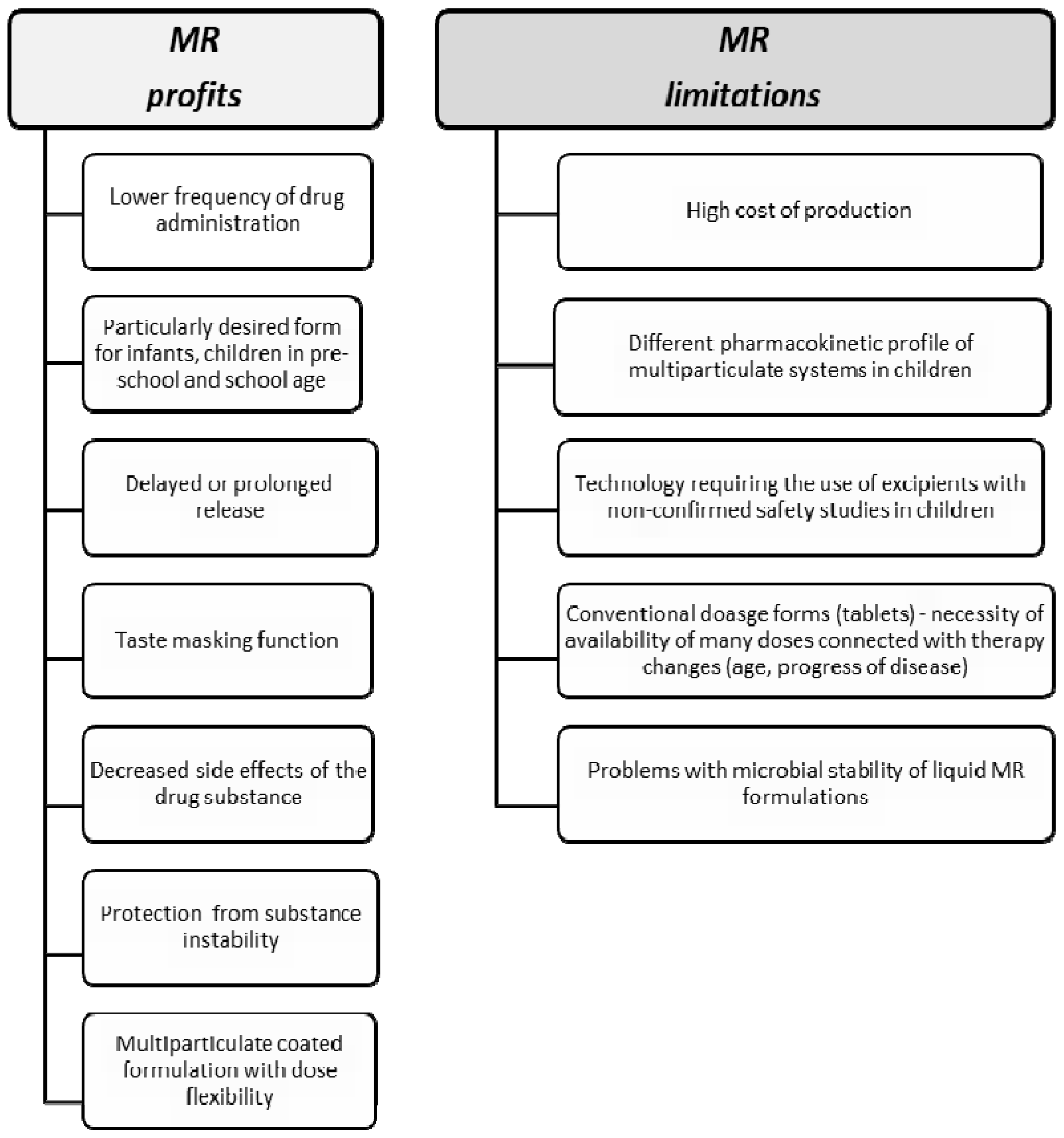
4. Excipients Utilized in MR—Safety of Use in Children
5. Novel Technologies 3D Printing for MR Formulations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MR | Modified Release |
| EMA | European Medicines Agency |
| BPCA | The Best Pharmaceuticals for Children |
| PIP | Paediatric Investigation Plan |
| STEP | Step&Toxicity of Excipients for Paediatric Patients Databaseb |
| FDA | Food and Drug Administration |
| ADHD | Attention-Deficit Hyperactivity Disorder |
| NA | No Data Available |
| HIV | Human Immunodeficiency Virus |
| MultiP | Multiparticulates |
| MUPS | Multi-Unit Pellet System |
| GERD | Gastro-Esophageal Reflux Disease |
| GRAS | Generally Recognized as Safe |
References
- Gore, R.; Chugh, P.K.; Tripathi, C.D.; Lhamo, Y.; Gautam, S. Paediatric off-label and unlicensed drug use and its implications. Curr. Clin. Pharmacol. 2017, 12, 18–25. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.; Conroy, S.; Avery, A.; Corns, H.; Choonara, I. Unlicensed and of label prescribing of drugs in general practice. Arch. Dis. Child. 2000, 83, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Frattarelli, D.A.; Galinkin, J.L.; Green, T.P.; Johnson, T.D.; Neville, K.A.; Paul, I.M.; Van Den Anker, J.N. American Academy of Paediatrics Committee on Drugs.: Off-label use of drugs in children. Paediatrics 2014, 133, 563–567. [Google Scholar]
- Salunke, S.; Tuleu, C. Formulating better medicines for children—Setting the pace for the future. Int. J. Pharm. 2013, 457, 308–309. [Google Scholar] [CrossRef] [PubMed]
- List of drugs for which paediatric studies are needed. Federal Register Notices; 2003; p. 68. Available online: https://bpca.nichd.nih.gov/prioritization/status/Documents/Federal_Register_01-21-2003.pdf (accessed on 5 April 2019).
- Reflection paper: Formulations of choice for the paediatric population, EMEA/CHMP/PEG/194810/2005. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf (accessed on 5 April 2019).
- Regulation (EC) No 1901/2006. Available online: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf (accessed on 5 April 2019).
- Salunke, S.; Liu, F.; Batchelor, H.; Walsh, J.; Turner, R.; Ju, T.R.; Tuleu, C. European Paediatric Formulation Initiative (EuPFI)-formulating ideas for better medicines for children. AAPS PharmSciTech. 2017, 18, 257–262. [Google Scholar] [CrossRef] [PubMed]
- State of paediatric medicines in the EU 10 years of the EU paediatric regulation, report from the Commission to the European Parliament and the Council, COM (2017) 626. Available online: https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf (accessed on 5 April 2019).
- Safety & Toxicity of Excipients for Paediatrics, STEP database. Available online: http://www.eupfi.org/step-database-info/ (accessed on 5 April 2019).
- Breslow, L.H. The best pharmaceuticals for children act of 2002: The rise of the voluntary incentive structure and congressional refusal to require paediatric testing. Harvard J. Legis. 2003, 40, 133–193. [Google Scholar] [PubMed]
- The BPCA priority list of needs in paediatric therapeutics. Federal Register Notices; 2014; 79. Available online: http://www.gpo.gov/fdsys/pkg/FR-2014-08-25/html/2014-20156.htm (accessed on 5 April 2019).
- Best Pharmaceuticals for Children Act (BPCA) Paediatric Formulation Initiative (PFI) Working Meeting. 6–7 December 2005. Available online: https://bpca.nichd.nih.gov/collaborativeefforts/documents/pfi_meeting_12-06-2005.pdf (accessed on 5 April 2019).
- Best Pharmaceuticals for Children Act Paediatric Formulations Initiative Workshop. 1–2 November 2011. Available online: https://bpca.nichd.nih.gov/collaborativeefforts/Documents/pfi_workshop_11-1-2011.pdf (accessed on 5 April 2019).
- Guideline on Pharmaceutical Development of Medicines for Paediatric Use, EMA/CHMP/QWP/805880/2012 Rev. 2. 2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf (accessed on 5 April 2019).
- Better Medicines for Children from Concept to Reality PROGRESS REPORT ON THE PAEDIATRIC REGULATION (EC) N°1901/2006. Available online: https://ec.europa.eu/health/sites/health/files/files/paediatrics/2013_com443/paediatric_report-com%282013%29443_en.pdf (accessed on 5 April 2019).
- Joseph, P.D.; Craig, J.C.; Caldwell, P.H.Y. Clinical trials in children. Br. J. Clin. Pharmacol. 2015, 79, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.R. Clinical trials for children: Some concerns. Indian J. Pharmacol. 2014, 46, 145–146. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to paediatric oral drug delivery: Benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef]
- Richey, R.H.; Shah, U.U.; Peak, M.; Craig, J.V.; Ford, J.L.; Barker, C.E.; Nunn, A.J.; Turner, M.A. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Richey, R.H. The Manipulation of Dosage Forms of Medications, With the Aim of Achieving the Required Dose, for Administration to Children. Ph.D. Thesis, University of Liverpool, Liverpool, UK, 2013. Available online: https://livrepository.liverpool.ac.uk/15475/4/RicheyRob_May2013_15475.pdf (accessed on 5 April 2019).
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable acceptance of mini-tablets compared with syrup: A randomized controlled trial in infants and preschool children. J. Pediatr. 2013, 163, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Seitz, A.; Meissner, T.; Breitkreutz, J.; Moeltner, A.; Bosse, H.M. Acceptability of uncoated mini-tablets in neonates-a randomized controlled trial. J. Pediatr. 2015, 167, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Kluk, A.; Sznitowska, M.; Brandt, A.; Sznurkowska, K.; Plata-Nazar, K.; Mysliwiec, M.; Kaminska, B.; Kotłowska, H. Can preschool-aged children swallow several minitablets at a time? Results from a clinical pilot study. Int. J. Pharm. 2015, 485, 1–6. [Google Scholar] [CrossRef]
- Feldman, M.; Bélanger, S. Extended-release medications for children and adolescents with attention-deficit hyperactivity disorder. Paediatr. Child Health. 2009, 14, 593–597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Riet-Nales, D.A.; Schobben, A.F.A.M.; Vromans, H.; Egberts, T.C.G.; Rademaker, C.M.A. Safe and effective pharmacotherapy in infants and preschool children: Importance of formulation aspects. Arch. Dis. Child. 2016, 101, 662–669. [Google Scholar] [CrossRef]
- Milap, C.N.; Loyd, V.A. Extemporaneous drug formulations. Clin. Ther. 2008, 30, 2112–2119. [Google Scholar]
- Riet-Nalesa, D.A.; Ferreira, J.A.; Schobben, A.F.A.M.; Neef, B.J.; Egberts, T.C.G.; Rademaker, C.D.A. Methods of administering oral formulations and child acceptability. Int. J. Pharm. 2015, 491, 261–267. [Google Scholar] [CrossRef]
- Loyd, V.A. Dosage form design and development. Clin. Ther. 2008, 30, 2102–2111. [Google Scholar]
- Flament, M.P. Extended release dosage: Recent advances and potential in paediatric medicine. Ther. Deliver. 2016, 7, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, V.; Rademaker, C.M.A.; Dijk, L.; Mantel-Teeuwisse, A.K. Paediatric drug formulations: A review of challenges and progress. Paediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Y.; Talegaonkar, S.; Iqbal, Z.; Ahmed, F.J.; Khar, R.K. Multiple emulsions: An overview. Curr. Drug Deliv. 2006, 3, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Kathpalia, H.; Phadke, C. Novel oral suspensions: A review. Curr. Drug Deliv. 2014, 11, 338–358. [Google Scholar] [CrossRef] [PubMed]
- Full Prescribing Information Delsym®. Available online: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm085592.pdf (accessed on 5 April 2019).
- Full Prescribing Information Dyanavel XR®. Available online: http://dyanavelxr.com/pdfs/pi.pdf (accessed on 5 April 2019).
- Full Prescribing Information MST® Continous®. Available online: https://www.medicines.org.uk/emc/product/1015/smpc#PHARMACOKINETIC_PROPS (accessed on 5 April 2019).
- Full Prescribing Information Quillivant XR®. Available online: https://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM602794.pdf (accessed on 5 April 2019).
- Tussionex® Drug Information: Description, User Reviews, Drug Side Effects, Interactions—Prescribing Information. Available online: https://www.rxlist.com/tussionex-drug.htm (accessed on 5 April 2019).
- Full Prescribing Information Zmax®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050797s016lbl.pdf (accessed on 5 April 2019).
- Saber/Oradur Technology on Durect Platform Information. Available online: http://www.durect.com/science-technologies/long-acting-injectables/saber-long-acting-injectables/ (accessed on 5 April 2019).
- Clinical trials of bupivacaine. Available online: https://clinicaltrials.gov/ct2/show/NCT01052012 (accessed on 5 April 2019).
- Ekelund, A.; Peredistijs, A.; Grohs, J.; Ellis, D.; Verity, N.; Rasmussen, S. SABER-Bupivacaine Reduces Postoperative Pain and Opioid Consumption Following Arthroscopic Subacromial Decompression. Personal Communication. 2016. Available online: http://www.durect.com/files/1914/6376/6227/EFORT2016_PostopPainReduction_Shoulder.pdf (accessed on 5 April 2019).
- ORADUR®-Methylphenidate ER Information. Available online: http://www.durect.com/pipeline/development/oradur-methylphenidate-er/ (accessed on 5 April 2019).
- Clinical Trials of Methylphenidate. Available online: https://clinicaltrials.gov/ct2/show/NCT02450890 (accessed on 5 April 2019).
- Kasashima, Y.; Uchida, S.; Yoshihara, K.; Yasuji, T.; Namiki, N. Oral sustained-release suspension based on a lauryl sulfate salt/complex. Int. J. Pharm. 2016, 515, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/variation-report/betmiga-h-c-2388-p46-0008-epar-assessment-report_en.pdf (accessed on 5 April 2019).
- REMOXY® (ORADUR®-Oxycodone) ER Capsules Information. Available online: http://www.durect.com/pipeline/development/remoxy/ (accessed on 5 April 2019).
- Clinical Trials of Oxycodone. Available online: https://clinicaltrials.gov/ct2/show/NCT01559701 (accessed on 5 April 2019).
- Pain Therapeutics Announces Feedback From Recent Meeting with FDA on Remoxy. Available online: https://www.drugs.com/nda/remoxy_er_190205.html (accessed on 5 April 2019).
- Singh, I.; Rehni, A.K.; Kalra, R.; Joshi, G.; Kumar, M.; Aboul-Enein, H.Y. Ion exchange resins: Drug delivery and therapeutic applications. FABAD J. Pharm. Sci. 2007, 32, 91–100. [Google Scholar]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Kojima, Y.; Ohta, T.; Shiraki, K.; Takano, R.; Maeda, H.; Ogawa, Y. Effects of spray drying process parameters on the solubility behavior and physical stability of solid dispersions prepared using a laboratory-scale spray dryer. Drug Dev. Ind. Pharm. 2013, 39, 1484–1493. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.D.; Selomuyla, C. On the spray drying of uniform functional microparticles. Particuology 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2009, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J.A. In situ forming polymeric drug delivery systems. Indian J. Pharm. Sci. 2009, 71, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Binu, C.; Surajpal, V. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. ScientificWorldJournal 2014, 1–7. [Google Scholar]
- Makwana, S.B.; Patel, V.A.; Parmar, S.J. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma. Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Salatin, S.; Naderinia, A.; Jalvehgari, M. Novel pentablock copolymers as thermosensitive self-assembling micelles for ocular drug delivery. Adv. Pharm. Bull. 2017, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sarada, K.; Firoz, S.; Padmini, K. In-situ gelling system: A review. Int. J. Curr. Pharm. Res. 2015, 5, 76–90. [Google Scholar]
- Jaya, R.K.K.; Selvadurai, M.; Sokkalingam, A.D. A review: Polymeric in-situ gel system. Res. Rev. J. Pharm. Pharm. Sci. 2013, 2, 1–7. [Google Scholar]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Orubu, E.S.F.; Tuleua, C. Medicines for children: Flexible solid oral formulations. Bull. World Health Organ. 2017, 95, 238–240. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Raja, S.; Patel, P.; Asare-Addo, K. The role of oral controlled release matrix tablets in drug delivery systems. Bioimpacts. 2012, 2, 175–187. [Google Scholar]
- Mondal, N. The role of matrix tablet in drug delivery system. Int. J. App. Pharm. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Development of Paediatric Medicines: Points to Consider in Formulation. Available online: https://www.who.int/childmedicines/partners/SabineKopp_Partners.pdf (accessed on 5 April 2019).
- Full Prescribing Information Lamictal® XR. Available online: https://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm215664.pdf (accessed on 5 April 2019).
- Full Prescribing Information Aciphex® Sprinkle™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020973s035204736s005lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Adderall XR®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf (accessed on 5 April 2019).
- Adzenys XR-ODT® Tablets Information. Available online: https://www.adzenysxrodt.com/ (accessed on 5 April 2019).
- Full Prescribing Information Adzenys XR-ODT®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204326s002lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Azulfidine®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/007073s124lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Concerta®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021121s038lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Coreg CR®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022012s010s013lbl.pdf (accessed on 5 April 2019).
- Cotempla XR-ODT® tablets information. Available online: https://www.cotemplaxrodt.com/ (accessed on 5 April 2019).
- Full Prescribing Information Cotempla XR-ODT®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205489s000lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Creon. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020725s000lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Finlepsin. Available online: http://www.ecopharm.bg/images/product/product_13_44_file2.pdf (accessed on 5 April 2019).
- Full Prescribing Information Focalin™ XR. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021802lbl.pdf (accessed on 5 April 2019).
- GranuPAS® Granules Information. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/granupas-previously-para-aminosalicylic-acid-lucane (accessed on 5 April 2019).
- Full Prescribing Information Kapvay®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022331s001s002lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Keppra®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080,021505s021s024lbl.pdf (accessed on 5 April 2019).
- Losec MUPS Capsules Information. Available online: https://www.medicines.org.uk/emc/files/pil.1493.pdf (accessed on 5 April 2019).
- Full Prescribing Information Metadate CD®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021259s021lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Moxatag™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050813lbl.pdf (accessed on 5 April 2019).
- Moxatag™ Tablets Information. Available online: http://www.moxatag.com/hcp_delivery.htm (accessed on 5 April 2019).
- Orfiril Long Minitablets Information. Available online: https://ec.europa.eu/health/documents/community-register/2018/20180531140837/anx_140837_en.pdf (accessed on 5 April 2019).
- Full Prescribing Information Pancrease MT®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022523Orig1s000ClinPharmR.pdf (accessed on 5 April 2019).
- Full Prescribing Information Pentasa. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020049s031lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Prevacid®, Prevacid® Solutab™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020406s078-021428s025lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Prevacid®, Prevacid® Solutab™. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020406s058,021281s017,021428s006lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Ritalin® LA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021284s010lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Tegretol® -XR. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/016608s101,018281s048lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Toprol-XL®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019962s038lbl.pdf (accessed on 5 April 2019).
- Full Prescribing Information Viramune®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020636s039_020933s030lbl.pdf (accessed on 5 April 2019).
- Martínez-Terán, M.E.; Hoang-Thi, T.H.; Flament, M.P. Multi-particulate dosage forms for paediatric use. Pediatr. Ther. 2017, 7, 314. [Google Scholar] [CrossRef]
- Shajahan, A.A.; Chandewar, V.; Jaiswal, S.B. A flexible technology for modified-release drugs: Multiple-unit pellet system (MUPS). J. Control Release 2010, 147, 2–16. [Google Scholar]
- Full Prescribing Information Prilosec (Omeprazole Magnesium) Delayed-Release Oral Suspension. Available online: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PaediatricAdvisoryCommittee/UCM610733.pdf (accessed on 5 April 2019).
- Milić, J.; Radojković, B.; Jančić-Stojanović, B.; Drašković, J.; Mirašević, S.; Čalija, B. Investigation of omeprazole stability in oral suspensions for paediatric use prepared extemporaneously from omeprazole capsules. Arh. Farm. 2017, 67, 14–25. [Google Scholar]
- Garg, S.; Svirskis, D.; Al-Kabban, M.; Farhan, S.; Komeshi, M.; Lee, J.; Liu, Q.; Naidoo, S.; Kairuz, T. Chemical stability of extemporaneously compounded omeprazole formulations: A comparison of two methods of compounding. Int. J. Pharm. Compd. 2009, 13, 250–253. [Google Scholar] [PubMed]
- Moodley, K.; Pillay, V.; Choonara, Y.E.; du Toit, L.C.; Ndesendo, V.M.K.; Kumar, P.; Cooppan, S.; Bawa, P. Oral drug delivery systems comprising altered geometric configurations for controlled drug delivery. Int. J. Mol. Sci. 2012, 13, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Venkata, P.D.B. Spheroidal oral drug absorption system (SODAS). J. Glob. Pharma Technol. 2011, 3, 1–5. [Google Scholar]
- Elan Drug Technologies. Spheroidal Drug Absorption System (SODAS®). Available online: http://www.elandrugtechnologies.com/oral_controlled_release/sodas (accessed on 5 April 2019).
- Weil, A.J. Cyclobenzaprine extended-release: The difference is in the formulation. Pharm. Times. 2009. Available online: https://www.pharmacytimes.com/p2p/cyclobenzaprine-extended-release (accessed on 5 April 2019).
- Kluk, A.; Sznitowska, M. Application properties of oral gels as media for administration of minitablets and pellets to paediatric patients. Int. J. Pharm. 2014, 460, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Preis, M. Orally Disintegrating films and mini-tablets—innovative dosage forms of choice for paediatric use. APS PharmSciTech. 2015, 16, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V. Acceptability of mini-tablets in young children: Results from three prospective cross-over studies. AAPS PharmSciTech. 2017, 18, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, A.; Fossler, M.J.; Barrett, J.S.; Worthington, J.H.; Ternik, R.; Charkoftaki, G.; Lum, S.; Breitkreutz, J.; Baltezor, M.; Macheras, P.; et al. Report from the paediatric formulations task force: Perspectives on the state of child-friendly oral dosage form. AAPS J. 2013, 15, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Van Riet-Nales, D.A.; Kozarewicz, P.; Aylward, B.; de Vries, R.; Egberts, T.C.; Rademaker, C.M.; Schobben, A.F. Paediatric drug development and formulation design—An European perspective. AAPS PharmSciTech. 2017, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- FDA, CDER, Guidance for industry—orally disintegrating tablets. 2008. Available online: https://www.fda.gov/downloads/Drugs/.../Guidances/ucm070578.pdf (accessed on 5 April 2019).
- The European Pharmacopoeia, 9th ed.; Council of Europe: Strasburg, France, 2016.
- Visser, J.C.; Woerdenbag, H.J.; Hanff, L.M.; Frijlink, H.W. Personalized medicine in paediatrics: The clinical potential of orodispersible films. AAPS PharmSciTech. 2017, 18, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, K.; Winnicka, K. How to assess orodispersible film quality? A review of applied methods and their modifications. Acta Pharm. 2019, 69, 155–176. [Google Scholar] [CrossRef]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Bilayered buccal films as child-appropriate dosage form for systemic administration of propranolol. Formulation and characterization of fast dissolving buccal films: A review. Int. J. Pharm. 2017, 5, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.A.; Salunke, S.; Thompson, K.; Baer, G.; Fegley, D.; Turner, M.A. Challenges and strategies to facilitate formulation development of paediatric drug products: Safety qualification of excipients. Int. J. Pharm. 2018, 536, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J. Excipients for the formulation of medicines for children. Eur. Ind. Pharm. 2012, 13, 14–16. [Google Scholar]
- Nahata, M.C. Safety of “inert” additives or excipients in paediatric medicines. Arch. Dis. Child. Fetal. Neonatal. Ed. 2009, 94, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, N. Ethanol exposure through medicines commonly used in paediatrics. Arch. Dis. Child. Educ. Pract. Ed. 2015, 100, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A. Stability of Compounded Formulations, 5th ed.; American Pharmacists Association: Washington, DC, USA, 2012. [Google Scholar]
- Emami, J.; Varshozas, J.; Ahmadi, F. Preparation and evaluation of a liquid sustained-release drug delivery system for theophylline using spray-drying technique. Res. Pharm. Sci. 2007, 2, 1–11. [Google Scholar]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of printing technologies for oral film formulations. Printing technologies in fabrication of drug delivery systems. Int. J. Pharm. 2015, 305, 78–584. [Google Scholar]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 30, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control Release 2015, 10, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” drug loaded dosage forms for paediatric applications. Pharm Res. 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Spritam® Drug Information. Available online: https://www.spritam.com/#/patient/about-spritam/what-is-spritam (accessed on 5 April 2019).
- Full Prescribing Information Spritam®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2016/207958Orig1s002ltr.pdf (accessed on 5 April 2019).

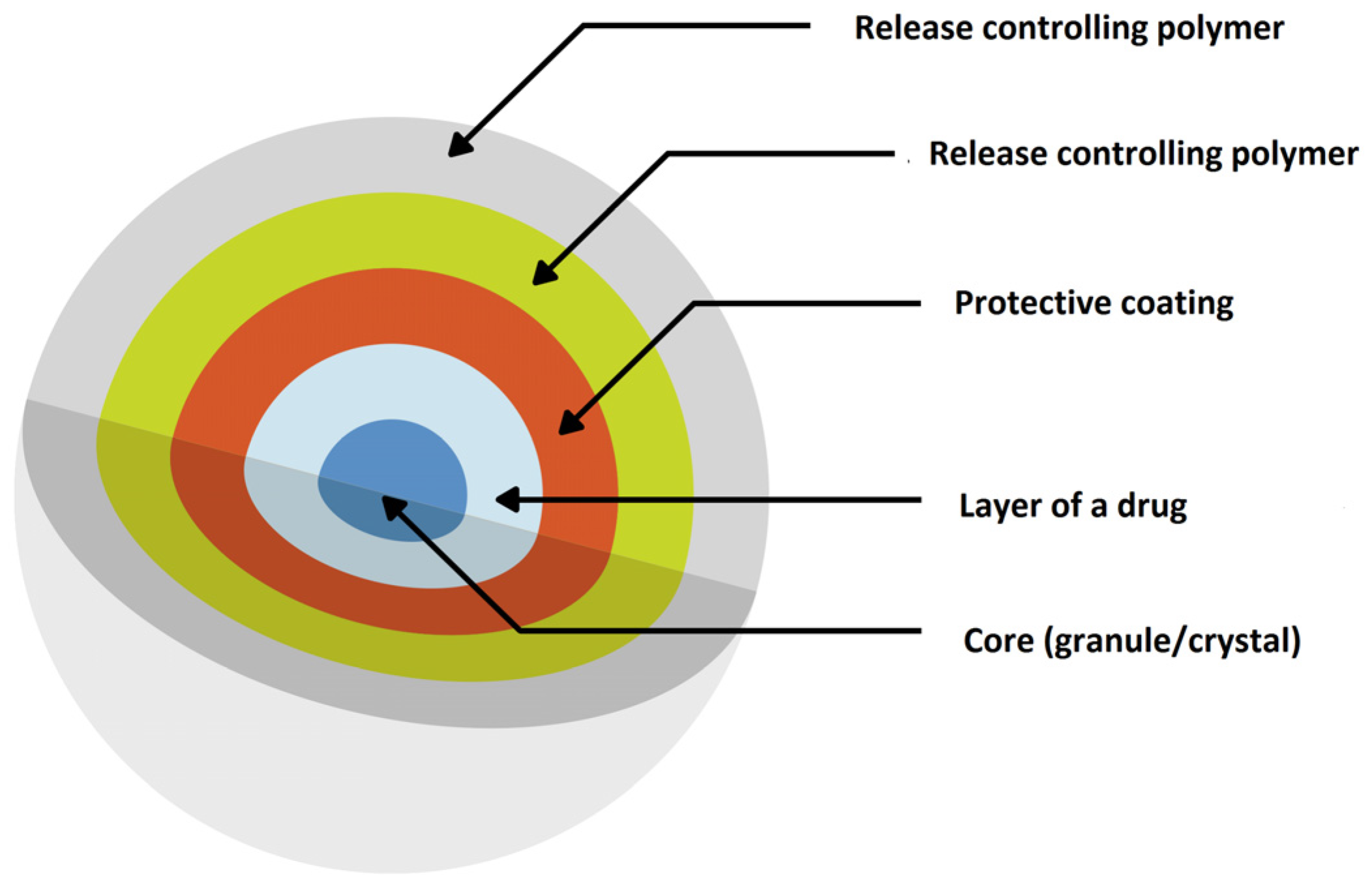
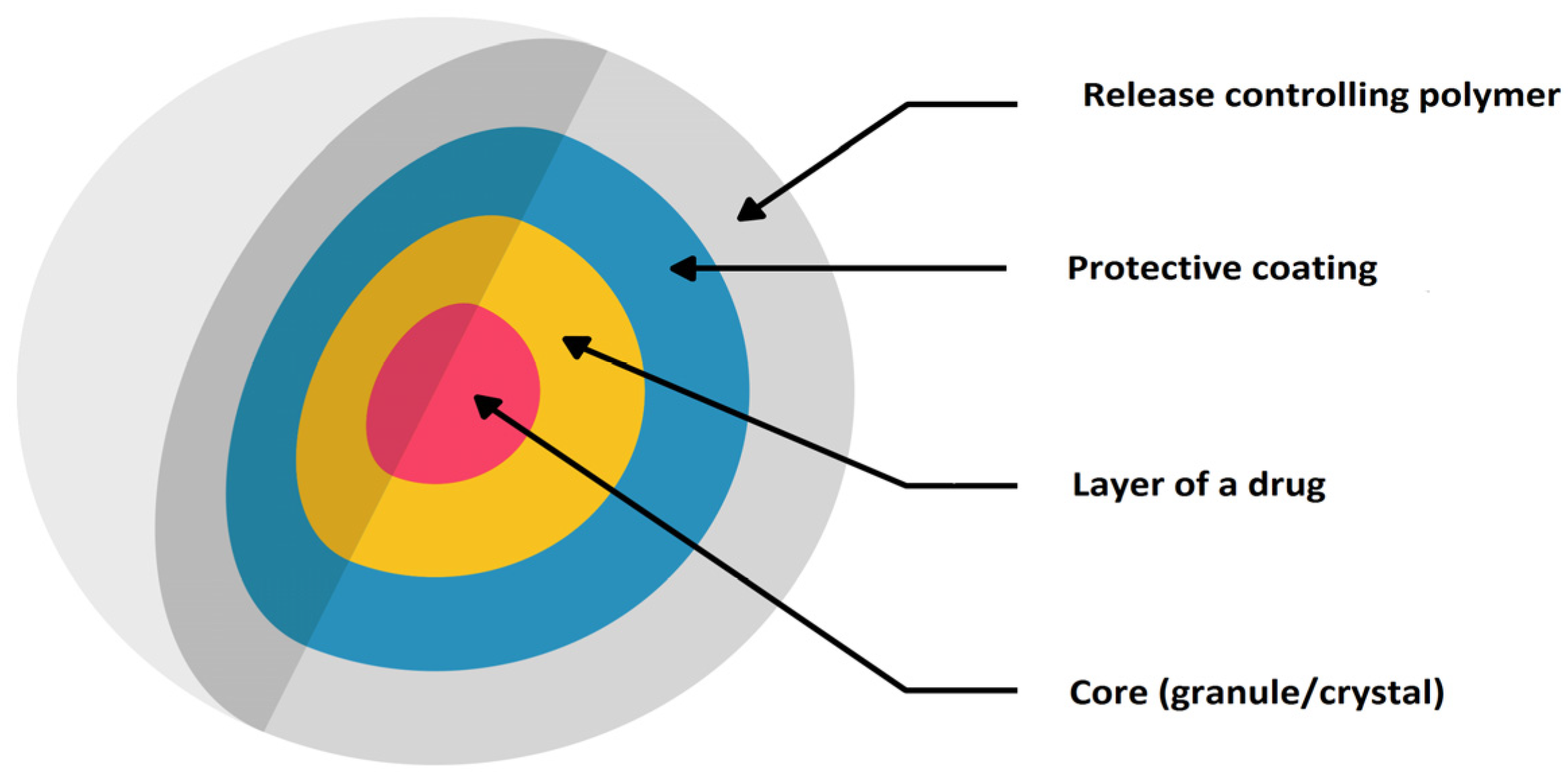
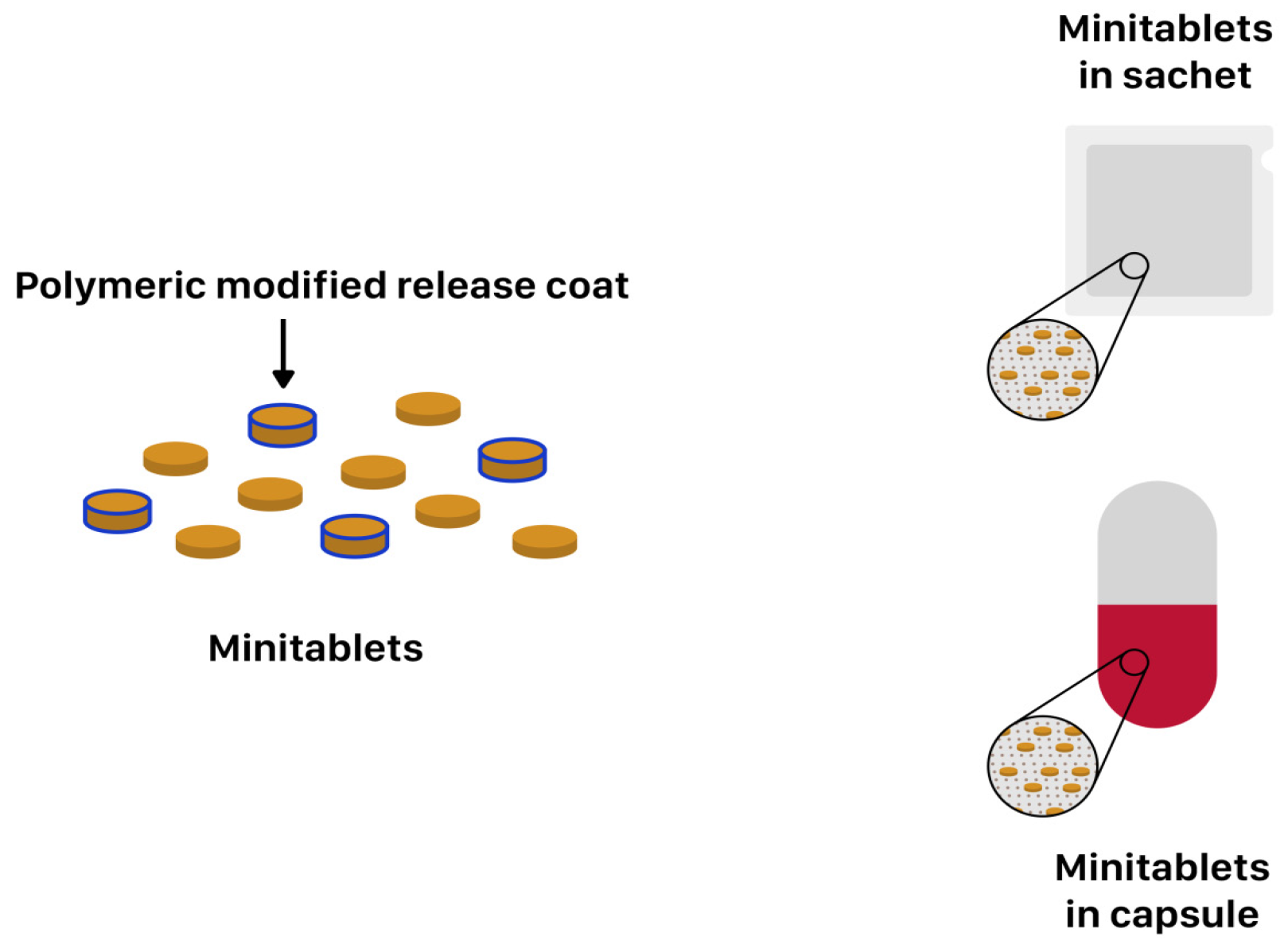
| Product (Manufacturer) | Drug | Dosage Form | Polymer (MR Technique) | Paediatric Licence | Indication | References |
|---|---|---|---|---|---|---|
| Delsym® (Reckitt Benckiser LLC.) | dextromethorphan | extended-release suspension | ion exchange resin drug/polymer complexation | ≥4 year | Cough | [36] |
| Dyanavel XR (TrisPharma) | amphetamine | extended-release suspension | sodium polystyrene sulfonate drug/polymer complexation | ≥6 years | attention-deficit hyperactivity disorder (ADHD) | [37] |
| MST® Continus® (Napp Pharmaceuticals Limited) | morphine | prolonged-release suspension | cationinc exchange resin drug/polymer complexation | ≥1 year | Pain | [38] |
| Quillivant XR (Pfizer) | methylphenidate | extended-release suspension | sodium polystyrene sulfonate drug/polymer complexation | ≥6 years | ADHD | [39] |
| Tussionex®(UCB) | Hydrocodone chlorpheniramine | extended-release suspension | ion exchange resin drug/polymer complexation microparticles | ≥6 years | common cold, flu | [40] |
| Zmax® (Pfizer) | azithromycin | extended-release suspension | glyceryl behenate and poloxamer 407 microspheres | ≥6 month | bacterial infections | [41] |
| under approval registration process by FDA | bupivacaine | NA * | SABER™ Delivery System in situ gel formation | NA * | local anesthetic | [42,43,44] |
| under approval registration process by FDA | methylphenidate | NA * | ORADUR™ in situ gel formation | NA * | pain | [45,46] |
| under approval registration process by FDA | mirabegron | sustained-release suspensions | microspheres with lauryl sulfate salt/complex | NA * | urinary incontinence | [47,48] |
| under approval registration process by FDA (Remoxy®) | oxycodone | NA * | ORADUR™ in situ gel formation | NA * | Pain | [49,50,51] |
| Product (Manufacturer) | Drug | Dosage Form | Polymer/MR Technique | Paediatric Licence | Indication | References |
|---|---|---|---|---|---|---|
| Aciphex® Sprinkle™ (Eisai Management Co., Ltd.) | rabeprazole | capsules with granules | ethylcellulose (delayed release) | ≥1 year | reflux | [70] |
| Adderall XR® (Shire US Inc) | mixed salts of single-entity amphetamine product | capsules with granules | hypromellose, methacrylic acid copolymer (immediate and delayed release) | ≥6 years | attention deficit hyperactivity disorder (ADHD) | [71] |
| Adzenys XR-ODT™ (Neos Therapeutics) | amphetamine | extended release orally disintegrating tablets (ODT) | methacrylic acid, ethylcellulose (ion resin technology) | ≥6 years | ADHD | [72,73] |
| Azulfidine® (Pfizer) | sulfasalazine | tablets | cellulose acetate phthalate (delayed release) | ≥6 years | mild to moderate ulcerative colitis Crohn disease – off labell use | [74] |
| Concerta® (Janssen-Cilag) | methylphenidate | tablets | cellulose acetate (matrix, extended release) | ≥6 years | ADHD | [75] |
| Coreg CR® (GSK) | carvedilol | capsules | methacrylic acid copolymers (controled release) | ≥6 years | hypertension | [76] |
| Cotempla XR-ODT® (Neos Therapeutics) | methylphenidate | orodispersible tablets | methacrylic acid, ethylcellulose, polystyrene sulfate (immediate and extended release) | ≥6 years | ADHD | [77,78] |
| Creon® (Solvay Pharmaceuticals) | pancreatic enzymes | Minitablets (in capsules) | dibuthyl phthalate, hypromellose phthalate (delayed-release) | ≥6 years | chronic pancreatitis, cystic fibrosis | [79] |
| Finlepsin® (Teva Pharmaceuticals) | carbamazepine | tablets | Eudragit RS 30D, Eudragit L 30 D (extended release) | ≥6 years | epilepsy | [80] |
| Focalin™ XR (Elan Holdings Inc.) | dexmethylphenidate | capsules | ammonio methacrylate copolymer (extended release) | ≥6 years | ADHD | [81] |
| GranuPAS® (Lucane Pharma) | para-aminosalicylic acid | gastro-resistant granules in sachet | methacrylic acid – ethyl acrylate copolymer (1:1) (extended release) | ≥1 year | tuberculosis | [82] |
| Kapvay ® (Concordia Pharmaceuticals Inc.) | clonidine | tablets | hypromellose (diffusion from gel matrix structure -extended release) | ≥6 years | ADHD | [83] |
| Keppra XR® (UCB) | levetiracetam | tablets | hypromellose, polyvinyl alcohol- (diffusion from gel matrix structure -extended release) | ≥12 years | epilepsy | [84] |
| Lamictal® XR (GSK) | lamotrigine | tablets | methacrylic acid copolymers (extended release) | ≥12 years | epilepsy | [69] |
| Losec MUPS (AstraZeneca AB) | omeprazole | gastro-resistant tablets with coated pellets | methacrylic acid – ethyl acrylate copolymer (1:1) dispersion (delayed-release) | ≥1 year | reflux | [85] |
| Metadate CD® (UCB Manufacturing, Inc.) | methylphenidate | capsules with granules | hypromellose, polyethylene glycol, ethylcellulose (immediate and extended release) | ≥6 years | ADHD | [86] |
| Moxatag™ (MiddleBrook Pharmaceuticals, Inc.) | amoxicilin | tablets | prolonged-release pulsatile delivery technology MUPS | ≥12 years | tonsillitis, pharyngitis | [87,88] |
| Orfiril Long (Desitin Arzneimittel GmbH) | natrii valproas | Minitablets (in sachet or capsule) | ethylcellulose, ammonium methacrylate copolymer (extended release) | ≥6 years | epilepsy | [89] |
| Pancrease MT® (McNeil) | pancreatic enzymes | enteric-coated minitablets in capsule | methacrylic acid ethyl acrylate copolymers (delayed release) | from birth | chronic pancreatitis, cystic fibrosis | [90] |
| Pentasa® (Ferring GmbH) | mesalazine | granules | Ethylcellulose (prolonged release) | ≥6 years | Crohn’s disease | [91] |
| Prevacid® SoluTab™ (Takeda) | lansoprazole | orodispersible tablets | methacrylic acid copolymer (delayed relese) | ≥1 year | reflux | [92,93] |
| Ritalin® LA (Novartis) | methylphenidate | capsules | ammonio methacrylate copolymer, gelatin, methacrylic acid copolymer (delayed relese) | ≤ 6 years | ADHD | [94] |
| Tegretol® XL (Novartis) | carbamazepine | tablets | ethylcellulose dispersion (matrix, extended release) | ≤ 6 years | epilepsy | [95] |
| TOPROL-XL® (Aralez Pharmaceuticals) | metoprolol | tablets | cellulose compounds (extended release) | ≥6 years | hypertension | [96] |
| Viramune® (Boehringer Ingelheim International GmbH) | nevirapine | tablets | hypromellose (prolonged release) | ≥3 years | human immunodeficieny virus (HIV) infection | [97] |
| Excipient | Paediatric Safety Data Use | Main Function in Formulation | |
|---|---|---|---|
| Cellulos derivatives | cellulose acetate | NA * | MR |
| cellulose acetate phthalate | NA * | MR | |
| carmellose sodium | yes | suspending agent | |
| ethylcellulose | yes | MR taste masking | |
| hypromellose | yes | suspending agent MR taste masking | |
| methylcellulose | yes | suspending agent | |
| ion exchange resin | NA * | drug/polymer complexation | |
| methacrylic acid copolymers | NA * | MR | |
| sodium polystyrene sulfate | NA * | drug/polymer complexation MR | |
| sodium alginate | NA * | MR | |
| calcium sulfate | NA * | MR | |
| lauryl sulfate | NA * | MR | |
| polyvinyl alcohol | NA * | MR | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimiuk, M.; Wasilewska, K.; Winnicka, K. How to Modify Drug Release in Paediatric Dosage Forms? Novel Technologies and Modern Approaches with Regard to Children’s Population. Int. J. Mol. Sci. 2019, 20, 3200. https://doi.org/10.3390/ijms20133200
Trofimiuk M, Wasilewska K, Winnicka K. How to Modify Drug Release in Paediatric Dosage Forms? Novel Technologies and Modern Approaches with Regard to Children’s Population. International Journal of Molecular Sciences. 2019; 20(13):3200. https://doi.org/10.3390/ijms20133200
Chicago/Turabian StyleTrofimiuk, Monika, Katarzyna Wasilewska, and Katarzyna Winnicka. 2019. "How to Modify Drug Release in Paediatric Dosage Forms? Novel Technologies and Modern Approaches with Regard to Children’s Population" International Journal of Molecular Sciences 20, no. 13: 3200. https://doi.org/10.3390/ijms20133200
APA StyleTrofimiuk, M., Wasilewska, K., & Winnicka, K. (2019). How to Modify Drug Release in Paediatric Dosage Forms? Novel Technologies and Modern Approaches with Regard to Children’s Population. International Journal of Molecular Sciences, 20(13), 3200. https://doi.org/10.3390/ijms20133200