Natural Mating Differentially Triggers Expression of Glucocorticoid Receptor (NR3C1)-Related Genes in the Preovulatory Porcine Female Reproductive Tract
Abstract
1. Introduction
2. Results
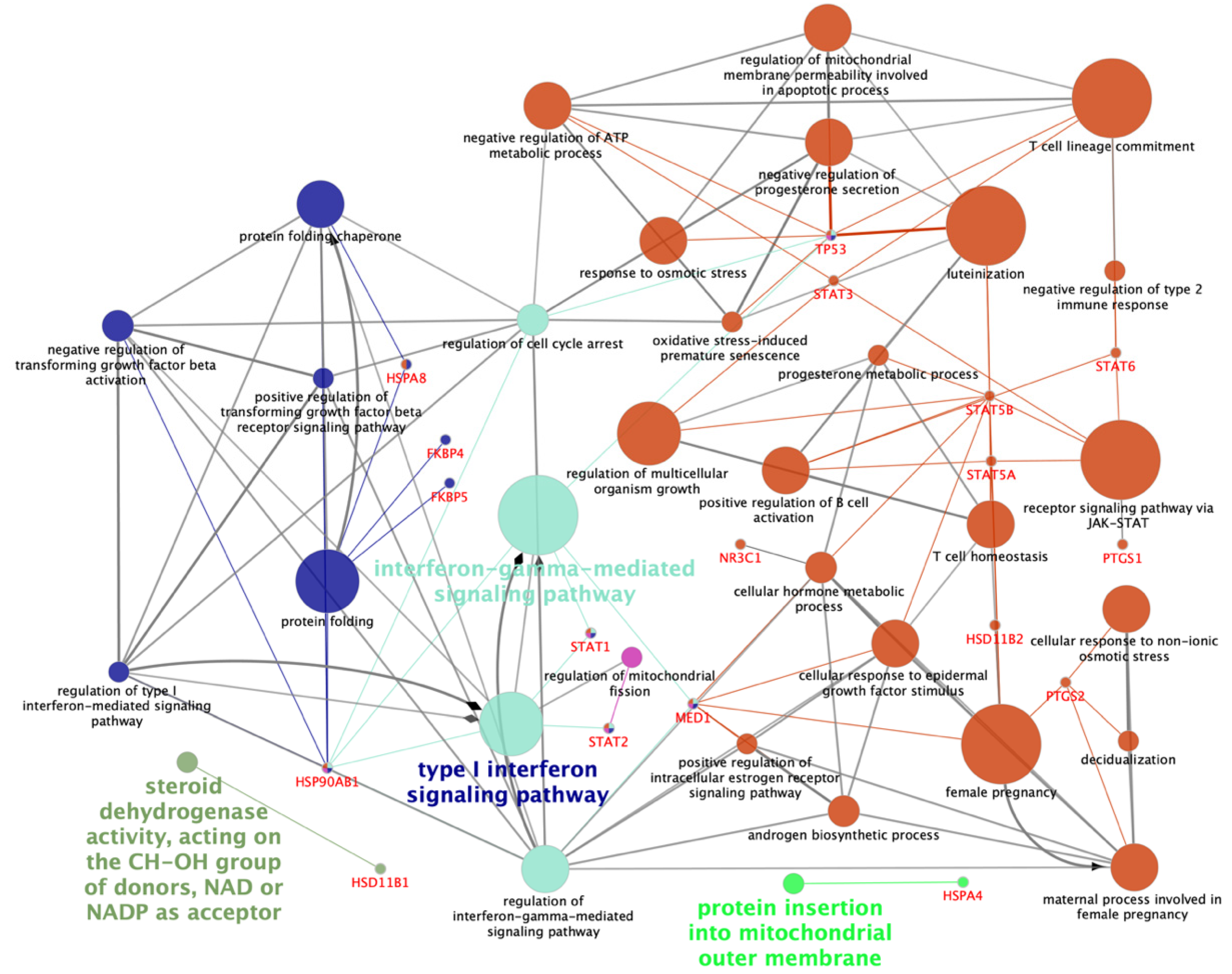
2.1. Gene Ontology of the Genes Related to the Glucocorticoid Receptor NR3C1
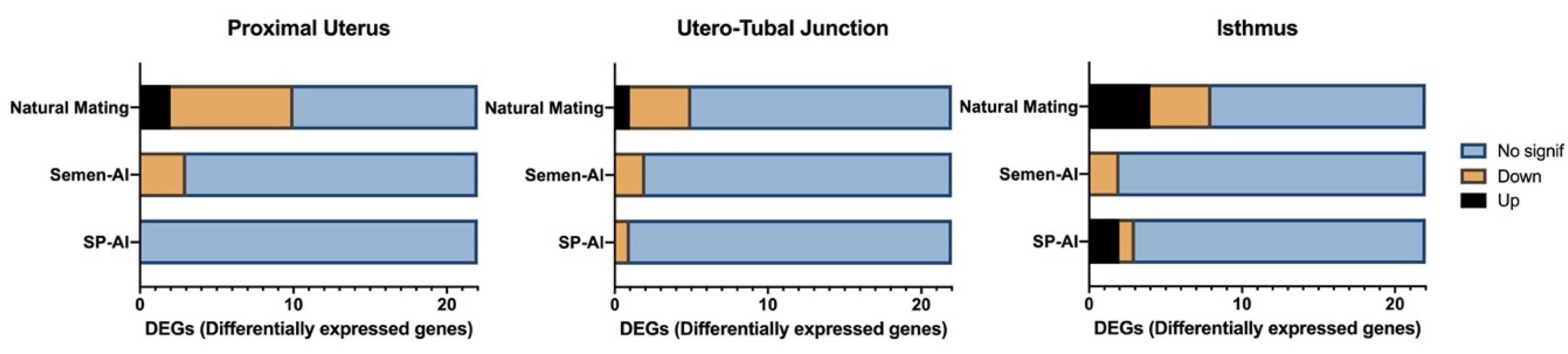
2.2. Natural Mating and AI of Semen Components Altered the Expression of Genes Related to the Glucocorticoid Receptor NR3C1
2.3. KEGG Pathways Analysis
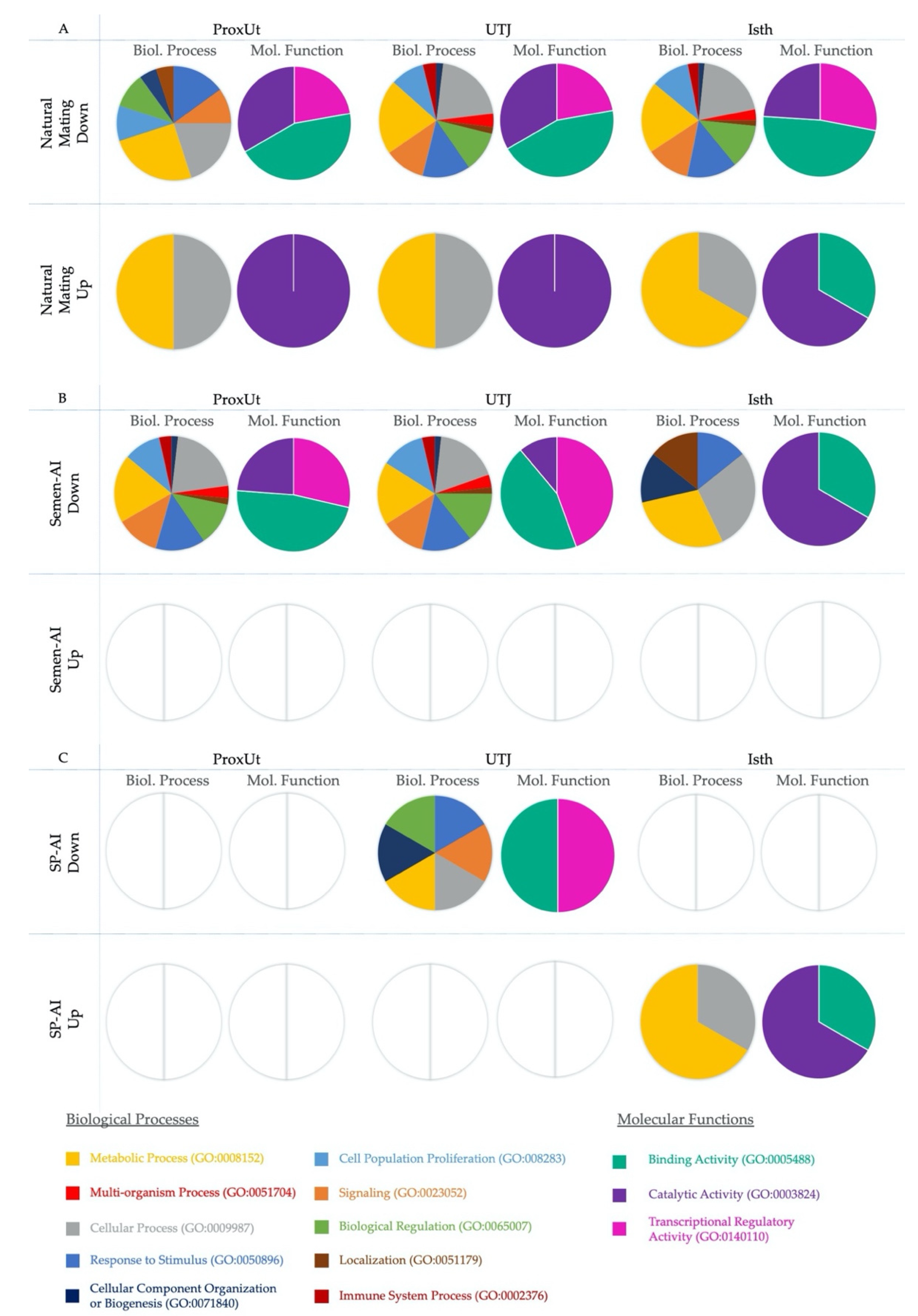
2.4. PANTHER Gene Ontology Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Design of the Study
4.3. Animal Management
4.4. Collection and Handling of Semen and Tissue Samples
4.5. Microarray Hybridization and Scanning
4.6. Microarray Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol. Metab. 2017, 28, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Tilbrook, A.J.; Turner, A.I.; Clarke, I.J. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev. Reprod. 2000, 5, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Whirledge, S.; Cidlowski, J.A. A role for glucocorticoids in stress-impaired reproduction: Beyond the hypothalamus and pituitary. Endocrinology 2013, 154, 4450–4468. [Google Scholar] [CrossRef] [PubMed]
- Fanson, K.V.; Parrott, M.L. The value of eutherian-marsupial comparisons for understanding the function of glucocorticoids in female mammal reproduction. Horm. Behav. 2015, 76, 41–47. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010, 35, 109–125. [Google Scholar]
- Yazawa, H.; Sasagawa, I.; Nakada, T. Apoptosis of testicular germ cells induced by exogenous glucocorticoid in rats. Hum. Reprod. 2000, 15, 1917–1920. [Google Scholar] [CrossRef]
- Kowal, B.F.; Turco, J.; Nangia, A.K. Addison’s disease presenting as male infertility. Fertil. Steril. 2006, 85, e1–e1059. [Google Scholar] [CrossRef]
- Whirledge, S.D.; Oakley, R.H.; Myers, P.H.; Lydon, J.P.; DeMayo, F.; Cidlowski, J.A. Uterine glucocorticoid receptors are critical for fertility in mice through control of embryo implantation and decidualization. Proc. Natl. Acad. Sci. USA 2015, 112, 15166–15171. [Google Scholar] [CrossRef]
- Lee, H.Y.; Acosta, T.J.; Tanikawa, M.; Sakumoto, R.; Komiyama, J.; Tasaki, Y.; Piskula, M.; Skarzynski, D.J.; Tetsuka, M.; Okuda, K. The role of glucocorticoid in the regulation of prostaglandin biosynthesis in non-pregnant bovine endometrium. J. Endocrinol. 2007, 193, 127–135. [Google Scholar] [CrossRef]
- Simmons, R.M.; Satterfield, M.C.; Welsh, T.H.; Bazer, F.W.; Spencer, T.E. HSD11B1, HSD11B2, PTGS2, and NR3C1 Expression in the Peri-Implantation Ovine Uterus: Effects of Pregnancy, Progesterone, and Interferon Tau1. Biol. Reprod. 2010, 82, 35–43. [Google Scholar] [CrossRef]
- Siemieniuch, M.J.; Majewska, M.; Takahashi, M.; Sakatani, M.; Łukasik, K.; Okuda, K.; Skarzynski, D.J. Are glucocorticoids auto- and/or paracrine factors in early bovine embryo development and implantation? Reprod. Biol. 2010, 10, 249–256. [Google Scholar] [CrossRef]
- Michael Romero, L. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002, 128, 1–24. [Google Scholar] [CrossRef]
- Wang, J.; Harris, C. Glucocorticoid Signaling From Molecules to Mice to Man; Springer: New York, NY, USA, 2015; ISBN 9781493928941. [Google Scholar]
- Vanderbilt, J.N.; Miesfeld, R.; Maler, B.A.; Yamamoto, K.R. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol. Endocrinol. 1987, 1, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ratman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.M.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.B.; Hong, Y.; Dhe-Paganon, S.; Yoon, H.S. FKBP family proteins: Immunophilins with versatile biological functions. NeuroSignals 2008, 16, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Franchimont, D.; Hiroi, N.; Frey, G.; Boettner, A.; Ehrhart-Bornstein, M.; O’shea, J.J.; Chrousos, G.P.; Bornstein, S.R. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002, 16, 61–71. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Schmidt, M.; Lax, E.; Zhou, R.; Cheishvili, D.; Ruder, A.M.; Ludiro, A.; Lapert, F.; Macedo da Cruz, A.; Sandrini, P.; Calzoni, T.; et al. Fetal glucocorticoid receptor (Nr3c1) deficiency alters the landscape of DNA methylation of murine placenta in a sex-dependent manner and is associated to anxiety-like behavior in adulthood. Transl. Psychiatry 2019, 9. [Google Scholar] [CrossRef]
- Orihuela, P.A.; Ríos, M.; Croxatto, H.B. Disparate Effects of Estradiol on Egg Transport and Oviductal Protein Synthesis in Mated and Cyclic Rats1. Biol. Reprod. 2001, 65, 1232–1237. [Google Scholar] [CrossRef]
- Shafik, A.; Shafik, I.; El Sibai, O.; Shafik, A.A. Oviductal motile response to penile cervical buffeting. Arch. Gynecol. Obstet. 2006, 273, 216–220. [Google Scholar] [CrossRef]
- Apichela, S.A.; Argañaraz, M.E.; Giuliano, S.; Zampini, R.; Carretero, I.; Miragaya, M.; Miceli, D.C. Llama oviductal sperm reservoirs: Involvement of bulbourethral glands. Andrologia 2014, 46, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, A.; Affara, N.A.; Hubank, M.; Holt, W.V. Sperm-Induced Modification of the Oviductal Gene Expression Profile After Natural Insemination in Mice1. Biol. Reprod. 2004, 71, 60–65. [Google Scholar] [CrossRef]
- Almiñana, C.; Caballero, I.; Heath, P.R.; Maleki-Dizaji, S.; Parrilla, I.; Cuello, C.; Gil, M.A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; et al. The battle of the sexes starts in the oviduct: Modulation of oviductal transcriptome by X and Y-bearing spermatozoa. BMC Genomics 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Parada-Bustamante, A.; Oróstica, M.L.; Reuquen, P.; Zuñiga, L.M.; Cardenas, H.; Orihuela, P.A. The role of mating in oviduct biology. Mol. Reprod. Dev. 2016, 83, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, M.; Martinez, C.A.; Wright, D.; Rodríguez-Martinez, H. The role of semen and seminal plasma in inducing large-scale genomic changes in the female porcine peri-ovulatory tract. Sci. Rep. 2020, 10, 5061. [Google Scholar] [CrossRef] [PubMed]
- Atikuzzaman, M.; Alvarez-Rodriguez, M.; Carrillo, A.V.; Johnsson, M.; Wright, D.; Rodriguez-Martinez, H. Conserved gene expression in sperm reservoirs between birds and mammals in response to mating. BMC Genomics 2017, 18. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H. Role of the oviduct in sperm capacitation. Theriogenology 2007, 68. [Google Scholar] [CrossRef]
- Jollife, I. Principal component analysis. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannisson, A.; Sanz, L.; Peña, F.J.; Martínez, E.A.; Roca, J.; Vázquez, J.M.; et al. The physiological roles of the boar ejaculate. Soc. Reprod. Fertil. Suppl. 2009, 66, 1–21. [Google Scholar]
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Atikuzzaman, M.; Bhai, R.M.; Fogelholm, J.; Wright, D.; Rodriguez-Martinez, H. Mating induces the expression of immune- and pH-regulatory genes in the utero-vaginal junction containing mucosal sperm-storage tubuli of hens. Reproduction 2015, 150, 473–483. [Google Scholar] [CrossRef]
- Brandt, Y.; Lang, A.; Madej, A.; Rodriguez-Martinez, H.; Einarsson, S. Impact of ACTH administration on the oviductal sperm reservoir in sows: The local endocrine environment and distribution of spermatozoa. Anim. Reprod. Sci. 2006, 92, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 2014, 5. [Google Scholar] [CrossRef]
- Gross, K.L.; Cidlowski, J.A. Tissue-specific glucocorticoid action: A family affair. Trends Endocrinol. Metab. 2008, 19, 331–339. [Google Scholar] [CrossRef]
- Michael, A.E.; Thurston, L.M.; Rae, M.T. Glucocorticoid metabolism and reproduction: A tale of two enzymes. Reproduction 2003, 126, 425–441. [Google Scholar] [CrossRef]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-hydroxysteroid dehydrogenases intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Gong, S.; Sun, G.-Y.; Zhang, M.; Yuan, H.-J.; Zhu, S.; Jiao, G.-Z.; Luo, M.-J.; Tan, J.-H. Mechanisms for the species difference between mouse and pig oocytes in their sensitivity to glucorticoids†. Biol. Reprod. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Yang, J.-G.; Chen, W.-Y.; Li, P.S. Effects of Glucocorticoids on Maturation of Pig Oocytes and Their Subsequent Fertilizing Capacity In Vitro1. Biol. Reprod. 1999, 60, 929–936. [Google Scholar] [CrossRef]
- Webb, R.J.; Sunak, N.; Wren, L.; Michael, A.E. Inactivation of glucocorticoids by 11β-hydroxysteroid dehydrogenase enzymes increases during the meiotic maturation of porcine oocytes. Reproduction 2008, 136, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Acosta, T.J.; Tetsuka, M.; Matsui, M.; Shimizu, T.; Berisha, B.; Schams, D.; Miyamoto, A. In vivo evidence that local cortisol production increases in the preovulatory follicle of the cow. J. Reprod. Dev. 2005, 51, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Conca, M.; Gardela, J.; Alvarez-Rodríguez, M.; Mogas, T.; López-Béjar, M. Immunofluorescence analysis of NR3C1 receptor following cortisol exposure during bovine in vitro oocyte maturation. Anim. Reprod. Sci. 2019, 16, 753. [Google Scholar]
- Tetsuka, M.; Tanakadate, M. Activation of hsd11b1 in the bovine cumulus-oocyte complex during ivm and ivf. Endocr. Connect. 2019, 8, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, N.N.; Brito, K.N.; Santana, P.D.; da Silva Cordeiro, M.; Silva, T.V.; Santos, A.X.; do Carmo Ramos, P.; Santos, S.D.; King, W.A.; dos Santos Miranda, M.; et al. Effect of cortisol on bovine oocyte maturation and embryo development in vitro. Theriogenology 2016, 85, 323–329. [Google Scholar] [CrossRef]
- Scarlet, D.; Ille, N.; Ertl, R.; Alves, B.G.; Gastal, G.D.A.; Paiva, S.O.; Gastal, M.O.; Gastal, E.L.; Aurich, C. Glucocorticoid metabolism in equine follicles and oocytes. Domest. Anim. Endocrinol. 2017, 59, 11–22. [Google Scholar] [CrossRef]
- Whirledge, S.; Xu, X.; Cidlowski, J.A. Global Gene Expression Analysis in Human Uterine Epithelial Cells Defines New Targets of Glucocorticoid and Estradiol Antagonism1. Biol. Reprod. 2013, 89. [Google Scholar] [CrossRef]
- Yang, K.; Fraser, M.; Yu, M.; Krkosek, M.; Challis, J.R.G.; Lamming, G.E.; Campbell, L.E.; Darnel, A. Pattern of 11β-Hydroxysteroid Dehydrogenase Type 1 Messenger Ribonucleic Acid Expression in the Ovine Uterus during the Estrous Cycle and Pregnancy1. Biol. Reprod. 1996, 55, 1231–1236. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Acosta, T.J.; Skarzynski, D.J.; Okuda, K. Prostaglandin F2alpha Stimulates 11Beta-Hydroxysteroid Dehydrogenase 1 Enzyme Bioactivity and Protein Expression in Bovine Endometrial Stromal Cells1. Biol. Reprod. 2009, 80, 657–664. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Saravia, F.; Wallgren, M.; Tienthai, P.; Johannisson, A.; Vázquez, J.M.; Martínez, E.; Roca, J.; Sanz, L.; Calvete, J.J. Boar spermatozoa in the oviduct. Theriogenology 2005, 63, 514–535. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y. Identification of key genes, regulatory factors, and drug target genes of recurrent implantation failure (RIF). Gynecol. Endocrinol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.V.; Batchen, E.J.; Denvir, M.A.; Gray, G.A.; Chapman, K.E. Cardiac GR and MR: From development to pathology. Trends Endocrinol. Metab. 2016, 27, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hähle, A.; Merz, S.; Meyners, C.; Hausch, F. The many faces of FKBP51. Biomolecules 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Gassen, N.C.; Rein, T. The FKBP51 glucocorticoid receptor co-chaperone: Regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2017, 18, 2614. [Google Scholar] [CrossRef]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef]
- Schiene-Fischer, C.; Yu, C. Receptor accessory folding helper enzymes: The functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 2001, 495, 1–6. [Google Scholar] [CrossRef]
- Davies, T.H.; Ning, Y.M.; Sánchez, E.R. A new first step in activation of steroid receptors. Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002, 277, 4597–4600. [Google Scholar] [CrossRef]
- Wochnik, G.M.; Rüegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef]
- Sacta, M.A.; Chinenov, Y.; Rogatsky, I. Glucocorticoid Signaling: An Update from a Genomic Perspective. Annu. Rev. Physiol. 2016, 78, 155–180. [Google Scholar] [CrossRef]
- Denny, W.B.; Valentine, D.L.; Reynolds, P.D.; Smith, D.F.; Scammell, J.G. Squirrel Monkey Immunophilin FKBP51 Is a Potent Inhibitor of Glucocorticoid Receptor Binding. Endocrinology 2000, 141, 4107–4113. [Google Scholar] [CrossRef]
- Ratajczak, T.; Cluning, C.; Ward, B.K. Steroid receptor-associated immunophilins: A gateway to steroid signalling. Clin. Biochem. Rev. 2015, 36, 31–52. [Google Scholar]
- Riggs, D.L.; Roberts, P.J.; Chirillo, S.C.; Cheung-Flynn, J.; Prapapanich, V.; Ratajczak, T.; Gaber, R.; Picard, D.; Smith, D.F. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003, 22, 1158–1167. [Google Scholar] [CrossRef]
- Scammell, J.G.; Denny, W.B.; Valentine, D.L.; Smiths, D.F. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol. 2001, 124, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; De Leo, S.A.; Mazaira, G.I.; Molinari, A.M.; Camisay, M.F.; Fontana, V.; Cox, M.B.; Piwien-Pilipuk, G.; Galigniana, M.D. NF-κB transcriptional activity is modulated by FK506-Binding proteins FKBP51 and FKBP52:A role for peptidyl-prolyl isomerase activity. J. Biol. Chem. 2014, 289, 26263–26276. [Google Scholar] [CrossRef]
- Zgajnar, N.R.; De Leo, S.A.; Lotufo, C.M.; Erlejman, A.G.; Pilipuk, G.P.; Galigniana, M.D. Biological actions of the hsp90-binding immunophilins FKBP51 and FKBP52. Biomolecules 2019, 9, 52. [Google Scholar] [CrossRef]
- Langenbach, R.; Morham, S.G.; Tiano, H.F.; Loftin, C.D.; Ghanayem, B.I.; Chulada, P.C.; Mahler, J.F.; Lee, C.A.; Goulding, E.H.; Kluckman, K.D.; et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 1995, 83, 483–492. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Crawford, M.C.; FitzGerald, G.A.; Funk, C.D. Isoform-Specific Compensation of Cyclooxygenase (Ptgs) Genes during Implantation and Late-Stage Pregnancy. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Pockley, A.G. Heat shock proteins as regulators of the immune response. Lancet 2003, 362, 469–476. [Google Scholar] [CrossRef]
- Geng, J.; Li, H.; Huang, C.; Chai, J.; Zheng, R.; Li, F.; Jiang, S. Functional analysis of HSPA1A and HSPA8 in parturition. Biochem. Biophys. Res. Commun. 2017, 483, 371–379. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Maj, T.; Chelmonska-Soyta, A. Pleiotropy and redundancy of STAT proteins in early pregnancy. Reprod. Domest. Anim. 2007, 42, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Bednorz, N.L.; Brill, B.; Klein, A.; Gäbel, K.; Groner, B. Tracking the Activation of Stat5 through the Expression of an Inducible Reporter Gene in a Transgenic Mouse Line. Endocrinology 2011, 152, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Petta, I.; Dejager, L.; Ballegeer, M.; Lievens, S.; Tavernier, J.; De Bosscher, K.; Libert, C. The Interactome of the Glucocorticoid Receptor and Its Influence on the Actions of Glucocorticoids in Combatting Inflammatory and Infectious Diseases. Microbiol. Mol. Biol. Rev. 2016, 80, 495–522. [Google Scholar] [CrossRef]
- Ezz, M.A.; Marey, M.A.; Elweza, A.E.; Kawai, T.; Heppelmann, M.; Pfarrer, C.; Balboula, A.Z.; Montaser, A.; Imakawa, K.; Zaabel, S.M.; et al. TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Akthar, I.; Suarez, S.S.; Morillo, V.A.; Sasaki, M.; Ezz, M.A.; Takahashi, K.I.; Shimada, M.; Marey, M.A.; Miyamoto, A. Sperm enter glands of preovulatory bovine endometrial explants and initiate inflammation. Reproduction 2020, 159, 181–192. [Google Scholar] [CrossRef]
- Choi, Y.; Johnson, G.A.; Burghardt, R.C.; Berghman, L.R.; Joyce, M.M.; Taylor, K.M.; David Stewart, M.; Bazer, F.W.; Spencer, T.E. Interferon Regulatory Factor-Two Restricts Expression of Interferon-Stimulated Genes to the Endometrial Stroma and Glandular Epithelium of the Ovine Uterus1. Biol. Reprod. 2001, 65, 1038–1049. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Sharkey, A.M.; Tan, Y.L.; Salamonsen, L.A.; Robert, J.R.A. Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod. Biol. Endocrinol. 2007, 5, 44. [Google Scholar] [CrossRef]
- Martinez, C.A.; Rubér, M.; Rodriguez-Martinez, H.; Alvarez-Rodriguez, M. Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death? Biomolecules 2020, 10, 554. [Google Scholar] [CrossRef]
- Bluyssen, H. STAT2-directed pathogen responses. Oncotarget 2015, 6, 28525–28526. [Google Scholar] [CrossRef]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.Z.; Bautista, D.M.; Kirichok, Y.; Lishko, P.V. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science (80-) 2016, 352, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Miller, M.R.; Lishko, P.V. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl. Acad. Sci. USA 2017, 114, 5743–5748. [Google Scholar] [CrossRef]
- Machado, S.A.; Sharif, M.; Wang, H.; Bovin, N.; Miller, D.J. Release of Porcine Sperm from Oviduct Cells is Stimulated by Progesterone and Requires CatSper. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Oren-Benaroya, R.; Orvieto, R.; Gakamsky, A.; Pinchasov, M.; Eisenbach, M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum. Reprod. 2008, 23, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–392. [Google Scholar] [CrossRef]
- Martinez, C.A.; Alvarez-Rodriguez, M.; Wright, D.; Rodriguez-Martinez, H. Does the pre-ovulatory pig oviduct rule sperm capacitation in vivo mediating transcriptomics of catsper channels? Int. J. Mol. Sci. 2020, 21, 1840. [Google Scholar] [CrossRef]
- Pursel, V.G.; Johnson, L.A. Freezing of Boar Spermatozoa: Fertilizing Capacity with Concentrated Semen and a New Thawing Procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Atikuzzaman, M.; Venhoranta, H.; Wright, D.; Rodriguez-Martinez, H. Expression of immune regulatory genes in the porcine internal genital tract is differentially triggered by spermatozoa and seminal plasma. Int. J. Mol. Sci. 2019, 20, 513. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Conca, M.; Gardela, J.; Martínez, C.A.; Wright, D.; López-Bejar, M.; Rodríguez-Martínez, H.; Álvarez-Rodríguez, M. Natural Mating Differentially Triggers Expression of Glucocorticoid Receptor (NR3C1)-Related Genes in the Preovulatory Porcine Female Reproductive Tract. Int. J. Mol. Sci. 2020, 21, 4437. https://doi.org/10.3390/ijms21124437
Ruiz-Conca M, Gardela J, Martínez CA, Wright D, López-Bejar M, Rodríguez-Martínez H, Álvarez-Rodríguez M. Natural Mating Differentially Triggers Expression of Glucocorticoid Receptor (NR3C1)-Related Genes in the Preovulatory Porcine Female Reproductive Tract. International Journal of Molecular Sciences. 2020; 21(12):4437. https://doi.org/10.3390/ijms21124437
Chicago/Turabian StyleRuiz-Conca, Mateo, Jaume Gardela, Cristina Alicia Martínez, Dominic Wright, Manel López-Bejar, Heriberto Rodríguez-Martínez, and Manuel Álvarez-Rodríguez. 2020. "Natural Mating Differentially Triggers Expression of Glucocorticoid Receptor (NR3C1)-Related Genes in the Preovulatory Porcine Female Reproductive Tract" International Journal of Molecular Sciences 21, no. 12: 4437. https://doi.org/10.3390/ijms21124437
APA StyleRuiz-Conca, M., Gardela, J., Martínez, C. A., Wright, D., López-Bejar, M., Rodríguez-Martínez, H., & Álvarez-Rodríguez, M. (2020). Natural Mating Differentially Triggers Expression of Glucocorticoid Receptor (NR3C1)-Related Genes in the Preovulatory Porcine Female Reproductive Tract. International Journal of Molecular Sciences, 21(12), 4437. https://doi.org/10.3390/ijms21124437








