Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using Pparβ/δ-Deficient Mice
Abstract
1. Introduction
2. Results
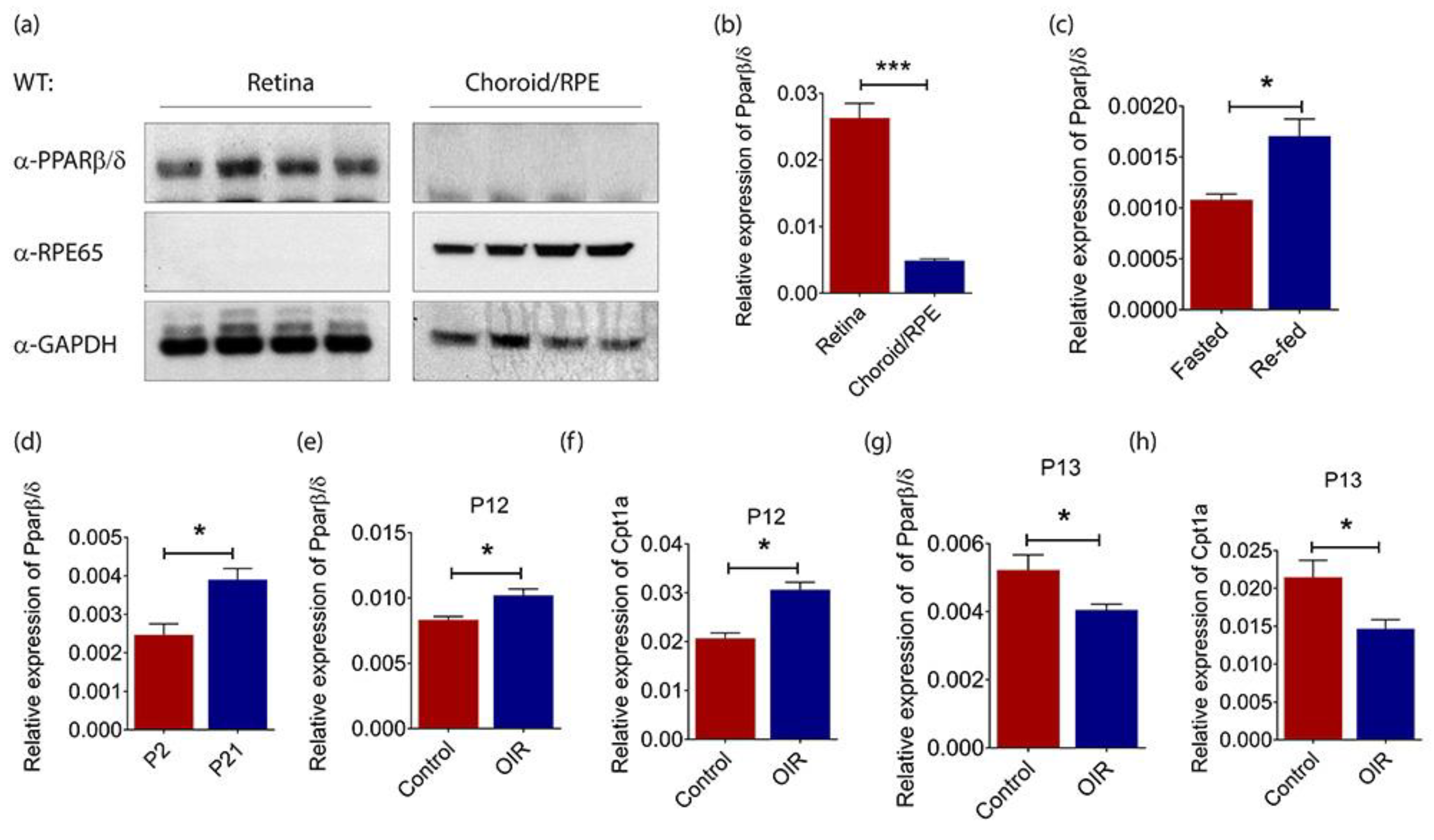
2.1. PPARβ/δ Is Highly Expressed in the Mouse Retina
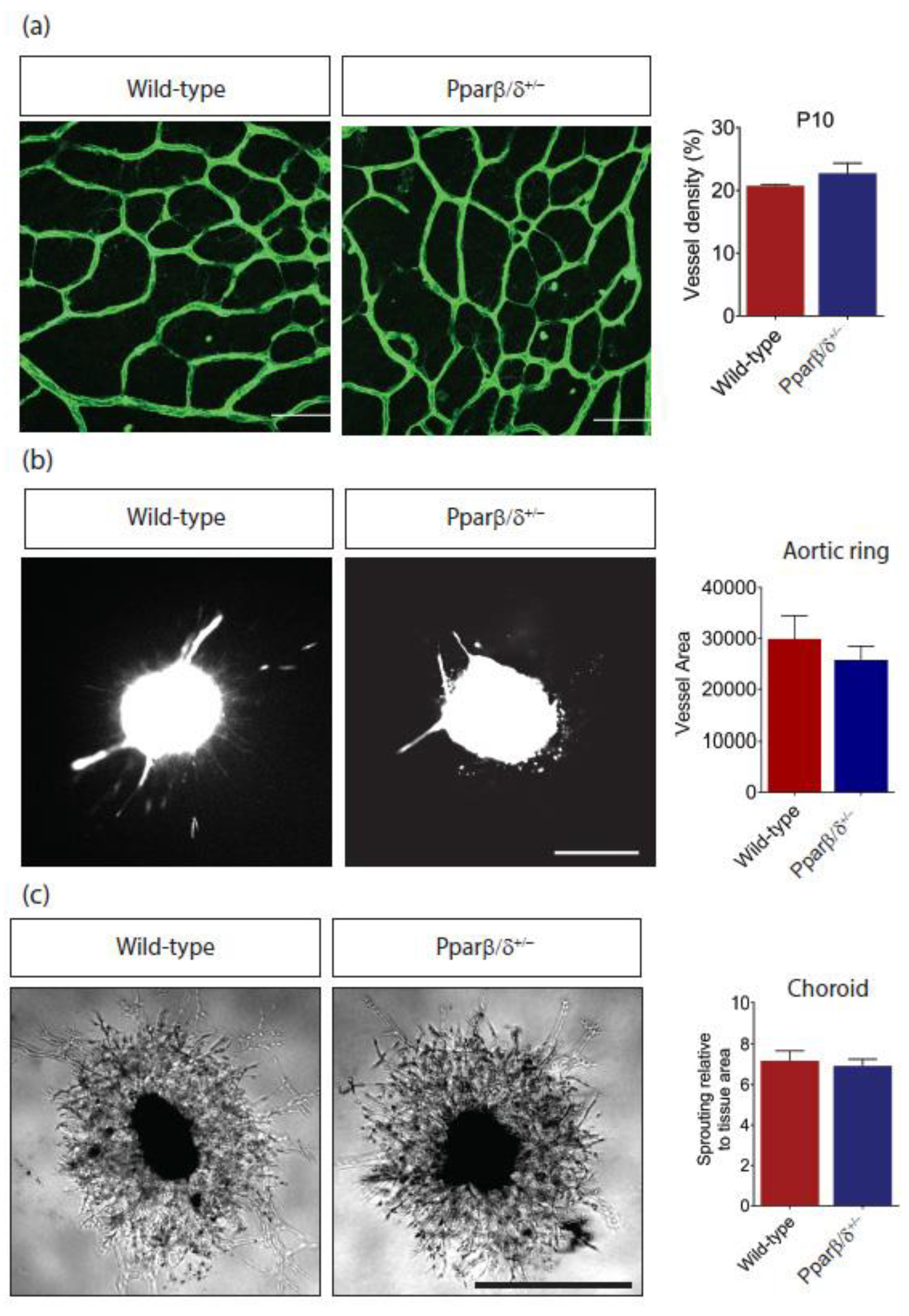
2.2. Pparβ/δ Deficiency Does Not Affect Retinal Angiogenesis
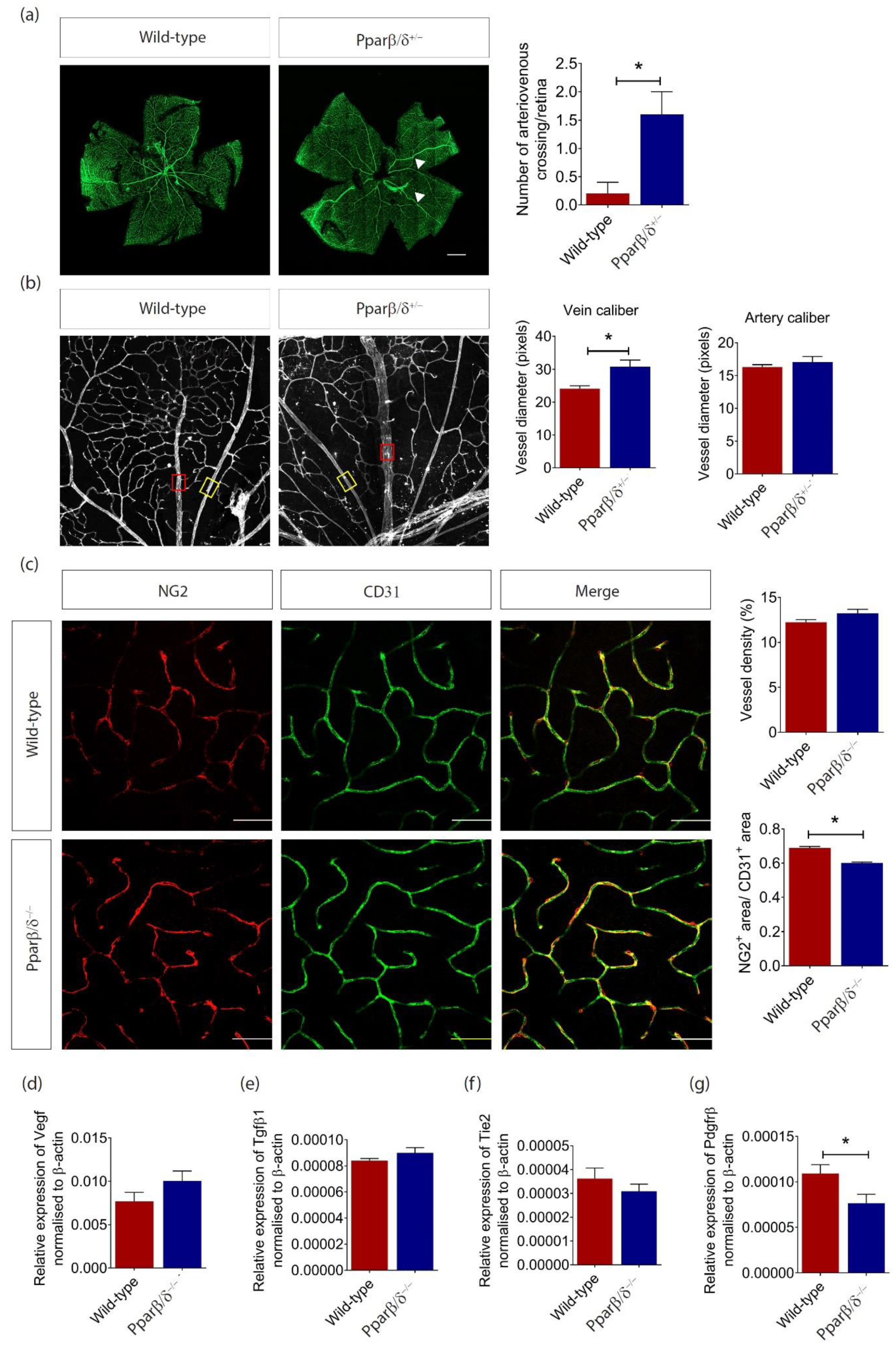
2.3. PPARβ/δ Mediates Blood Vessel Remodeling
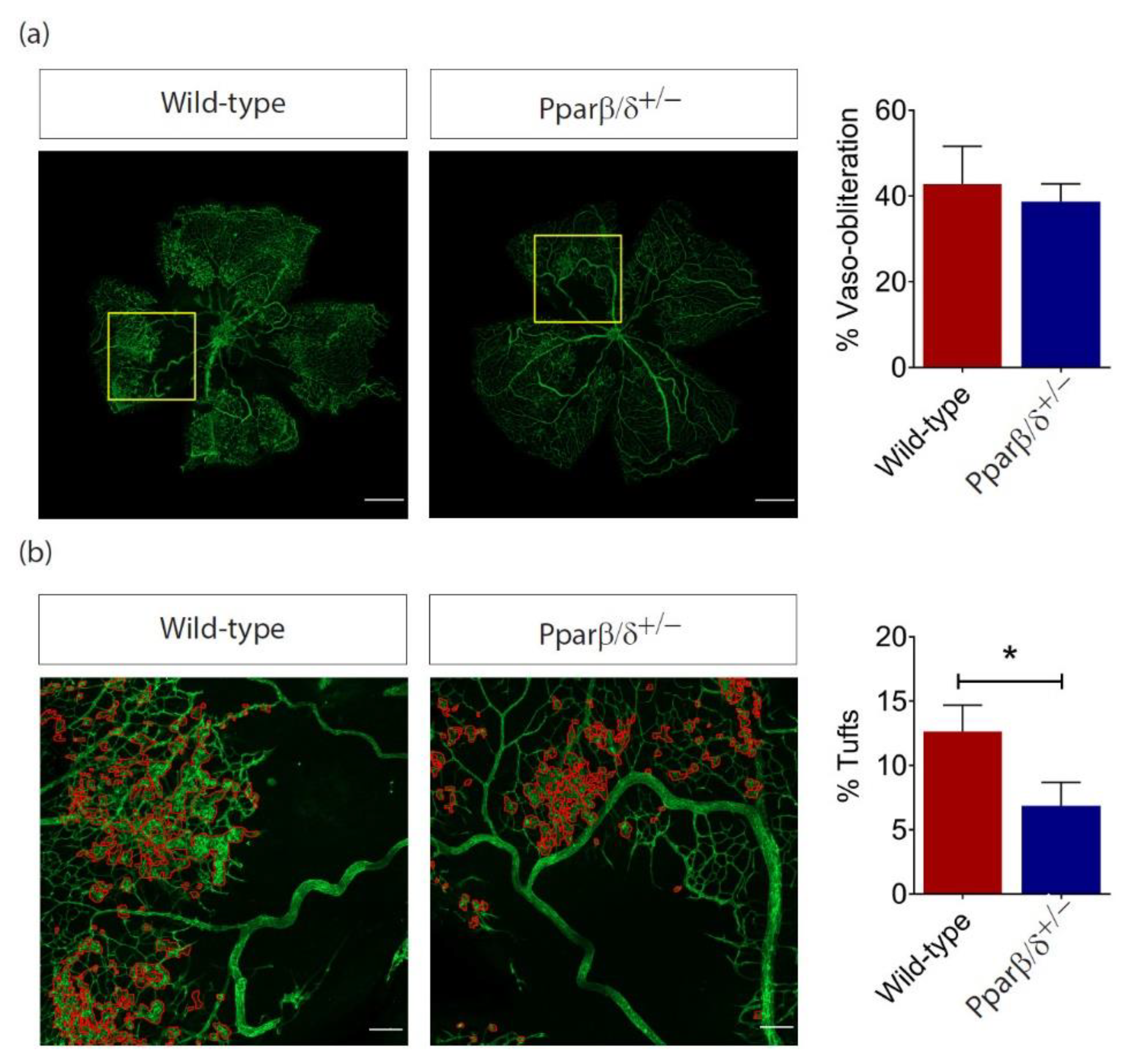
2.4. PPARβ/δ Is Specifically Required for Pathological Retinal Angiogenesis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. Drug Treatment
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Protein Extraction and Western Blot Analysis
4.6. Mouse Model of Laser-Induced Choroidal Neovascularization
4.7. Mouse Model of Oxygen-Induced Retinopathy (OIR)
4.8. Retina Flat Mount Immunohistochemistry
4.9. Aortic Ring Assay
4.10. Choroid Sprouting Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PPARβ/δ | Peroxisome proliferator-activated receptorβ/δ |
| CNV | Choroidal neovascularization |
| OIR | Oxygen-induced retinopathy |
| VEGF | Vascular endothelial growth factor |
| PDR | Proliferative diabetic retinopathy |
| DME | Diabetic macular edema |
| nAMD | Neovascular age-related macular degeneration |
| RVO | Retinal vein occlusion |
| PPARs | Peroxisome proliferator-activated receptors |
| ECs | Endothelial cells |
| RPE | Retinal pigment epithelium |
| kDa | kilodalton |
| RPE65 | Retinal pigment epithelium-specific protein 65 kDa |
| FAO | Fatty acid oxidation |
| Angptl4 | Angiopoietin-like 4 |
| Cpt1a | Carnitine palmitoyltransferase 1A |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| RT-qPCR | Quantitative real-time polymerase chain reaction |
| Pdk4 | Pyruvate dehydrogenase kinase 4 |
| DMSO | Dimethyl sulfoxide |
| µm | micrometer |
| nM | nanomolar |
| Tgfβ1 | Transforming Growth Factor Beta 1 |
| Tie2 | TEK Receptor Tyrosine Kinase |
| Pdgfrβ | Platelet Derived Growth Factor Receptor Beta Polypeptide |
| ATP | adenosine triphosphate |
| PCR | Polymerase chain reaction |
| DTT | dithiothreitol |
| PMSF | phenylmethylsulfonyl fluoride |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene Fluoride |
| HRP | Horseradish peroxidase |
| PFA | paraformaldehyde |
| DAPI | 4′,6-diamidino-2-phenylindole |
| PBS | phosphate-buffered saline |
| IB4 | isolectin B4 |
References
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Wong, T.Y.; Scott, I.U. Clinical practice. Retinal-vein occlusion. N. Engl. J. Med. 2010, 363, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Kokame, G.T.; Decarlo, T.E.; Kaneko, K.N.; Omizo, J.N.; Lian, R. Anti-Vascular Endothelial Growth Factor Resistance in Exudative Macular Degeneration and Polypoidal Choroidal Vasculopathy. Ophthalmol. Retina 2019, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Cabral, T.; Mello, L.G.M.; Lima, L.; Polido, J.; Regatieri, C.V.; Belfort, R.; Mahajan, V.B. Retinal and choroidal angiogenesis: A review of new targets. Int. J. Retina Vitreous 2017, 3, 31. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Devel. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Falavarjani, K.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Osaadon, P.; Fagan, X.J.; Lifshitz, T.; Levy, J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 2014, 28, 510–520. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular Mediators of Angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Nishijima, K.; Ng, Y.-S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P.; et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007, 171, 53–67. [Google Scholar] [CrossRef]
- Daniel, E.; Toth, C.A.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Fine, S.L.; Huang, J.; Ying, G.-S.; Hagstrom, S.A.; Winter, K.; et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2013, 121, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Antonetti, D.A. Using the Past to Inform the Future: Anti-VEGF Therapy as a Road Map to Develop Novel Therapies for Diabetic Retinopathy. Diabetes 2013, 62, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Draoui, N.; De Zeeuw, P.; Carmeliet, P. Angiogenesis revisited from a metabolic perspective: Role and therapeutic implications of endothelial cell metabolism. Open Biol. 2017, 7, 170219. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.L.C.; Siersbæk, M.; Mandrup, S. PPARs: Fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012, 23, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.P.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Michalik, L.; Wahli, W. Transcriptional Regulation of Metabolism. Physiol. Rev. 2006, 86, 465–514. [Google Scholar] [CrossRef]
- Bishop-Bailey, D. PPARs and angiogenesis. Biochem. Soc. Trans. 2011, 39, 1601–1605. [Google Scholar] [CrossRef]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARβ/δ Induces Endothelial Cell Proliferation and Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 63–69. [Google Scholar] [CrossRef]
- Capozzi, M.; Mccollum, G.W.; Savage, S.R.; Penn, J.S. Peroxisome Proliferator-Activated Receptor-β/δ Regulates Angiogenic Cell Behaviors and Oxygen-Induced Retinopathy. Invest. Ophthalmol. Vis. Sci. 2013, 54, 4197–4207. [Google Scholar] [CrossRef]
- Suárez, S.; Mccollum, G.W.; Bretz, C.A.; Yang, R.; Capozzi, M.; Penn, J.S. Modulation of VEGF-Induced Retinal Vascular Permeability by Peroxisome Proliferator-Activated Receptor-β/δ. Invest. Ophthalmol. Vis. Sci. 2014, 55, 8232–8240. [Google Scholar] [CrossRef]
- Choudhary, M.; Ding, J.-D.; Qi, X.; Boulton, M.E.; Yao, P.-L.; Peters, J.M.; Malek, G. PPARβ/δ selectively regulates phenotypic features of age-related macular degeneration. Aging 2016, 8, 1952–1971. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Barish, G.D.; Narkar, V.A.; Evans, R.M. PPARδ: A dagger in the heart of the metabolic syndrome. J. Clin. Investig. 2006, 116, 590–597. [Google Scholar] [CrossRef]
- Escher, P.; Braissant, O.; Basu-Modak, S.; Michalik, L.; Wahli, W.; Desvergne, B. Rat PPARs: Quantitative Analysis in Adult Rat Tissues and Regulation in Fasting and Refeeding. Endocrinology 2001, 142, 4195–4202. [Google Scholar] [CrossRef]
- Connolly, S.; Hores, T.; Smith, L.; D’Amore, P.A. Characterization of vascular development in the mouse retina. Microvasc. Res. 1988, 36, 275–290. [Google Scholar] [CrossRef]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.S.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef]
- Shearer, B.G.; Steger, D.J.; Way, J.M.; Stanley, T.B.; Lobe, D.C.; Grillot, D.A.; Iannone, M.A.; Lazar, M.A.; Willson, T.M.; Billin, A. Identification and Characterization of a Selective Peroxisome Proliferator-Activated Receptor β/δ (NR1C2) Antagonist. Mol. Endocrinol. 2008, 22, 523–529. [Google Scholar] [CrossRef]
- Barak, Y.; Liao, D.; He, W.; Ong, E.S.; Nelson, M.C.; Olefsky, J.M.; Boland, R.; Evans, R.M. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. 2001, 99, 303–308. [Google Scholar] [CrossRef]
- Nadra, K.; Anghel, S.I.; Joye, E.; Tan, N.S.; Basu-Modak, S.; Trono, D.; Wahli, W.; Desvergne, B. Differentiation of Trophoblast Giant Cells and Their Metabolic Functions Are Dependent on Peroxisome Proliferator-Activated Receptor β/δ. Mol. Cell. Biol. 2006, 26, 3266–3281. [Google Scholar] [CrossRef]
- Toth, P.M.; Naruhn, S.; Pape, V.F.; Dörr, S.M.A.; Klebe, G.; Müller, R.; Diederich, W.E. Development of Improved PPARβ/δ Inhibitors. Chem. Med. Chem. 2011, 7, 159–170. [Google Scholar] [CrossRef]
- Haigh, J.; I Morelli, P.; Gerhardt, H.; Haigh, K.; Tsien, J.; Damert, A.; Miquerol, L.; Mühlner, U.; Klein, R.; Ferrara, N.; et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Bio. 2003, 262, 225–241. [Google Scholar] [CrossRef]
- Muraoka, Y.; Tsujikawa, A. Arteriovenous crossing associated with branch retinal vein occlusion. Jpn. J. Ophthalmol. 2019, 63, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, N.L.; Larsen, M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology 1999, 106, 2054–2062. [Google Scholar] [CrossRef]
- Subramani, M.; Ponnalagu, M.; Krishna, L.; Jeyabalan, N.; Chevour, P.; Sharma, A.; Jayadev, C.; Shetty, R.; Begum, N.; Archunan, G.; et al. Resveratrol reverses the adverse effects of bevacizumab on cultured ARPE-19 cells. Sci. Rep. 2017, 7, 12242. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2019, 3, CD005139. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Vandekeere, S.; Dewerchin, M.; Carmeliet, P. Angiogenesis Revisited: An Overlooked Role of Endothelial Cell Metabolism in Vessel Sprouting. Microcirculation 2015, 22, 509–517. [Google Scholar] [CrossRef]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of Fatty Acid Oxidation in Mouse Cumulus-Oocyte Complexes during Maturation and Modulation by PPAR Agonists. PLoS ONE 2014, 9, e87327. [Google Scholar] [CrossRef]
- Narravula, S.; Colgan, S.P. Hypoxia-Inducible Factor 1-Mediated Inhibition of Peroxisome Proliferator-Activated Receptor α Expression during Hypoxia. J. Immunol. 2001, 166, 7543–7548. [Google Scholar] [CrossRef]
- Li, X.; Kimura, H.; Hirota, K.; Sugimoto, H.; Kimura, N.; Takahashi, N.; Fujii, H.; Yoshida, H. Hypoxia reduces the expression and anti-inflammatory effects of peroxisome proliferator-activated receptor- in human proximal renal tubular cells. Nephrol. Dial. Transplant. 2007, 22, 1041–1051. [Google Scholar] [CrossRef]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator–activated receptor α mediates the adaptive response to fasting. J. Clin. Invest. 1999, 103, 1489–1498. [Google Scholar] [CrossRef]
- Wong, T.Y.; McIntosh, R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br. Med Bull. 2005, 73, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Mitchell, P. Hypertensive Retinopathy. N. Engl. J. Med. 2004, 351, 2310–2317. [Google Scholar] [CrossRef]
- Klein, A.P.; Klein, B.E.; Moss, S.E.; Wong, T.Y. Retinal Vessel Caliber and Microvascular and Macrovascular Disease in Type 2 Diabetes. Ophthalmology 2007, 114, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.K.; De Jong, F.J.; Bos, M.J.; Vingerling, J.R.; Hofman, A.; Koudstaal, P.J.; De Jong, P.T.; Breteler, M. Retinal vessel diameters and risk of stroke: The Rotterdam Study. Neurology 2006, 66, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- McGeechan, K.; Liew, G.; Macaskill, P.; Irwig, L.; Klein, R.; Klein, B.E.K.; Wang, J.J.; Mitchell, P.; Vingerling, J.R.; De Jong, P.T.V.M.; et al. Prediction of Incident Stroke Events Based on Retinal Vessel Caliber: A Systematic Review and Individual-Participant Meta-Analysis. Am. J. Epidemiol. 2009, 170, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.-T.; De Silva, D.A.; Cheung, C.Y.-L.; Chang, H.-M.; Chen, C.P.; Wong, M.C.; Wong, T.Y.; Ikram, M.K. Microvascular Structure and Network in the Retina of Patients With Ischemic Stroke. Stroke 2013, 44, 2121–2127. [Google Scholar] [CrossRef]
- McGeechan, K.; Liew, G.; Macaskill, P.; Irwig, L.; Klein, A.P.; Klein, B.E.; Wang, J.J.; Mitchell, P.; Vingerling, J.R.; DeJong, P.T.; et al. Retinal Vessel Caliber and Risk for Coronary Heart Disease: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2009, 151, 404–413. [Google Scholar] [CrossRef]
- Wong, T.Y.; Kamineni, A.; Klein, A.P.; Sharrett, A.R.; Klein, B.E.; Siscovick, D.S.; Cushman, M.; Duncan, B.B. Quantitative Retinal Venular Caliber and Risk of Cardiovascular Disease in Older Persons. Arch. Intern. Med. 2006, 166, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Mitchell, P.; Wong, T.; Wang, J. Retinal Microvascular Signs Are Associated with Chronic Kidney Disease in Persons with and without Diabetes. Kidney Blood Press. Res. 2012, 35, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Broe, R.; Rasmussen, M.L.; Frydkjaer-Olsen, U.; Olsen, B.S.; Mortensen, H.B.; Hodgson, L.; Wong, T.Y.; Peto, T.; Grauslund, J. Retinal Vessel Calibers Predict Long-term Microvascular Complications in Type 1 Diabetes: The Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Diabetes 2014, 63, 3906–3914. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, A.P.; Klein, B.E.; Tielsch, J.M.; Hubbard, L.; Nieto, F. Retinal Microvascular Abnormalities and their Relationship with Hypertension, Cardiovascular Disease, and Mortality. Surv. Ophthalmol. 2001, 46, 59–80. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Infusino, F.; Maestrini, V.; Mancone, M.; Fedele, F. Myocardial Ischemia and Diabetes Mellitus: Role of Oxidative Stress in the Connection between Cardiac Metabolism and Coronary Blood Flow. J. Diabetes Res. 2019, 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Wawrzyniak, M.; Pich-Bavastro, C.; Gross, B.; Schütz, F.; Fleury, S.; Quemener, S.; Sgandurra, M.; Bouchaert, E.; Moret, C.; Mury, L.; et al. Endothelial, but not smooth muscle, peroxisome proliferator-activated receptor β/δ regulates vascular permeability and anaphylaxis. J. Allergy Clin. Immunol. 2015, 135, 1625–1635.e5. [Google Scholar] [CrossRef][Green Version]
- Eelen, G.; De Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef]
- Sng, M.K.; Chan, J.S.K.; Teo, Z.; Phua, T.; Tan, E.H.P.; Wee, J.W.K.; Koh, N.J.N.; Tan, C.K.; Chen, J.P.; Pal, M.; et al. Selective deletion of PPARβ/δ in fibroblasts causes dermal fibrosis by attenuated LRG1 expression. Cell Discov. 2018, 4, 15. [Google Scholar] [CrossRef]
- Wang, X.; Abraham, S.; McKenzie, J.A.G.; Jeffs, N.; Swire, M.; Tripathi, V.B.; Luhmann, U.F.; Lange, C.A.K.; Zhai, Z.; Arthur, H.M.; et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature 2013, 499, 306–311. [Google Scholar] [CrossRef]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; I Guerin, K.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E.H. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A Computational Tool for Quantitative Analysis of Vascular Networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Friedlander, M.; Hurst, C.G.; Cui, Z.; Pei, D.T.; Evans, L.P.; Juan, A.M.; Tahir, H.; Duhamel, F.; Chen, J.; et al. Choroid Sprouting Assay: An Ex Vivo Model of Microvascular Angiogenesis. PLoS ONE 2013, 8, e69552. [Google Scholar] [CrossRef]

| Primer | Sequence (5′–3′) |
|---|---|
| PBX9 | AGACAATGATGGTGTGCTCA |
| PBX10 | GCAGCTGCTGCTCAGCTGCCTGC |
| Rev1 | CCTGAGACAGACTGCGCA |
| UMS | GCTCCTGAAGTCCACAATTCACAGTCC |
| Target (Mouse) | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Amplicon Size (bp) |
| Pparβ/δ (NM_011145.3) | CGGCAGCCTCAACATGG | AGATCCGATCGCACTTCTCATAC | 143 |
| Cpt1a (NM_013495.2) | AACACCATCCACGCCATACTG | TCCCAGAAGACGAATAGGTTTGAG | 75 |
| Pdgfrb (NM_001146268.1) | AGGAGTGATACCAGCTTTAGTCC | CCGAGCAGGTCAGAACAAAGG | 152 |
| Vegfa (NM_001025250.3) | TAGAGTACATCTTCAAGCCG | TCTTTCTTTGGTCTGCATTC | 199 |
| Tek (Tie2) (NM_013690.3) | GGCATTCCAGAACGTGAGAGAA | GATCCGGATTGTTTTTGGCCT | 89 |
| Tgfb1 (NM_011577.2) | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC | 183 |
| Actb (β-actin) (NM_007393.5) | CCTTCTTGGGTATGGAATCCTGT | CACTGTGTTGGCATAGAGGTCTTTAC | 101 |
| Gapdh (NM_001289726.1) | ACTGAGGACCAGGTTGTCTCC | CTGTAGCCGTATTCATTGTCATAC | 134 |
| Target (Human) | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Amplicon Size (bp) |
| GAPDH (NM_002046.7) | GGTCTCCTCTGACTTCAACA | AGCCAAATTCGTTGTCATAC | 116 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, S.Y.; Kwan, Y.P.; Qiu, B.; Tan, A.; Murray, H.L.; Barathi, V.A.; Tan, N.S.; Cheung, C.M.G.; Wong, T.Y.; Wahli, W.; et al. Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using Pparβ/δ-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 4403. https://doi.org/10.3390/ijms21124403
Ho SY, Kwan YP, Qiu B, Tan A, Murray HL, Barathi VA, Tan NS, Cheung CMG, Wong TY, Wahli W, et al. Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using Pparβ/δ-Deficient Mice. International Journal of Molecular Sciences. 2020; 21(12):4403. https://doi.org/10.3390/ijms21124403
Chicago/Turabian StyleHo, Sze Yuan, Yuet Ping Kwan, Beiying Qiu, Alison Tan, Hannah Louise Murray, Veluchamy Amutha Barathi, Nguan Soon Tan, Chui Ming Gemmy Cheung, Tien Yin Wong, Walter Wahli, and et al. 2020. "Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using Pparβ/δ-Deficient Mice" International Journal of Molecular Sciences 21, no. 12: 4403. https://doi.org/10.3390/ijms21124403
APA StyleHo, S. Y., Kwan, Y. P., Qiu, B., Tan, A., Murray, H. L., Barathi, V. A., Tan, N. S., Cheung, C. M. G., Wong, T. Y., Wahli, W., & Wang, X. (2020). Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using Pparβ/δ-Deficient Mice. International Journal of Molecular Sciences, 21(12), 4403. https://doi.org/10.3390/ijms21124403







