Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model
Abstract
1. Introduction
2. Results
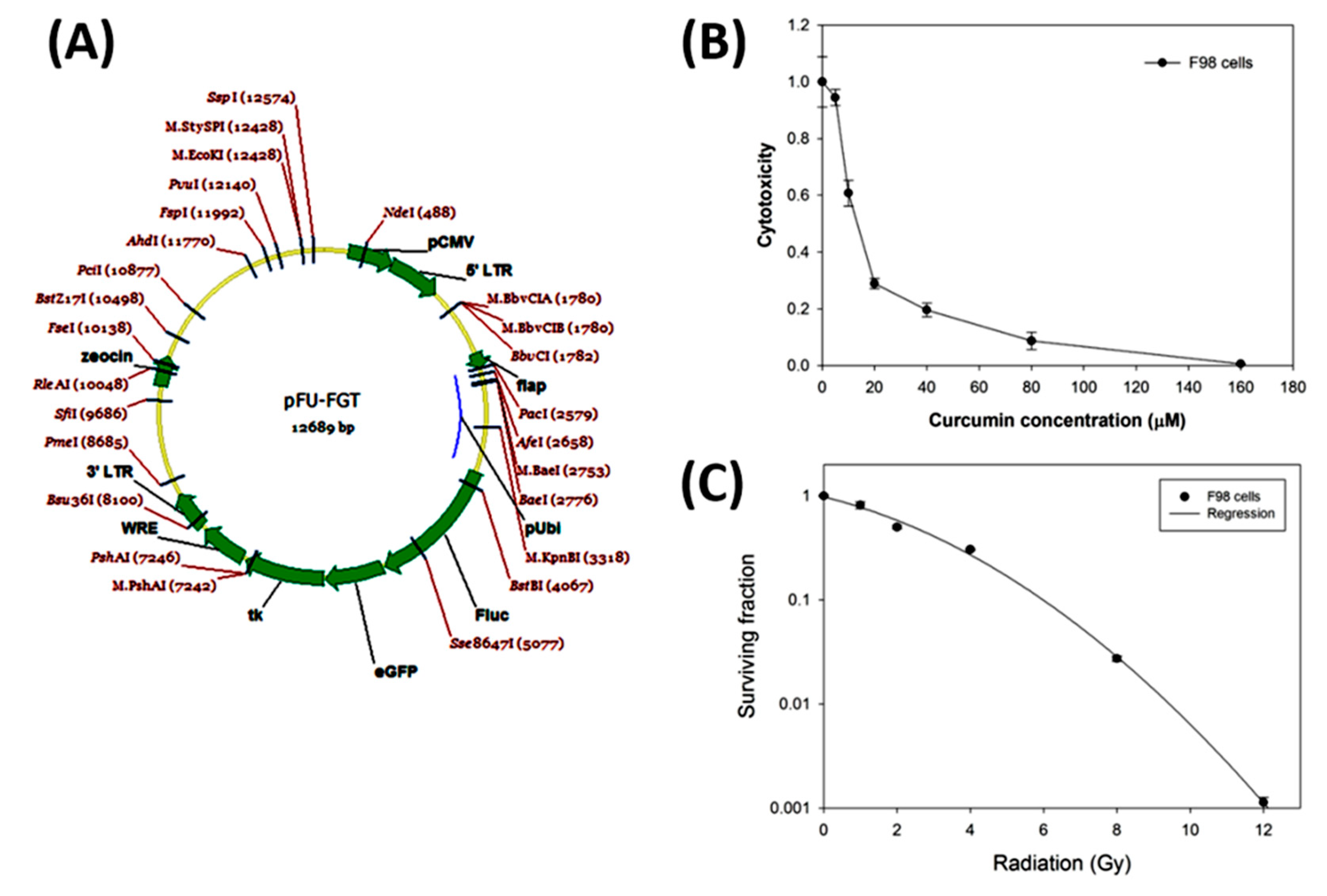
2.1. The Gene Map of the Lentiviral-Based Trifusion Reporter Vector and Survival Curves of F98 Glioblastoma Cells with Curcumin Alone and Radiation Alone
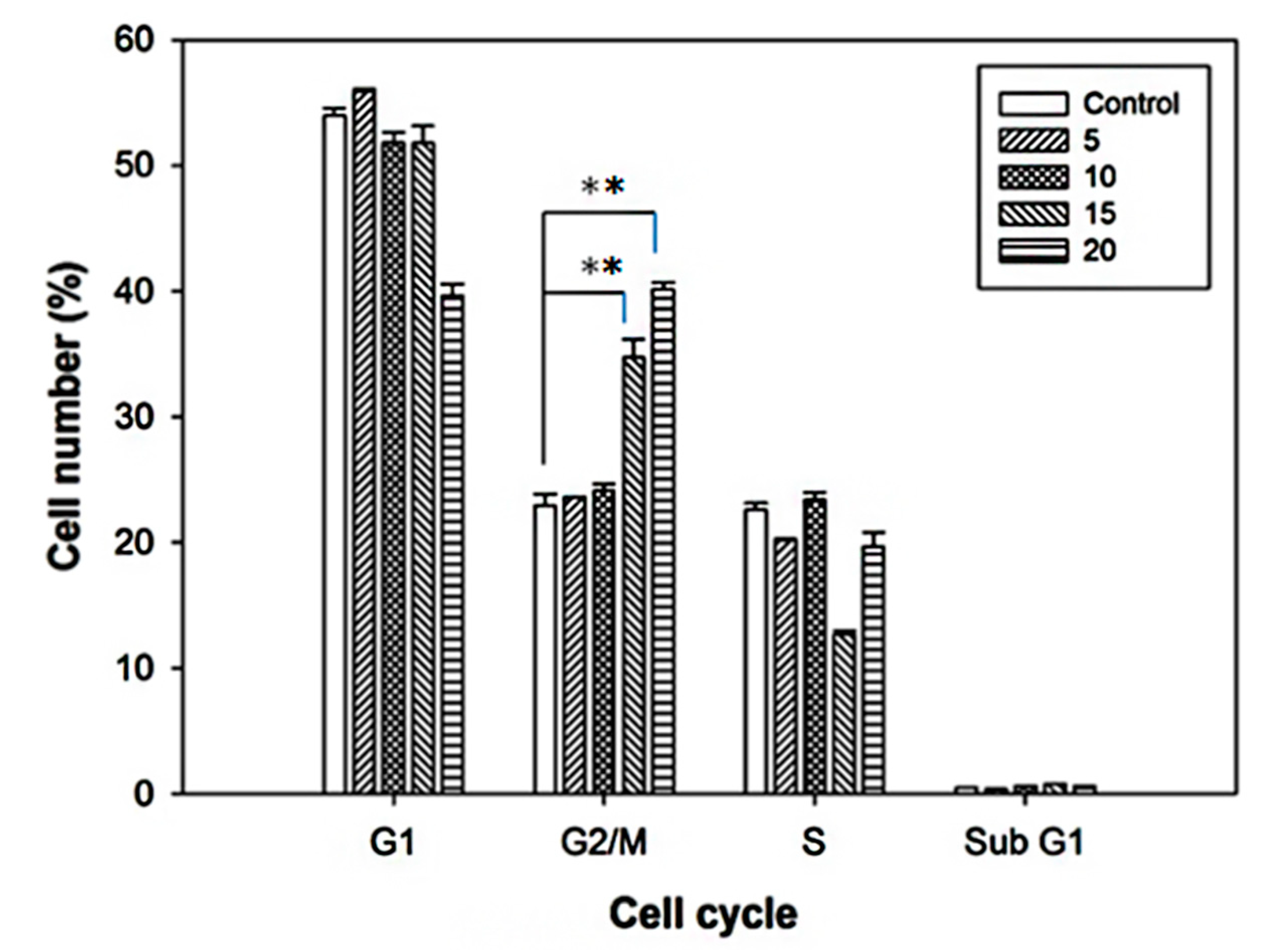
2.2. Cell Cycle Alterations after Curcumin Treatment
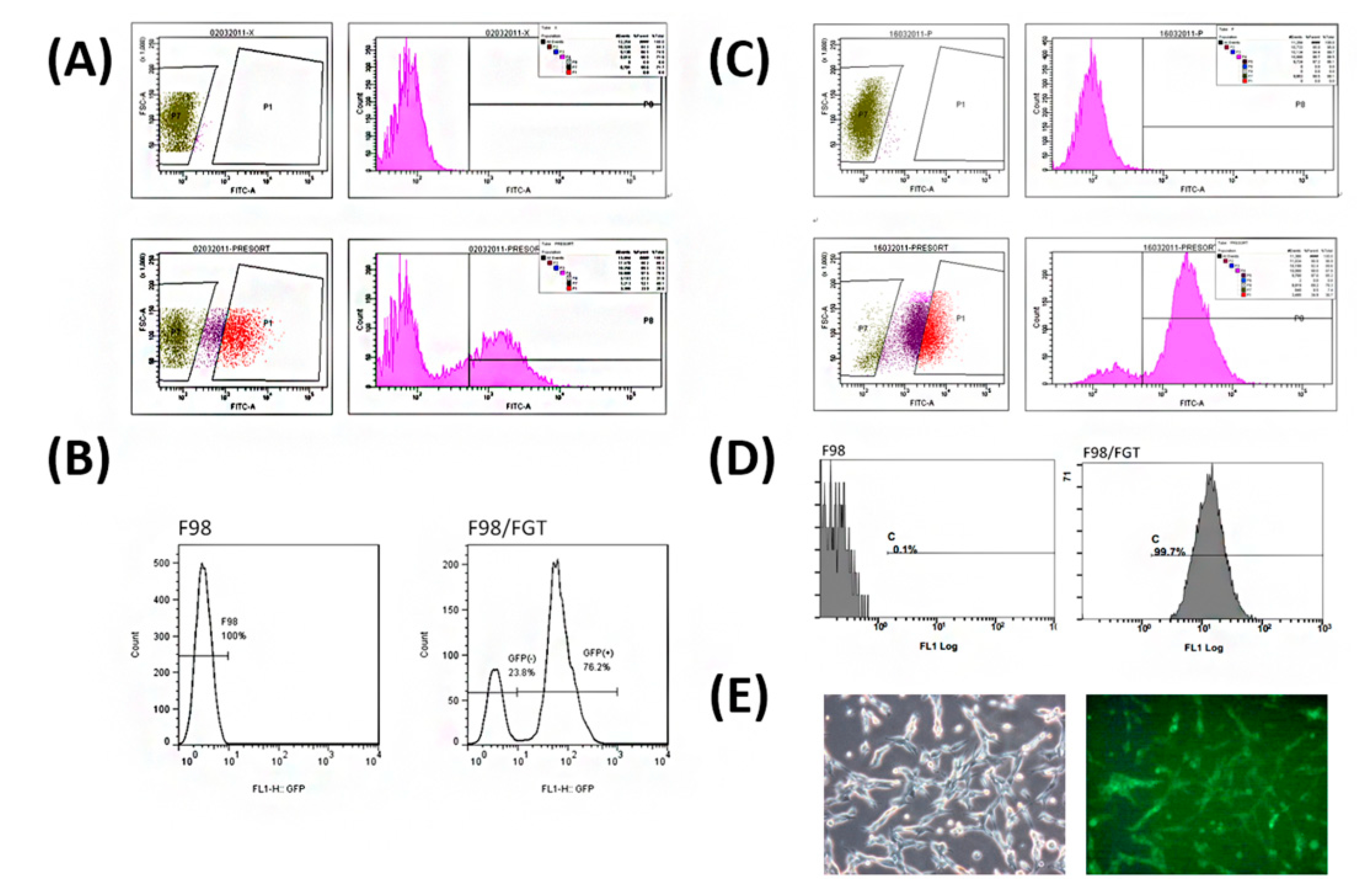
2.3. Transfection of pFu-FGT Lentivirus into F98 Cell Line to Form Unsorted Primary F98/FGT Cells
2.4. Screening and Sorting of High Purity of F98/FGT Cells with GFP Reporter
2.5. Using Luciferase Activity for Monitoring the Cell Growth of F98/FGT Cells
2.6. Thymidine Kinase Activity Distinguishes F98/FGT from F98 after Ganciclovir Treatment
2.7. Noninvasive Luminescence and MRI Images Successfully Track the Tumor Growth, and Curcumin indeed Acts as a Radiosensitizer
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transfection and Selection of F98 with pFu-FGT Lentivirus
4.3. Assay of Luciferase Activity of F98/FGT Cells
4.4. Assay of Thymidine Kinase Activity
4.5. Viability of Curcumin-Treated Cells
4.6. Cell Cycle Analysis after Curcumin Treatment
4.7. Colony Formation Assay for Cells Irradiated with Radiation Alone and Combined with Curcumin
4.8. F98/FGT Animal Model
4.9. Treatment Strategy and Experimental Design Flow Chart
4.10. Noninvasive Luminescence Imaging for Tracking the Tumor Growth
4.11. MRI System for Tracking the Tumor Growth in Brain
4.12. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Goradel, N.H.; Ghiyami-Hour, F.; Jahangiri, S.; Negahdari, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. Nanoparticles as new tools for inhibition of cancer angiogenesis. J. Cell Physiol. 2018, 233, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Saadatpour, Z.; Bjorklund, G.; Chirumbolo, S.; Alimohammadi, M.; Ehsani, H.; Ebrahiminejad, H.; Pourghadamyari, H.; Baghaei, B.; Mirzaei, H.R.; Sahebkar, A.; et al. Molecular imaging and cancer gene therapy. Cancer Gene Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Sahebkar, A.; Sichani, L.S.; Moridikia, A.; Nazari, S.; Sadri Nahand, J.; Salehi, H.; Stenvang, J.; Masoudifar, A.; Mirzaei, H.R.; et al. Therapeutic application of multipotent stem cells. J. Cell Physiol. 2018, 233, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.R.; Sahebkar, A.; Salehi, R.; Nahand, J.S.; Karimi, E.; Jaafari, M.R.; Mirzaei, H. Boron neutron capture therapy: Moving toward targeted cancer therapy. J. Cancer Res. Ther. 2016, 12, 520–525. [Google Scholar] [CrossRef]
- Mirzaei, H.R.; Mirzaei, H.; Lee, S.Y.; Hadjati, J.; Till, B.G. Prospects for chimeric antigen receptor (CAR) gammadelta T cells: A potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 2016, 380, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Shahar, T.; Nossek, E.; Steinberg, D.M.; Rozovski, U.; Blumenthal, D.T.; Bokstein, F.; Sitt, R.; Freedman, S.; Corn, B.W.; Kanner, A.A.; et al. The impact of enrollment in clinical trials on survival of patients with glioblastoma. J. Clin. Neurosci. 2012, 19, 1530–1534. [Google Scholar] [CrossRef]
- Park, J.K.; Hodges, T.; Arko, L.; Shen, M.; Dello Iacono, D.; McNabb, A.; Olsen Bailey, N.; Kreisl, T.N.; Iwamoto, F.M.; Sul, J.; et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J. Clin. Oncol. 2010, 28, 3838–3843. [Google Scholar] [CrossRef]
- Senders, J.T.; Staples, P.; Mehrtash, A.; Cote, D.J.; Taphoorn, M.J.B.; Reardon, D.A.; Gormley, W.B.; Smith, T.R.; Broekman, M.L.; Arnaout, O. An Online Calculator for the Prediction of Survival in Glioblastoma Patients Using Classical Statistics and Machine Learning. Neurosurgery 2020, 86, E184–E192. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Park, C.G.; Ham, S.W.; Choi, S.H.; Lee, S.Y.; Kim, J.Y.; Seo, S.; Jin, X.; Kim, J.K.; Eun, K.; et al. BRM270, a Compound from Natural Plant Extracts, Inhibits Glioblastoma Stem Cell Properties and Glioblastoma Recurrence. J. Med. Food 2017, 20, 838–845. [Google Scholar] [CrossRef]
- Barani, I.J.; Larson, D.A. Radiation therapy of glioblastoma. Cancer Treat. Res. 2015, 163, 49–73. [Google Scholar]
- Storey, K.; Leder, K.; Hawkins-Daarud, A.; Swanson, K.; Ahmed, A.U.; Rockne, R.C.; Foo, J. Glioblastoma Recurrence and the Role of O(6)-Methylguanine-DNA Methyltransferase Promoter Methylation. JCO Clin. Cancer Inform. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef]
- Zanders, E.D.; Svensson, F.; Bailey, D.S. Therapy for glioblastoma: Is it working? Drug Discov. Today 2019, 24, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Garnier, D.; Meehan, B.; Kislinger, T.; Daniel, P.; Sinha, A.; Abdulkarim, B.; Nakano, I.; Rak, J. Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization. Neuro. Oncol. 2018, 20, 236–248. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Srivastava, G.; Mehta, J.L. Currying the heart: Curcumin and cardioprotection. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 22–27. [Google Scholar] [CrossRef]
- Mythri, R.B.; Bharath, M.M. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012, 18, 91–99. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Ahmed, T.; Gilani, A.H. Therapeutic potential of turmeric in Alzheimer’s disease: Curcumin or curcuminoids? Phytother. Res. 2014, 28, 517–525. [Google Scholar] [CrossRef]
- Pan, J.; Li, H.; Ma, J.F.; Tan, Y.Y.; Xiao, Q.; Ding, J.Q.; Chen, S.D. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl. Neurodegener. 2012, 1, 16. [Google Scholar] [CrossRef]
- Perry, M.C.; Demeule, M.; Regina, A.; Moumdjian, R.; Beliveau, R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010, 54, 1192–1201. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Kuo, C.L.; Chueh, F.S.; Liu, K.C.; Bau, D.T.; Chung, J.G. Curcuminoids Induce Reactive Oxygen Species and Autophagy to Enhance Apoptosis in Human Oral Cancer Cells. Am. J. Chin. Med. 2018, 46, 1145–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Liu, M.; Lan, X. Multimodality reporter gene imaging: Construction strategies and application. Theranostics 2018, 8, 2954–2973. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.; Chung, J.K. Reporter gene imaging. AJR Am. J. Roentgenol. 2013, 201, W206–W214. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tian, R.; Liu, T.; Liu, G. MRI Reporter Genes for Noninvasive Molecular Imaging. Molecules 2016, 21, 580. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.J.; Chuah, T.L.; Luff, J.; Lavin, M.F.; Walker, D.G. A novel rat model for glioblastoma multiforme using a bioluminescent F98 cell line. J. Clin. Neurosci. 2008, 15, 545–551. [Google Scholar] [CrossRef]
- Chien, Y.C.; Chen, J.C.; Lin, W.C.; Ding, H.J.; Wang, H.E.; Kao, C.H.; Hwang, J.J. Using [(1)(8)F]FBAU for imaging brain tumor progression in an F98/tk-luc glioma-bearing rat model. Oncol. Rep. 2014, 32, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Falke, J.; Parkkinen, J.; Vaahtera, L.; Hulsbergen-van de Kaa, C.A.; Oosterwijk, E.; Witjes, J.A. Curcumin as Treatment for Bladder Cancer: A Preclinical Study of Cyclodextrin-Curcumin Complex and BCG as Intravesical Treatment in an Orthotopic Bladder Cancer Rat Model. Biomed. Res. Int. 2018, 2018, 9634902. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Prabu, S.L.; Jordan, B.C.; Purushothaman, Y.; Umamaheswari, A.; Hosseini Zare, M.S.; Thilagavathi, R. Molecular mechanisms of curcumin and its analogs in colon cancer prevention and treatment. Life Sci. 2019, 239, 117032. [Google Scholar] [CrossRef] [PubMed]
- Ambegaokar, S.S.; Wu, L.; Alamshahi, K.; Lau, J.; Jazayeri, L.; Chan, S.; Khanna, P.; Hsieh, E.; Timiras, P.S. Curcumin inhibits dose-dependently and time-dependently neuroglial cell proliferation and growth. Neuro. Endocrinol. Lett. 2003, 24, 469–473. [Google Scholar] [PubMed]
- Park, K.S.; Yoon, S.Y.; Park, S.H.; Hwang, J.H. Anti-Migration and Anti-Invasion Effects of Curcumin via Suppression of Fascin Expression in Glioblastoma Cells. Brain Tumor Res. Treat. 2019, 7, 16–24. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Klafke, K.; Figueiro, F.; Terra, S.R.; Paludo, F.J.; Morrone, M.; Bristot, I.J.; Battastini, A.M.; Forcelini, C.M.; et al. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015, 358, 220–231. [Google Scholar] [CrossRef]
- Bangaru, M.L.; Chen, S.; Woodliff, J.; Kansra, S. Curcumin (diferuloylmethane) induces apoptosis and blocks migration of human medulloblastoma cells. Anticancer Res. 2010, 30, 499–504. [Google Scholar]
- Wang, L.; Ye, X.; Cai, X.; Su, J.; Ma, R.; Yin, X.; Zhou, X.; Li, H.; Wang, Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget 2015, 6, 18027–18037. [Google Scholar] [CrossRef]
- Liu, E.; Wu, J.; Cao, W.; Zhang, J.; Liu, W.; Jiang, X.; Zhang, X. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J. Neurooncol. 2007, 85, 263–270. [Google Scholar] [CrossRef]
- Minafra, L.; Porcino, N.; Bravata, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef]
- Sebastia, N.; Montoro, A.; Hervas, D.; Pantelias, G.; Hatzi, V.I.; Soriano, J.M.; Villaescusa, J.I.; Terzoudi, G.I. Curcumin and trans-resveratrol exert cell cycle-dependent radioprotective or radiosensitizing effects as elucidated by the PCC and G2-assay. Mutat. Res. 2014, 766–767, 49–55. [Google Scholar] [CrossRef]
- Chendil, D.; Ranga, R.S.; Meigooni, D.; Sathishkumar, S.; Ahmed, M.M. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 2004, 23, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Erices, J.I.; Torres, A.; Niechi, I.; Bernales, I.; Quezada, C. Current natural therapies in the treatment against glioblastoma. Phytother. Res. 2018, 32, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Berliner, A.; Fernando, S.S.; Ranasinghe, B.; Ray, I.; Tariq, H.; Banerjee, P. Curcumin blocks brain tumor formation. Brain Res. 2009, 1266, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Caretti, V.; Zondervan, I.; Meijer, D.H.; Idema, S.; Vos, W.; Hamans, B.; Bugiani, M.; Hulleman, E.; Wesseling, P.; Vandertop, W.P.; et al. Monitoring of tumor growth and post-irradiation recurrence in a diffuse intrinsic pontine glioma mouse model. Brain Pathol. 2011, 21, 441–451. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-H.; Shen, C.-Y.; Chien, Y.-C.; Chang, W.-S.; Tsai, C.-W.; Lin, Y.-H.; Hwang, J.-J. Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model. Int. J. Mol. Sci. 2020, 21, 4385. https://doi.org/10.3390/ijms21124385
Wang W-H, Shen C-Y, Chien Y-C, Chang W-S, Tsai C-W, Lin Y-H, Hwang J-J. Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model. International Journal of Molecular Sciences. 2020; 21(12):4385. https://doi.org/10.3390/ijms21124385
Chicago/Turabian StyleWang, Wei-Hsun, Chao-Yu Shen, Yi-Chun Chien, Wen-Shin Chang, Chia-Wen Tsai, Yi-Hsien Lin, and Jeng-Jong Hwang. 2020. "Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model" International Journal of Molecular Sciences 21, no. 12: 4385. https://doi.org/10.3390/ijms21124385
APA StyleWang, W.-H., Shen, C.-Y., Chien, Y.-C., Chang, W.-S., Tsai, C.-W., Lin, Y.-H., & Hwang, J.-J. (2020). Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model. International Journal of Molecular Sciences, 21(12), 4385. https://doi.org/10.3390/ijms21124385



