Differentiation and Establishment of Dental Epithelial-Like Stem Cells Derived from Human ESCs and iPSCs
Abstract
1. Introduction
2. Results
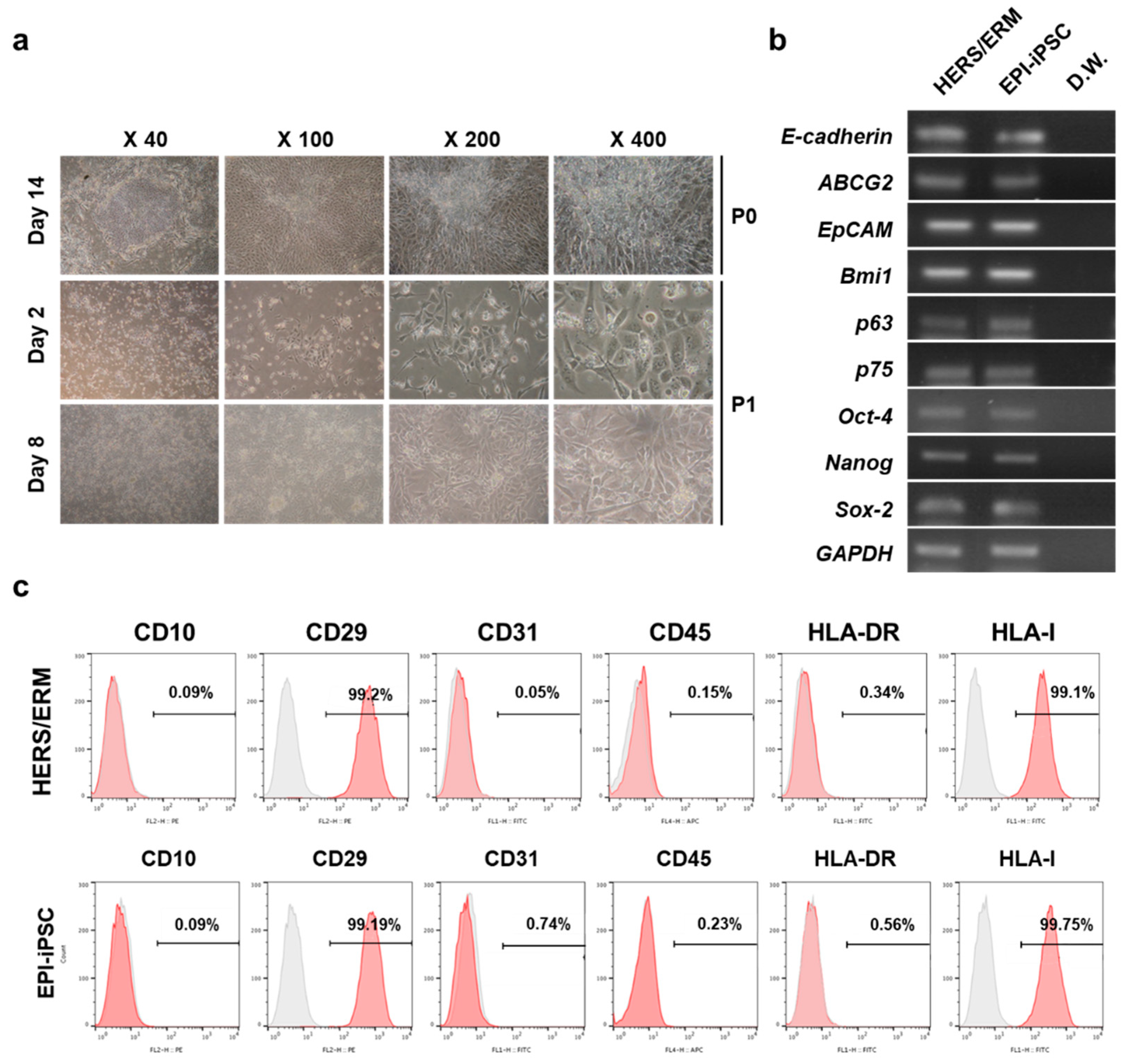
2.1. Differentiation and Characterization of hESCs into Epithelial-Like Stem Cells
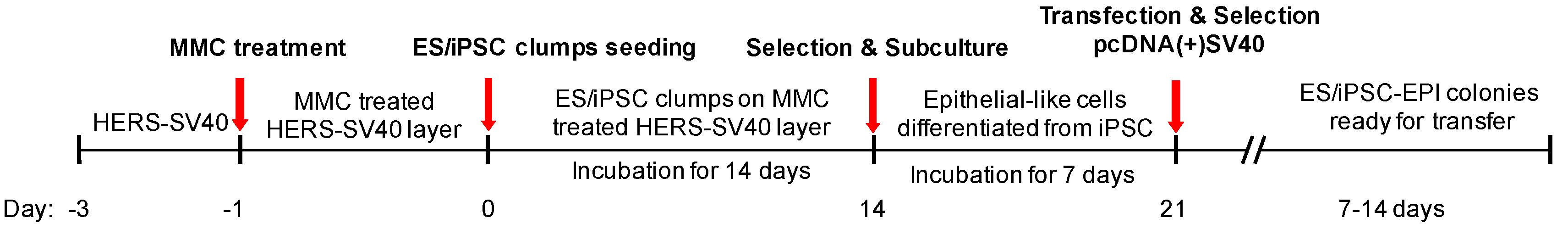
2.2. Establishment of Differentiated Dental Epithelial-Like Stem Cell Lines Derived from hiPSC
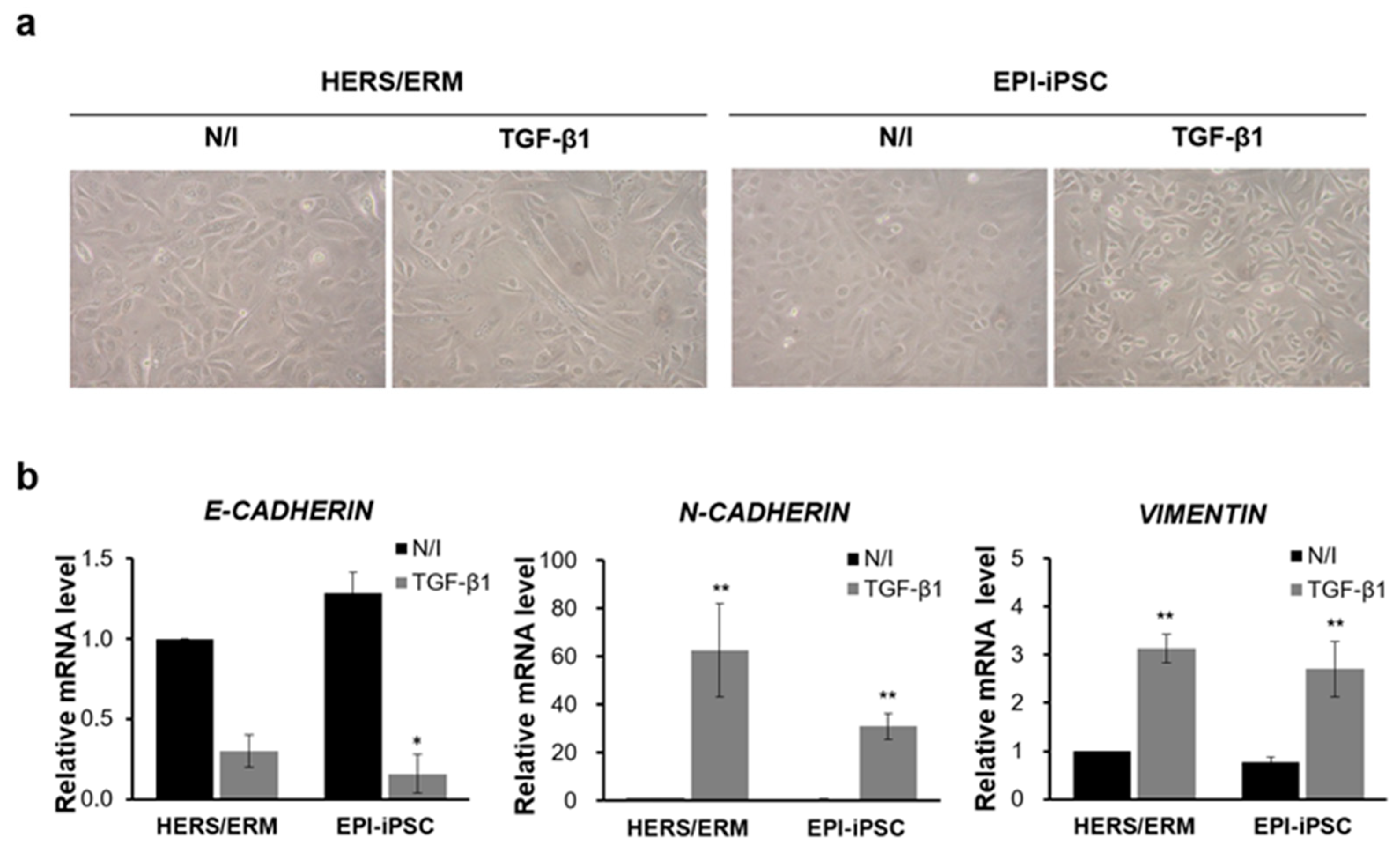
2.3. Induction of Epithelial-Mesenchymal Transition (EMT) in Dental Epithelial-Like Stem Cell Lines Derived from hiPSCs
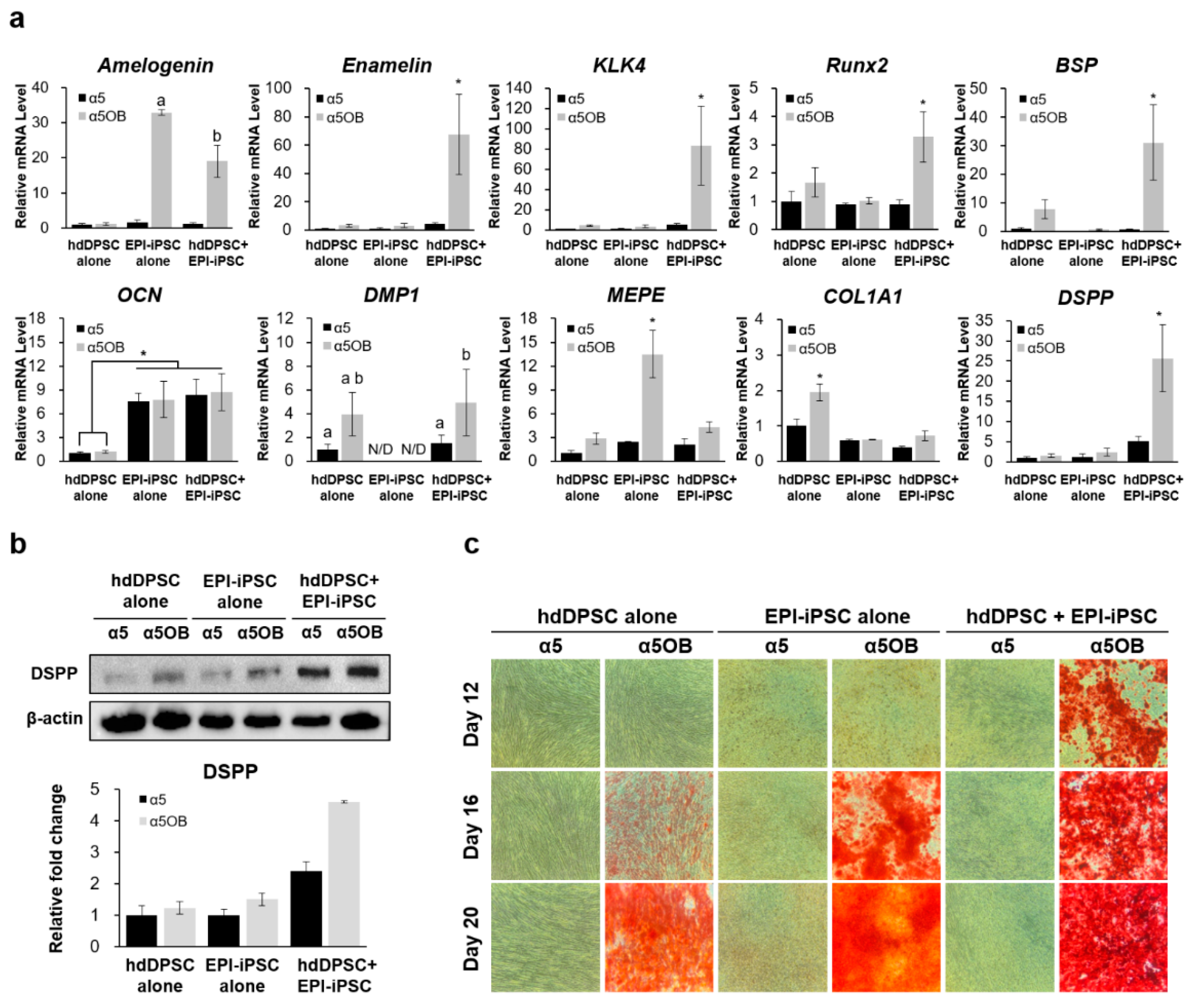
2.4. Differentiation Potential of Differentiated Dental Epithelial-Like Stem Cell Lines Derived From hiPSC
3. Discussion
4. Materials and Methods
4.1. Cell Preparation and Culture Conditions
4.2. Differentiation of Human ESCs and iPSCs into Epithelial-Like Cells
4.3. SV40 LT Transformation-Mediated Immortalization
4.4. Immunofluorescent Staining
4.5. Microsatellite (STR) Analysis
4.6. Total RNA Preparation and Reverse Transcription
4.7. Semi-Quantitative PCR
4.8. Flow Cytometry
4.9. Epithelial-Mesenchymal Transition (EMT) Induced by Transforming Growth Factor-β1 (TGF-β1)
4.10. Real-Time PCR
4.11. Odontogenic Differentiation
4.12. Western Blotting
4.13. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HERS/ERM | Hertwig’s epithelial root sheath/epithelial rests of Malassez |
| hESCs | Human embryonic stem cells |
| hiPSCs | Human induced pluripotent stem cells |
| hdDPSC | Dental pulp stem cells from human exfoliated deciduous teeth |
| EPI-ES EPI-iPSC | Dental epithelial-like stem cells derived from hESCs |
| Dental epithelial-like stem cells derived from hiPSCs |
References
- Grobstein, C. Mechanisms of organogenetic tissue interaction. Natl. Cancer Inst. Monogr. 1967, 26, 279–299. [Google Scholar] [PubMed]
- Thesleff, I.; Hurmerinta, K. Tissue interactions in tooth development. Differentiation 1981, 18, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, I.; Vaahtokari, A.; Kettunen, P.; Aberg, T. Epithelial-mesenchymal signaling during tooth development. Connect. Tissue Res. 1995, 32, 9–15. [Google Scholar] [CrossRef]
- Spouge, J.D. A new look at the rests of Malassez. A review of their embryological origin, anatomy, and possible role in periodontal health and disease. J. Periodontol. 1980, 51, 437–444. [Google Scholar] [CrossRef]
- Nam, H.; Lee, G. Identification of novel epithelial stem cell-like cells in human deciduous dental pulp. Biochem. Biophys. Res. Commun. 2009, 386, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Kim, J.; Park, J.; Park, J.C.; Kim, J.W.; Seo, B.M.; Lee, J.C.; Lee, G. Expression profile of the stem cell markers in human Hertwig’s epithelial root sheath/Epithelial rests of Malassez cells. Mol. Cells 2011, 31, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, Y.; Tsuchiya, S.; Hata, K.; Honda, M.J. Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. J. Cell Physiol. 2008, 217, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef]
- Nam, H.; Kim, J.-H.; Kim, J.-W.; Seo, B.-M.; Park, J.-C.; Kim, J.-W.; Lee, G. Establishment of Hertwig’s epithelial root sheath/epithelial rests of Malassez cell line from human periodontium. Mol. Cells 2014, 37, 562. [Google Scholar] [CrossRef]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Darimont, C.; Avanti, O.; Tromvoukis, Y.; Vautravers-Leone, P.; Kurihara, N.; Roodman, G.D.; Colgin, L.M.; Tullberg-Reinert, H.; Pfeifer, A.M.; Offord, E.A. SV40 T antigen and telomerase are required to obtain immortalized human adult bone cells without loss of the differentiated phenotype. Cell Growth Differ.-Publ. Am. Assoc. Cancer Res. 2002, 13, 59–68. [Google Scholar]
- Lee, C.H.; Kim, J.H.; Lee, H.J.; Jeon, K.; Lim, H.; Choi, H.; Lee, E.R.; Park, S.H.; Park, J.Y.; Hong, S.; et al. The generation of iPS cells using non-viral magnetic nanoparticle based transfection. Biomaterials 2011, 32, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, J.; Lai, H.; Yang, C.; Guo, C.; Wang, C. Reprogramming fibroblasts to pluripotency using arginine-terminated polyamidoamine nanoparticles based non-viral gene delivery system. Int. J. Nanomed. 2014, 9, 5837–5847. [Google Scholar]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef]
- Arakaki, M.; Ishikawa, M.; Nakamura, T.; Iwamoto, T.; Yamada, A.; Fukumoto, E.; Saito, M.; Otsu, K.; Harada, H.; Yamada, Y.; et al. Role of epithelial-stem cell interactions during dental cell differentiation. J. Biol. Chem. 2012, 287, 10590–10601. [Google Scholar] [CrossRef]
- Farea, M.; Husein, A.; Halim, A.S.; Berahim, Z.; Nurul, A.A.; Mokhtar, K.I.; Mokhtar, K. Cementoblastic lineage formation in the cross-talk between stem cells of human exfoliated deciduous teeth and epithelial rests of Malassez cells. Clin. Oral Investig. 2016, 20, 1181–1191. [Google Scholar] [CrossRef]
- Kulan, P.; Karabiyik, O.; Kose, G.; Kargul, B. The effect of accelerated mineral trioxide aggregate on odontoblastic differentiation in dental pulp stem cell niches. Int. Endod. J. 2018, 51, 758–766. [Google Scholar] [CrossRef]
- Louvrier, A.; Euvrard, E.; Nicod, L.; Rolin, G.; Gindraux, F.; Pazart, L.; Houdayer, C.; Risold, P.; Meyer, F.; Meyer, C. Odontoblastic differentiation of dental pulp stem cells from healthy and carious teeth on an original PCL-based 3D scaffold. Int. Endod. J. 2018, 51, e252–e263. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Kang, K.-J.; Ju, S.M.; Jang, Y.-J.; Kim, J. Indirect co-culture of stem cells from human exfoliated deciduous teeth and oral cells in a microfluidic platform. Tissue Eng. Regen. Med. 2016, 13, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharabi, N.; Xue, Y.; Fujio, M.; Ueda, M.; Gjerde, C.; Mustafa, K.; Fristad, I. Bone marrow stromal cell paracrine factors direct osteo/odontogenic differentiation of dental pulp cells. Tissue Eng. Part A 2014, 20, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.N.; Miyauchi, S.; Onishi, A.; Tanimoto, K.; Kato, K. Differentiation of mouse-induced pluripotent stem cells into dental epithelial-like cells in the absence of added serum. In Vitro Cell Dev. Biol. Anim. 2019, 55, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Attisano, L.; Wrana, J.L. Signal transduction by the TGF-β superfamily. Science 2002, 296, 1646–1647. [Google Scholar] [CrossRef] [PubMed]
- Beerling, E.; Seinstra, D.; de Wit, E.; Kester, L.; van der Velden, D.; Maynard, C.; Schäfer, R.; van Diest, P.; Voest, E.; van Oudenaarden, A. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep. 2016, 14, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Kim, R.; Green, J.B.A.; Klein, O.D. From snapshots to movies: Understanding early tooth development in four dimensions. Dev. Dyn. 2017, 246, 442–450. [Google Scholar] [CrossRef]
- Lee, H.-K.; Park, J.-W.; Seo, Y.-M.; Kim, H.H.; Lee, G.; Bae, H.-S.; Park, J.-C. Odontoblastic inductive potential of epithelial cells derived from human deciduous dental pulp. J. Mol. Histol. 2016, 47, 345–351. [Google Scholar] [CrossRef]
- Bluteau, G.; Luder, H.U.; De Bari, C.; Mitsiadis, T.A. Stem cells for tooth engineering. Eur. Cell Mater. 2008, 16, 1–9. [Google Scholar] [CrossRef]
- Duailibi, M.T.; Duailibi, S.E.; Young, C.S.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Bioengineered teeth from cultured rat tooth bud cells. J. Dent. Res. 2004, 83, 523–528. [Google Scholar] [CrossRef]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S. Stem Cells from Human Dental Tissue for Regenerative Medicine. Stem Cells Toxicol. Med. 2017, 481–501. [Google Scholar]
- Hyun, S.-Y.; Mun, S.; Kang, K.-J.; Lim, J.-C.; Kim, S.-Y.; Han, K.; Jang, Y.-J. Amelogenic transcriptome profiling in ameloblast-like cells derived from adult gingival epithelial cells. Sci. Rep. 2019, 9, 3736. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Guo, Y.; Tang, J.; Zhou, J.; Zhang, H.; Lu, W.; Gao, Y.; Wang, L.; Pei, D.; Duan, Y.; et al. Differentiation of mouse embryonic stem cells into dental epithelial-like cells induced by ameloblasts serum-free conditioned medium. Biochem. Biophys. Res. Commun. 2010, 394, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Yoon, K.S.; Arakaki, M.; Otsu, K.; Fukumoto, S.; Harada, H.; Green, D.W.; Lee, J.M.; Jung, H.S. Effective Differentiation of Induced Pluripotent Stem Cells Into Dental Cells. Dev. Dyn. 2019, 248, 129–139. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Y.; Liu, P.; Chen, S.; Wu, X.; Sun, Y.; Li, A.; Huang, K.; Luo, R.; Wang, L.; et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen. (Lond.) 2013, 2, 6. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Granja, P.L.; Soares, R.; Guerreiro, S.G. Cellular strategies to promote vascularisation in tissue engineering applications. Eur. Cell Mater. 2014, 28, 51–66, discussion 66-7. [Google Scholar] [CrossRef]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef]
- Kim, M.; Turnquist, H.; Jackson, J.; Sgagias, M.; Yan, Y.; Gong, M.; Dean, M.; Sharp, J.G.; Cowan, K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin. Cancer Res. 2002, 8, 22–28. [Google Scholar]
- Muyrers-Chen, I.; Paro, R. Epigenetics: Unforeseen regulators in cancer. Biochim. Biophys. Acta 2001, 1552, 15–26. [Google Scholar] [CrossRef]
- Raaphorst, F.M.; Otte, A.P.; Meijer, C.J. Polycomb-group genes as regulators of mammalian lymphopoiesis. Trends Immunol. 2001, 22, 682–690. [Google Scholar] [CrossRef]
- Jacobs, J.J.; van Lohuizen, M. Cellular memory of transcriptional states by Polycomb-group proteins. Semin. Cell Dev. Biol. 1999, 10, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gaddipati, S.; Muralidhar, R.; Sangwan, V.S.; Mariappan, I.; Vemuganti, G.K.; Balasubramanian, D. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J. Ophthalmol. 2014, 62, 644–648. [Google Scholar] [PubMed]
- Cherepanov, P.; Devroe, E.; Silver, P.A.; Engelman, A. Identification of an evolutionarily conserved domain in human lens epithelium-derived growth factor/transcriptional co-activator p75 (LEDGF/p75) that binds HIV-1 integrase. J. Biol. Chem. 2004, 279, 48883–48892. [Google Scholar] [CrossRef]
- Nakamura, T.; Endo, K.I.; Kinoshita, S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells 2007, 25, 628–638. [Google Scholar] [CrossRef]
- Li, C.Y.; Cha, W.; Luder, H.U.; Charles, R.P.; McMahon, M.; Mitsiadis, T.A.; Klein, O.D. E-cadherin regulates the behavior and fate of epithelial stem cells and their progeny in the mouse incisor. Dev. Biol. 2012, 366, 357–366. [Google Scholar] [CrossRef]
- Sonoyama, W.; Seo, B.-M.; Yamaza, T.; Shi, S. Human Hertwig’s epithelial root sheath cells play crucial roles in cementum formation. J. Dent. Res. 2007, 86, 594–599. [Google Scholar] [CrossRef]
- Katsuno, Y.; Qin, J.; Oses-Prieto, J.; Wang, H.; Jackson-Weaver, O.; Zhang, T.; Lamouille, S.; Wu, J.; Burlingame, A.; Xu, J. Arginine methylation of SMAD7 by PRMT1 in TGF-β–induced epithelial–mesenchymal transition and epithelial stem-cell generation. J. Biol. Chem.y 2018, 293, 13059–13072. [Google Scholar] [CrossRef]
- Boyd, N.L.; Robbins, K.R.; Dhara, S.K.; West, F.D.; Stice, S.L. Human embryonic stem cell–derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng. Part. A 2009, 15, 1897–1907. [Google Scholar] [CrossRef]
- Gordon, J.A.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, X.; Lu, X.; Zhang, C.; Yu, H.; Zhao, T. Cells derived from iPSC can be immunogenic—Yes or No? Protein Cell 2014, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]


| HERS-SV40 | Human iPSCs | EPI-iPSC | |||
|---|---|---|---|---|---|
| STR Locus | Genotype | STR Locus | Genotype | STR Locus | Genotype |
| D8S1179 | 13 | D8S1179 | 13 | D8S1179 | 13 |
| D21S11 | 29, 30 | D21S11 | 28, 32.2 | D21S11 | 28, 32.2 |
| D7S820 | 9, 11 | D7S820 | 7, 8 | D7S820 | 7, 8 |
| CSF1PO | 11, 12 | CSF1PO | 12 | CSF1PO | 12 |
| D3S1358 | 16, 17 | D3S1358 | 17 | D3S1358 | 17 |
| TH01 | 7, 9 | TH01 | 9 | TH01 | 9 |
| D13S317 | 9, 10 | D13S317 | 9, 12 | D13S317 | 9, 12 |
| D16S539 | 12, 14 | D16S539 | 9, 12 | D16S539 | 9, 12 |
| D2S1338 | 17,24 | D2S1338 | 18, 19 | D2S1338 | 18, 19 |
| D19S433 | 14, 15.2 | D19S433 | 13, 14 | D19S433 | 13, 14 |
| vWA | 14, 18 | vWA | 14, 16 | vWA | 14, 16 |
| TPOX | 8, 9 | TPOX | 8, 11 | TPOX | 8, 11 |
| D18S51 | 13, 15 | D18S51 | 13, 15 | D18S51 | 13, 15 |
| AMEL | X, Y | AMEL | X, Y | AMEL | X, Y |
| D5S818 | 10, 13 | D5S818 | 10, 11 | D5S818 | 10, 11 |
| FGA | 26 | FGA | 22 | FGA | 22 |
| Target Gene | 5′ Oligonucleotide | 3′ Oligonucleotide |
|---|---|---|
| GAPDH | GAT GCT GGC GCT GAG TAC G | GCT AAG CAG TTG GTG GTG C |
| N-cadherin | ACA GTG GCC ACC TAC AAA GG | CCG AGA TGG GGT TGA TAA TG |
| E-cadherin | TGC CCA GAA AAT GAA AAA GG | GTG TAT GTG GCA ATG CGT TC |
| Vimentin | TCT ACG AGG AGG AGA TGC GG | GGT CAA GAC GTG CCA GAG AC |
| ABCG2 | CCA CAG GTG GAG GCA AAT CT | TCG CGG TGC TCC ATT TAT CA |
| EpCAM | GCT GGC CGT AAA CTG CTT TG | ACA TTT GGC AGC CAG CTT TG |
| BMI1 | CAG CCC AGC AGG AGG TAT TC | GGA TGA GGA GAC TGC ACT GG |
| P63 | ATG TTG TAC CTG GAA AAC AAT GC | GTG ATG GAG AGA GAG CAT CGA A |
| P75 | ACC GAG CTG GAA GTC GAG | CTC ACC GCT GTG TGT GTA C |
| SOX2 | GAC TTC ACA TGT CCC AGC AC | GGG TTT TCT CCA TGC TGT TT |
| OCT4 | ACC CCT GGT GCC GTG AA | GGC TGA ATA CCT TCC CAA ATA |
| NANOG | CCT ATG CCT GTG ATT TGT GG | TTC TCT GCA GAA GTG GGT TG |
| Amelogenin | AAG AAC CAT CAA GAA ATG GGG | TGA TAT AAC CAG GGT GCC CA |
| Enamelin | TCC ACG GAA ATC CTC AGC AC | GGG GGT TGA GCT TCC TCT TC |
| KLK4 | GAT CGC TCG TCT CTG GTA GC | GAG TTC TGG AAA CAG TGT GCG |
| Runx2 | CCC AGT ATG AGA GTA GGT GTC C | GGG TAA GAC TGG TCA TAG GAC C |
| BSP | AAG GCT ACG ATG GCT ATG ATG GT | AAT GGT AGC CGG ATG CAA AG |
| OCN | ATC CTT TGG GGT TTG GCC TAC | GCC AAT AGG GCG AGG AGT G |
| DMP1 | ACA GGC AAA TGA AGA CCC | TTC ACT GGC TTG TAT GG |
| MEPE | CAA GAA GCC AGG TAT TCT GAA GG | TGT GGT TGA AAT GTT GGT GCT |
| Col1a1 | CAA AAA ATG GGA GAC AAT TTC ACA | TCA TGT TCG GTT GGT CAA AGA T |
| DSPP | GCC AGA GCA AGT CTG GTA ACG GT | TGT CTC TGC AGG AGT TAG GTC TTG GT |
| Components | Contents of α5 (Control Group) | Contents of α5OB (Differentiation Group) |
|---|---|---|
| Alpha MEM | - | - |
| FBS | 5% | 5% |
| Antibiotics-antimycotics | 1% | 1% |
| Dexamethasone | - | 0.1 µM |
| B-glycerophosphate | - | 10 mM |
| L- ascorbic acid phosphate | - | 50 µg/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-H.; Yang, J.; Jeon, D.-H.; Kim, J.-H.; Chae, G.Y.; Jang, M.; Lee, G. Differentiation and Establishment of Dental Epithelial-Like Stem Cells Derived from Human ESCs and iPSCs. Int. J. Mol. Sci. 2020, 21, 4384. https://doi.org/10.3390/ijms21124384
Kim G-H, Yang J, Jeon D-H, Kim J-H, Chae GY, Jang M, Lee G. Differentiation and Establishment of Dental Epithelial-Like Stem Cells Derived from Human ESCs and iPSCs. International Journal of Molecular Sciences. 2020; 21(12):4384. https://doi.org/10.3390/ijms21124384
Chicago/Turabian StyleKim, Gee-Hye, Jihye Yang, Dae-Hyun Jeon, Ji-Hye Kim, Geun Young Chae, Mi Jang, and Gene Lee. 2020. "Differentiation and Establishment of Dental Epithelial-Like Stem Cells Derived from Human ESCs and iPSCs" International Journal of Molecular Sciences 21, no. 12: 4384. https://doi.org/10.3390/ijms21124384
APA StyleKim, G.-H., Yang, J., Jeon, D.-H., Kim, J.-H., Chae, G. Y., Jang, M., & Lee, G. (2020). Differentiation and Establishment of Dental Epithelial-Like Stem Cells Derived from Human ESCs and iPSCs. International Journal of Molecular Sciences, 21(12), 4384. https://doi.org/10.3390/ijms21124384





