S(+)-(2E)-N-(2-Hydroxypropyl)-3-Phenylprop-2-Enamide (KM-568): A Novel Cinnamamide Derivative with Anticonvulsant Activity in Animal Models of Seizures and Epilepsy
Abstract
1. Introduction
2. Results
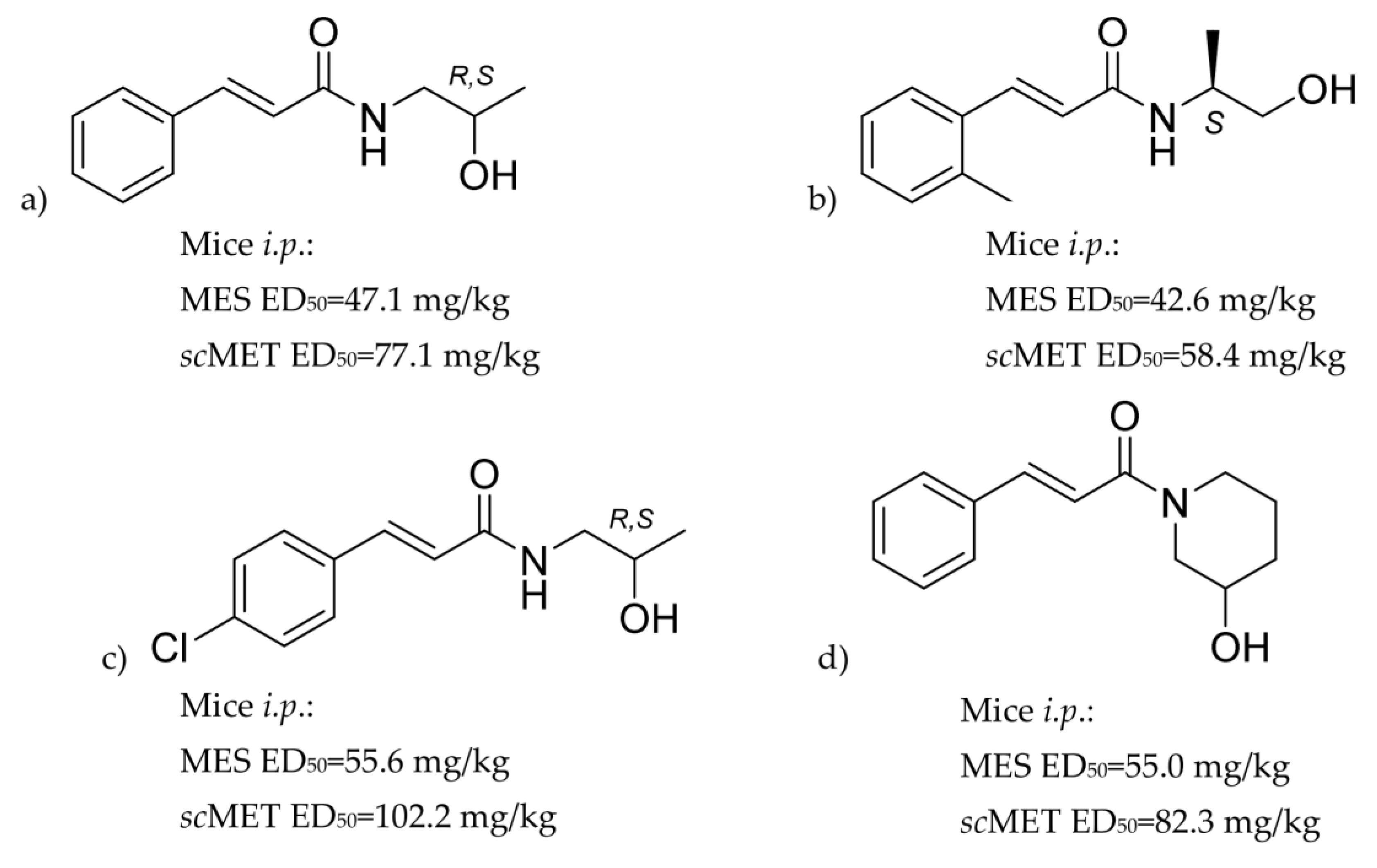
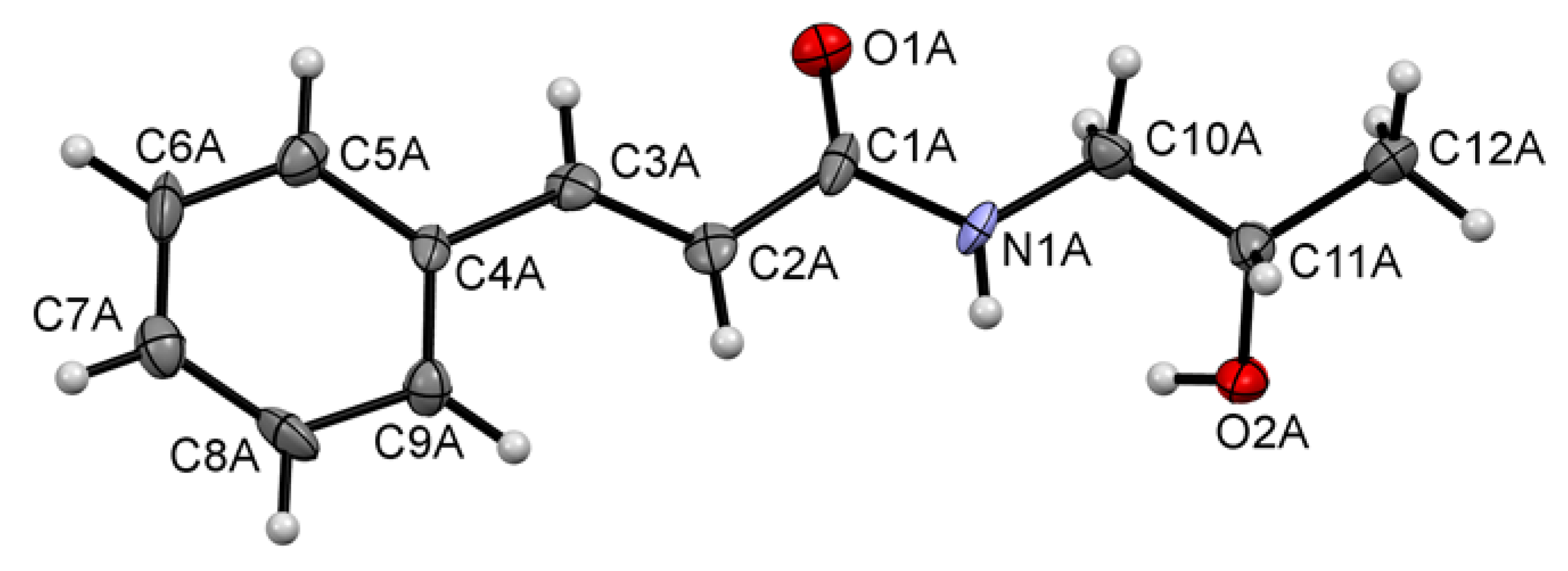
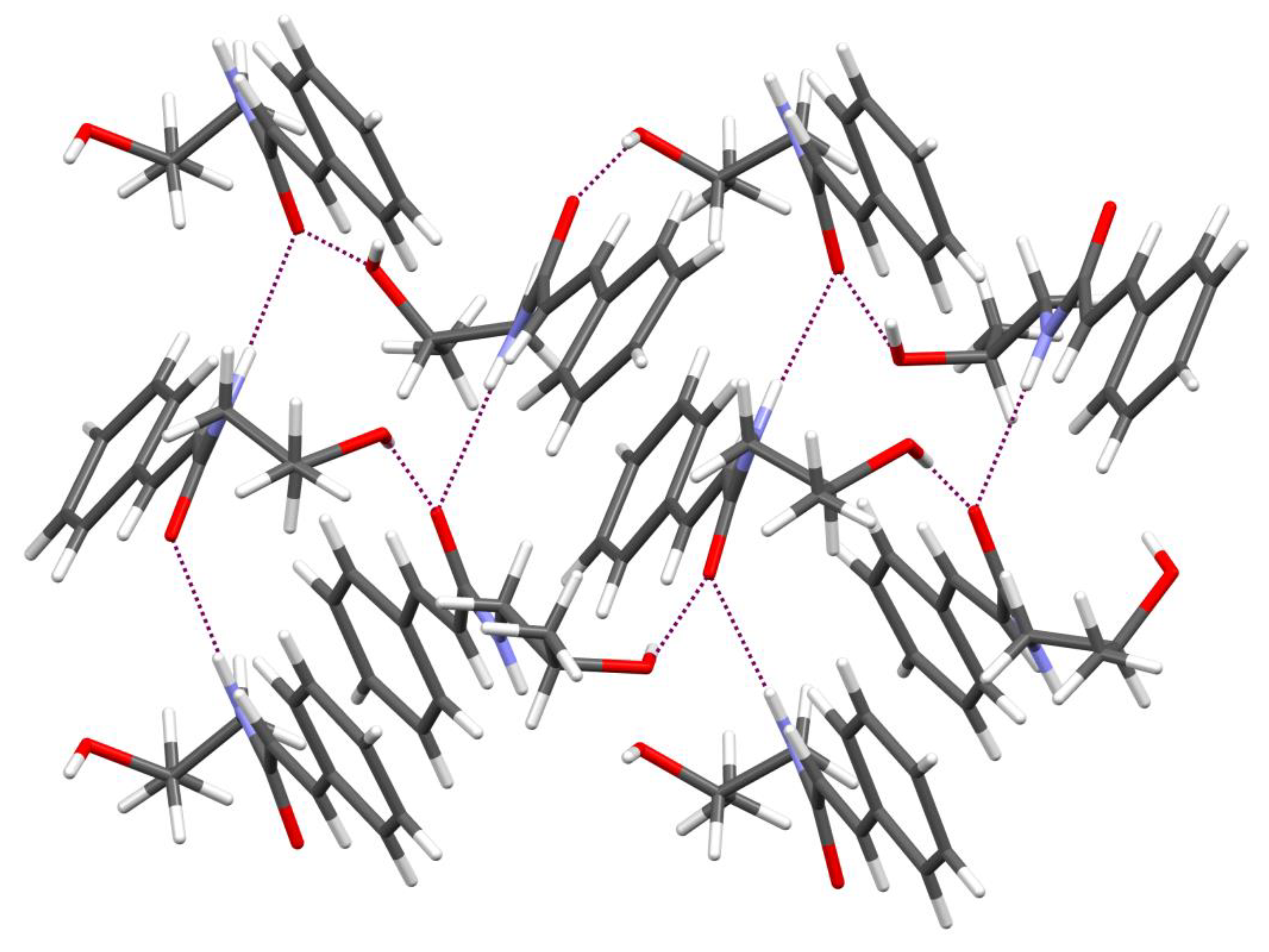
2.1. Synthesis and Crystal Structure
2.2. Anticonvulsant Activity
2.3. Test of Hyperalgesia
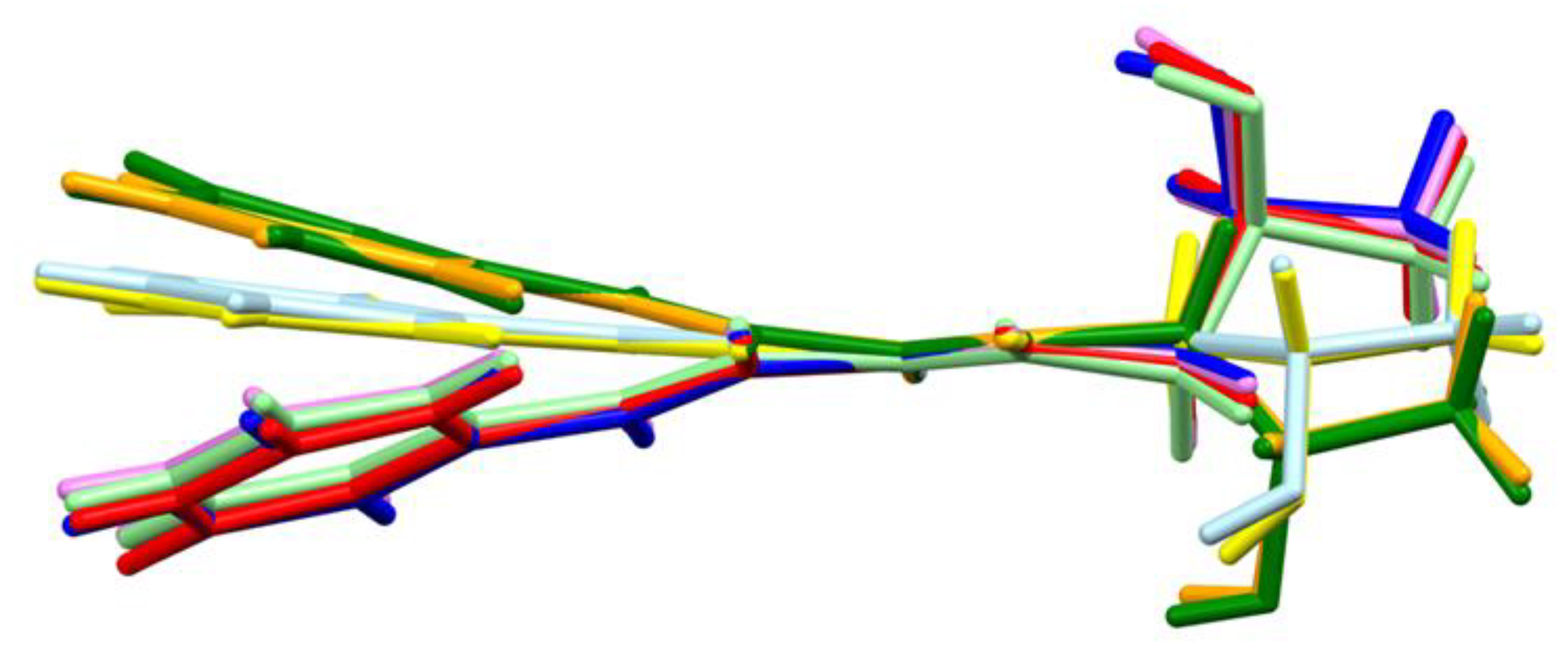
2.4. Molecular Mechanism of Action
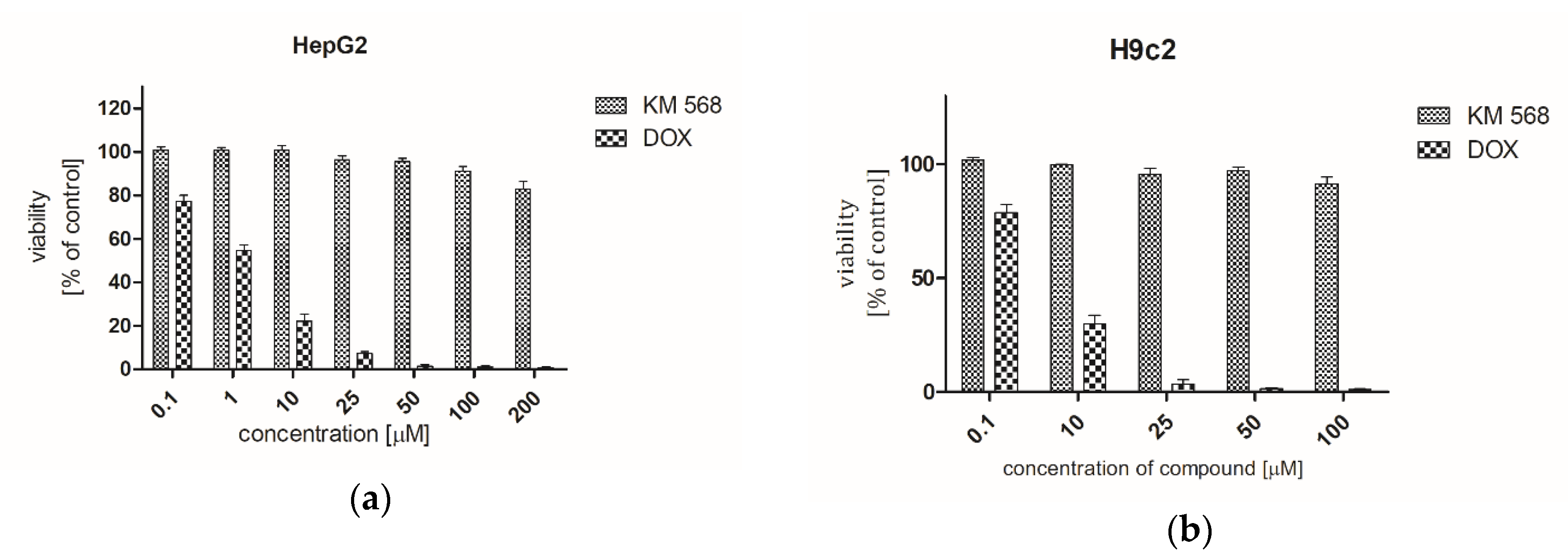
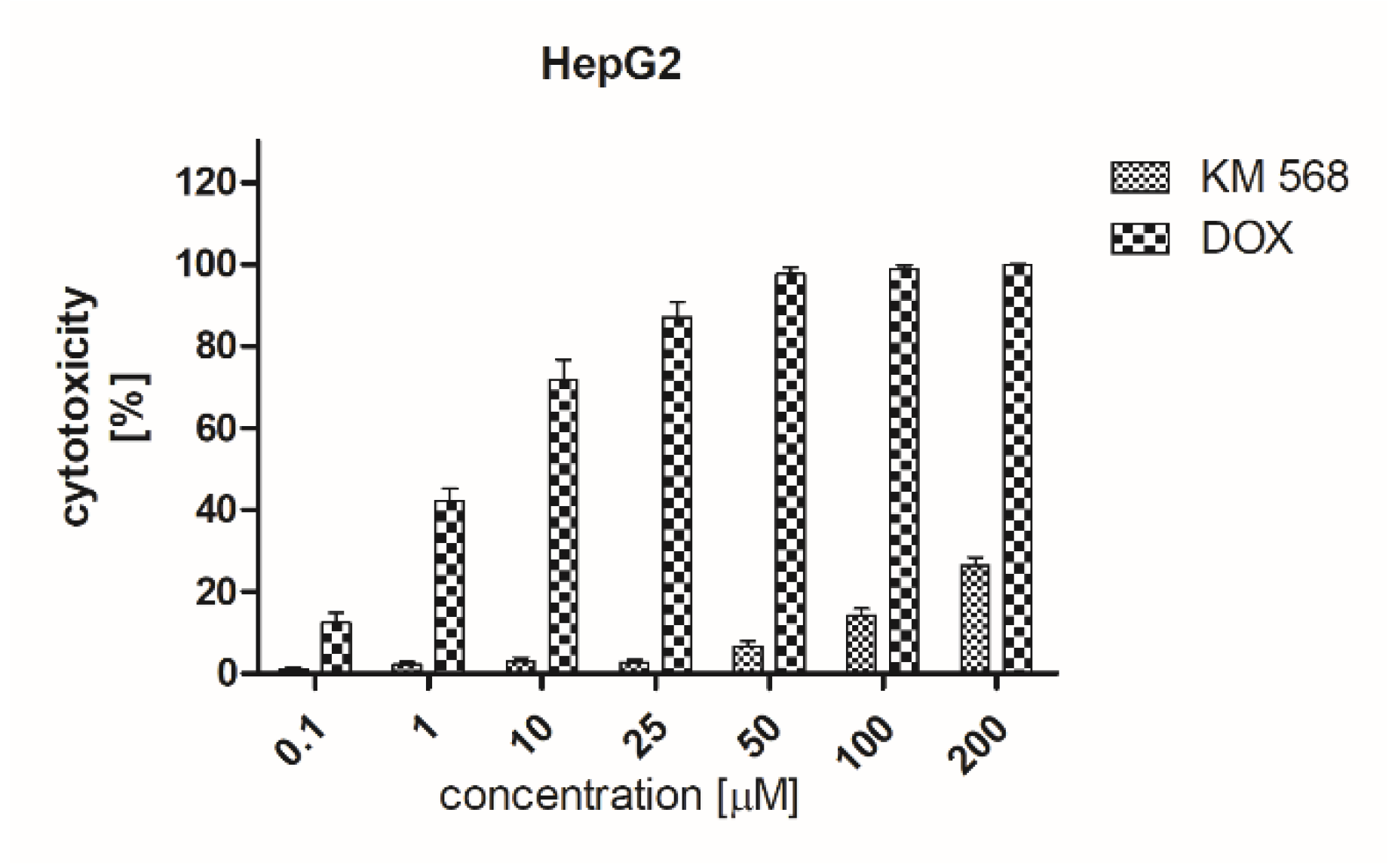
2.5. Preclinical Safety Evaluation
3. Discussion
4. Materials and Methods
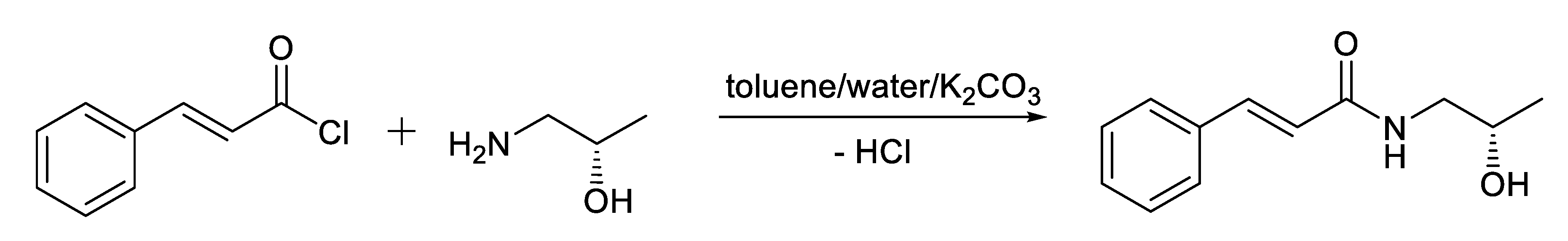
4.1. Chemistry
4.1.1. General Procedure of Preparation of Compound KM-568
4.1.2. Analytical Data for Compound KM-568 - S(+)-(2E)-N-(2-hydroxypropyl)-3-phenylprop-2-enamide
4.2. Crystallographic Data for KM-568
4.3. In Vivo Pharmacology—General
4.4. In Vivo Pharmacology Procedures
4.4.1. Maximal Electroshock Test (MES)
4.4.2. Subcutaneous Metrazol Seizure Threshold Test (scMET)
4.4.3. Neurotoxicity (TOX)
4.4.4. 6-Hz Psychomotor Seizure Model (6-Hz)
4.4.5. Chemoconvulsants Tests—Bicuculine and Picrotoxin
4.4.6. The Frings Audiogenic Seizure-Susceptible Mouse Model (AGS-Susceptible Mouse Model)
4.4.7. Intravenous Metrazol Seizure Threshold Test (ivMET)
4.4.8. Mesial Temporal Lobe Epilepsy Model (MTLE) in Mice
4.4.9. Corneal Kindled Seizure Model in Mice
4.4.10. Hippocampal Kindled Rat Model
4.4.11. Lamotrigine (LTG) Resistant Amygdala Kindled Seizure Model in Rats
4.4.12. Pilocarpine-Induced Status Epilepticus Model
4.4.13. Formalin Test of Hyperalgesia
4.5. Molecular Mechanism of Action
4.6. In Vitro Metabolism
4.7. Mutagenicity Assessment
4.8. Cytotoxicity Assessment
4.8.1. Cell Culture
4.8.2. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
4.8.3. Lactate Dehydrogenase (LDH) Assay
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sander, J.W. The epidemiology of epilepsy revisited. Curr. Opin. Neurol. 2003, 16, 165–170. [Google Scholar] [CrossRef]
- Rho, J.M.; White, H.S. Brief history of anti-seizure drug development. Epilepsia Open 2018, 3, 114–119. [Google Scholar] [CrossRef]
- Brodie, M.J. Antiepileptic drug therapy the story so far. Seizure 2010, 19, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.F. Design of innovative therapeutics for pharmacoresistant epilepsy: Challenges and needs. Epilepsia 2013, 54, 56–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef]
- Zaccara, G.; Franciotta, D.; Perucca, E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia 2007, 48, 1223–1244. [Google Scholar] [CrossRef]
- Chen, B.; Choi, H.; Hirsch, L.J.; Moeller, J.; Javed, A.; Kato, K.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Cosmetic side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2015, 42, 129–137. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Gunda, S.G.R.; Vashishtha, S.C.; Zello, G.A.; Das, U.; Nienaber, K.H.; Stables, J.P.; Allen, T.M.; Santos, C.L. Anticonvulsants containing the N-(3-aryl-2-propenoyl) amido pharmacophore. J. Enzyme Inhib. Med. Chem. 2004, 19, 303–312. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nanduri, S.; Madhavi, Y.V. Cinnamamide: An insight into the pharmacological advances and structure–activity relationships. Eur. J. Med. Chem. 2019, 181, 111561. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Pańczyk, K.; Waszkielewicz, A.M.; Marona, H. Cinnamamide derivatives for central and peripheral nervous system disorders - a review of structure-activity relationships. Chem.Med.Chem. 2015, 10, 1302–1325. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.P.; Wei, C.X.; Deng, X.Q.; Sui, X.; Piao, H.R.; Quan, Z.S. Synthesis and anticonvulsant activity of N-(2-hydroxyethyl) cinnamamide derivatives. Eur. J. Med. Chem. 2009, 44, 3654–3657. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, A.; Barili, P.L.; Crotti, P.; Macchia, B.; Macchia, F.; Pecchia, A. Structure-activity relationships in cinnamamides. 1. Synthesis and pharmacological evaluation of some (E)- and (Z)-N-alkyl-α,β-dimethylcinnamamides. J. Med. Chem. 1975, 18, 842–846. [Google Scholar] [CrossRef]
- Khom, S.; Strommer, B.; Schöffmann, A.; Hintersteiner, J.; Baburin, I.; Erker, T.; Schwarz, T.; Schwarzer, C.; Zaugg, J.; Hamburger, M.; et al. GABAA receptor modulation by piperine and a non-TRPV1 activating derivative. Biochem. Pharmacol. 2013, 85, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Schöffmann, A.; Wimmer, L.; Goldmann, D.; Khom, S.; Hintersteiner, J.; Baburin, I.; Schwarz, T.; Hintersteininger, M.; Pakfeifer, P.; Oufir, M.; et al. Efficient modulation of γ-aminobutyric acid type a receptors by piperine derivatives. J. Med. Chem. 2014, 57, 5602–5619. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.E.; Metzler, B.; Stafford, G.I.; Van Staden, J.; Jäger, A.K.; Rasmussen, H.B. Amides from Piper capense with CNS activity - A preliminary SAR analysis. Molecules 2009, 14, 3833–3843. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Żesławska, E.; Bareyre, F.M.; Nitek, W.; Waszkielewicz, A.M.; Marona, H. Physicochemical and biological evaluation of a cinnamamide derivative R,S-(2E)-1-(3-hydroxypiperidin-1-yl)-3-phenylprop-2-en-1-one (KM-608) for nervous system disorders. Chem. Biol. Drug Des. 2017, 90, 244–253. [Google Scholar] [CrossRef]
- Gunia, A.; Waszkielewicz, A.M.; Cegła, M.; Marona, H. Preliminary evaluation of anticonvulsant activity of some aminoalkanol and amino acid cinnamic acid derivatives. Lett. Drug Des. Discov. 2012, 9, 37–43. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Żesławska, E.; Słoczyńska, K.; Koczurkiewicz, P.; Nitek, W.; Zelaszczyk, D.; Szkaradek, N.; Waszkielewicz, A.M.; Pękala, E.; Marona, H. Anticonvulsant activity, crystal structures, and preliminary safety evaluation of N - trans -cinnamoyl derivatives of selected (un)modified aminoalkanols. Eur. J. Med. Chem. 2016, 107, 26–37. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Żesławska, E.; Nitek, W.; Popiół, J.; Marona, H. Anticonvulsant activity of enantiomeric N-trans-cinnamoyl derivatives of 2-aminopropan-1-ols and 2-aminobutan-1-ols. Chirality 2016, 28, 482–488. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Żelaszczyk, D.; Waszkielewicz, A.M.; Pańczyk, K.; Marona, H.; Rapacz, A.; Filipek, B.; Żesławska, E.; Słoczyńska, K.; Pękala, E.; et al. Structure-anticonvulsant activity studies in the group of (E)-N-cinnamoyl aminoalkanols derivatives monosubstituted in phenyl ring with 4-Cl, 4-CH3 or 2-CH3. Bioorganic Med. Chem. 2017, 25, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Bareyre, F.M.; Marona, H.; Waszkielewicz, A.M. Four N-(E)-cinnamoyl (cinnamamide) derivatives of aminoalkanols with promising anticonvulsant and analgesic activity. Bioorganic Med. Chem. Lett. 2019, 29, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Żesławska, E.; Nitek, W.; Marona, H.; Gunia-Krzyżak, A. Crystallographic studies of cinnamamide derivatives as a means of searching for anticonvulsant activity. Acta Crystallogr. Sect. C Struct. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Żesławska, E.; Nitek, W.; Marona, H.; Gunia-Krzyżak, A. Cinnamamide pharmacophore for anticonvulsant activity: Evidence from crystallographic studies. Acta Crystallogr. Sect. C Struct. Chem. 2018. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chemie Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Mandhane, S.N.; Aavula, K.; Rajamannar, T. Timed pentylenetetrazol infusion test: A comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure 2007, 16, 636–644. [Google Scholar] [CrossRef]
- Lothman, E.W.; Salerno, R.A.; Perlin, J.B.; Kaiser, D.L. Screening and characterization of antiepileptic drugs with rapidly recurring hippocampal seizures in rats. Epilepsy Res. 1988, 2, 367–379. [Google Scholar] [CrossRef]
- Löscher, W.; Schmidt, D. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia 2011, 52, 657–678. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef]
- Ganesh, T.; Jiang, J.; Yang, M.S.; Dingledine, R. Lead optimization studies of cinnamic amide EP2 antagonists. J. Med. Chem. 2014, 57, 4173–4184. [Google Scholar] [CrossRef]
- Bialer, M.; White, H.S. Key factors in the discovery and development of new antiepileptic drugs. Nat. Rev. Drug Discov. 2010, 9, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Wang, S.Y. Chemical modification and structure-activity relationship studies of piperine and its analogs: An example of drug development from folk medicine. In QSAR and Drug Design: New Developments and Applications; Fujita, T., Ed.; Elsevier Science, B.V.: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Perucca, E.; French, J.; Bialer, M. Development of new antiepileptic drugs: Challenges, incentives, and recent advances. Lancet Neurol. 2007, 6, 793–804. [Google Scholar] [CrossRef]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef]
- Holmes, G. Animal model studies application to human patients. Neurology 2007, 69, S28–S32. [Google Scholar] [CrossRef]
- Da Cruz, G.M.P.; Felipe, C.F.B.; Scorza, F.A.; Da Costa, M.A.C.; Tavares, A.F.; Menezes, M.L.F.; De Andrade, G.M.; Leal, L.K.A.M.; Brito, G.A.C.; Da Graça Naffah-Mazzacoratti, M.; et al. Piperine decreases pilocarpine-induced convulsions by GABAergic mechanisms. Pharmacol. Biochem. Behav. 2013, 104, 144–153. [Google Scholar] [CrossRef]
- Tamiz, A.P.; Cai, S.X.; Zhou, Z.L.; Yuen, P.W.; Schelkun, R.M.; Whittemore, E.R.; Weber, E.; Woodward, R.M.; Keana, J.F.W. Structure-activity relationship of N-(phenylalkyl)cinnamides as novel NR2B subtype-selective NMDA receptor antagonists. J. Med. Chem. 1999, 42, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Doherty, E.M.; Fotsch, C.; Bo, Y.; Chakrabarti, P.P.; Chen, N.; Gavva, N.; Han, N.; Kelly, M.G.; Kincaid, J.; Klionsky, L.; et al. Discovery of potent, orally available vanilloid receptor-1 antagonists. Structure-activity relationship of N-aryl cinnamides. J. Med. Chem. 2005, 48, 71–90. [Google Scholar] [CrossRef]
- Newland, C.F.; Cull-Candy, S.G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J. Physiol. 1992, 447, 191–213. [Google Scholar] [CrossRef]
- Snodgrass, S.R. GABA and epilepsy: Their complex relationship and the evolution of our understanding. J. Child Neurol. 1992, 7, 77–86. [Google Scholar] [CrossRef]
- Squires, R.F.; Saederup, E.; Crawley, J.N.; Skolnick, P.; Paul, S.M. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984, 35, 1439–1444. [Google Scholar] [CrossRef]
- Papp, A.; Fehér, O.; Erdélyi, L. The ionic mechanism of the pentylenetetrazol convulsions. Acta Biol. Hung. 1987, 38, 349–361. [Google Scholar] [PubMed]
- Słoczyńska, K.; Gunia-Krzyzak, A.; Koczurkiewicz, P.; Wójcik-Pszczoła, K.; Żelaszczyk, D.; Popiół, J.; Pȩkala, E. Metabolic stability and its role in the discovery of new chemical entities. Acta Pharm. 2019, 69, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol. Scand. 2008, 118, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gunia, A.; Waszkielewicz, A.M.; Marona, H. New Alkanolamide Derivatives of Cinnamic Acid and Use of Alkanolamide Derivatives of Cinnamic Acid for Preparation of Drugs. European Patent Claim No. PCT/PL2013/000139, 15 May 2014. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Stables, J.P.; Kupferberg, H.J. The NIH anticonvulsant drug development (ADD) program: Preclinical anticonvulsant screening project. In Molecular and Cellular Targets for Antiepileptic Drugs; Avanzini, G., Tanganelli, P., Avoli, M., Eds.; John Libbey&Comp. Ltd.: London, UK, 1997; pp. 191–198. [Google Scholar]
- White, H.S.; Woodhead, K.S.; Wilcox, J.P.; Stables, J.P.; Kupferberg, H.J.; Wolf, H.H. Discovery and preclinical development of antiepileptic drugs. In Antiepileptic Drugs Fifth Edition; Levy, R.H., Mattson, R.H., Meldrum, B.S., Perucca, E., Eds.; Lippincott Williams&Wilkins: Philadelphia, PA, USA, 2002; pp. 36–48. ISBN 9788578110796. [Google Scholar]
- PANAchE. Available online: https://panache.ninds.nih.gov/ (accessed on 22 April 2020).
- Frings, H.; Frings, M. Development of strains of albino mice with predictable susceptibilities to audiogenic seizures. Science (80-) 1953, 117, 283–284. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Matagne, A.; Klitgaard, H. Validation of corneally kindled mice: A sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998, 31, 59–71. [Google Scholar] [CrossRef]
- Turski, W.A. Pilocarpine-induced seizures in rodents - 17 years on. Pol. J. Pharmacol. 2000, 52, 63–65. [Google Scholar]
- White, H.S. Animal models of epileptogenesis. Neurology 2002, 59, S7–S14. [Google Scholar] [CrossRef] [PubMed]
- Tjolsen, A.; Berge, O.; Hunskaar, S.; Rosland, J.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Marona, H.; Gunia, A.; Słoczyska, K.; Rapacz, A.; Filipek, B.; Cegła, M.; Opoka, W. Preliminary evaluation of anticonvulsant activity and neurotoxicity of some 1,4-substituted piperazine derivatives. Acta Pol. Pharm. Drug Res. 2009, 66. [Google Scholar]
- Słoczyńska, K.; Pańczyk, K.; Waszkielewicz, A.M.; Marona, H.; Pękala, E. In vitro mutagenic, antimutagenic, and antioxidant activities evaluation and biotransformation of some bioactive 4-substituted 1-(2-methoxyphenyl)piperazine derivatives. J. Biochem. Mol. Toxicol. 2016, 30, 593–601. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development Test Guideline No. 471: Bacterial Reverse Mutation Test. 1997. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948418.pdf (accessed on 18 June 2020).
- Flückiger-Isler, S.; Baumeister, M.; Braun, K.; Gervais, V.; Hasler-Nguyen, N.; Reimann, R.; Van Gompel, J.; Wunderlich, H.G.; Engelhardt, G. Assessment of the performance of the Ames IITM assay: A collaborative study with 19 coded compounds. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 2004, 558, 181–197. [Google Scholar] [CrossRef]
- Flückiger-Isler, S.; Kamber, M. Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 2012, 747, 36–45. [Google Scholar] [CrossRef]
- XENOMETRIX. Available online: https://www.xenometrix.ch (accessed on 21 April 2020).
- Umbuzeiro, G.D.A.; Rech, C.M.; Correia, S.; Bergamasco, A.M.; Cardenette, G.H.L.; Flückiger-Isler, S.; Kamber, M. Comparison of the Salmonella/microsome microsuspension assay with the new microplate fluctuation protocol for testing the mutagenicity of environmental samples. Environ. Mol. Mutagen. 2010, 51, 31–38. [Google Scholar] [CrossRef]
- Reifferscheid, G.; Maes, H.M.; Allner, B.; Badurova, J.; Belkin, S.; Bluhm, K.; Brauer, F.; Bressling, J.; Domeneghetti, S.; Elad, T.; et al. International round-robin study on the Ames fluctuation test. Environ. Mol. Mutagen. 2012, 53, 185–197. [Google Scholar] [CrossRef]
- Smith, K.E.C.; Heringa, M.B.; Uytewaal, M.; Mayer, P. The dosing determines mutagenicity of hydrophobic compounds in the Ames II assay with metabolic transformation: Passive dosing versus solvent spiking. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 750, 12–18. [Google Scholar] [CrossRef]

| Mice, i.p. | Mice, p.o. | |||||
|---|---|---|---|---|---|---|
| Test | KM-568 (mg/kg) | Results a | ED50/TD50 (mg/kg) b | KM-568 (mg/kg) | Results a | ED50/TD50 (mg/kg) b |
| MES | 25 | 0/8 | 44.46 (42.50–47.93) | 50 | 0/8 | 86.6 (74.48–102.11) |
| 38 | 1/8 | 60 | 2/8 | |||
| 41 | 0/8 | 70 | 2/8 | |||
| 42 | 2/8 | 90 | 3/8 | |||
| 44 | 5/8 | 120 | 7/8 | |||
| 50 | 7/8 | 150 | 8/8 | |||
| scMET | 70 | 2/8 | 104.29 (74.63–130.51) | 50 | 1/8 | 107.27 (68.07–148.03) |
| 100 | 3/8 | 100 | 4/8 | |||
| 138 | 5/8 | 150 | 4/8 | |||
| 170 | 8/8 | 200 | 8/8 | |||
| 6-Hz (32 mA) | 50 | 1/8 | 71.55 (57.99–81.87) | |||
| 75 | 3/8 | |||||
| 87 | 7/8 | |||||
| 110 | 8/8 | |||||
| 6-Hz (44 mA) | 50 | 0/8 | 114.4 (81.75–138.57) | |||
| 100 | 3/8 | |||||
| 150 | 6/8 | |||||
| 200 | 8/8 | |||||
| Bicuculine | 130 | 0/8 | >130 | |||
| Picrotoxin | 70 | 1/8 | 94.11 (73.85–113.62) | |||
| 100 | 5/8 | |||||
| 130 | 7/8 | |||||
| TOX | 100 | 0/8 | 148.47 (140.08–158.84) | 200 | 0/8 | 344.14 (286.55–400.89) |
| 125 | 0/8 | 300 | 3/8 | |||
| 138 | 2/8 | 350 | 5/8 | |||
| 150 | 5/8 | 400 | 5/8 | |||
| 170 | 7/8 | 500 | 7/8 | |||
| 200 | 8/8 | |||||
| Rats, i.p. | Rats, p.o. | |||||
|---|---|---|---|---|---|---|
| Test | KM-568 (mg/kg) | Results a | ED50 or TD50 (mg/kg) b | KM-568 (mg/kg) | Results a | ED50 or TD50 (mg/kg) b |
| MES | 15 | 1/8 | 27.58 (18.84–41.74) | 20.0 | 1/8 | 30.81 (24.16–37.13) |
| 22 | 3/8 | 30.0 | 3/8 | |||
| 30 | 5/8 | 35.0 | 5/8 | |||
| 60 | 7/8 | 40.0 | 7/8 | |||
| scMET | 30 | 1/8 | 41.72 (33.54–49.37) | 62.5 | >250 | |
| 45 | 4/8 | 125.0 | ||||
| 60 | 8/8 | 250.0 | ||||
| 95 | 8/8 | |||||
| TOX | 60 | 0/8 | 95.21 (79.8–110.42) | 125.0 | 0/2 | >500 |
| 80 | 0/8 | 250.0 | 0/2 | |||
| 100 | 2/8 | 500.0 | 0/2 | |||
| 120 | 5/8 | |||||
| 140 | 7/8 | |||||
| KM-568 (mg/kg) | Results a | ED50 (mg/kg) |
|---|---|---|
| 10.0 | 1/8 | 13.21 (11.22–15.11) |
| 12.5 | 3/8 | |
| 15.0 | 6/8 | |
| 17.5 | 7/8 |
| KM-568 (mg/kg) | Time to twitch (sec) | Twitch * (mg/kg) | Time to clonus (sec) | Clonus * (mg/kg) |
|---|---|---|---|---|
| 0 | 26.1 ± 1.7 | 26.8 ± 1.5 | 28.7 ± 1.8 | 29.6 ± 1.6 |
| 44 | 35.8 ± 1.5 | 37.8 ± 1.4 | 48.2 ± 3.5 | 50.8 ± 3.5 |
| 148 | 57.9 ± 1.6 | 61.3 ± 2.2 | 75.9 ± 2.9 | 80.2 ± 3.2 |
| Recording Period | HPD * Counts | Mean HPD Counts | SEM | Effect (% of Baseline) |
|---|---|---|---|---|
| Baseline | 16; 18; 21; 14 | 17.3 | 1.49 | - |
| 20–40 min | 14; 8; 17; 12 | 12.8 | 1.89 | 73.9 |
| KM-568 (mg/kg) | Results a | Individual Seizure Score b | Average Seizure Score | ED50 (mg/kg) |
|---|---|---|---|---|
| 57 | 1/8 | 4,5,5,0,4,5,4,5 | 4.0 | 79.17 (60.34–98.26) |
| 84 | 5/8 | 4,5,3,3,3,3,3,5 | 3.625 | |
| 115 | 7/8 | 0,0,0,4,0,0,0,1 | 0.625 |
| KM-568 (mg/kg) | Time (min) | Results a | Seizure Score b ± SEM | Seizure Duration (sec) ± SEM | ED50 (mg/kg) |
|---|---|---|---|---|---|
| 23 | 0 | 5.0 ± 0.0 | 66.83 ± 10.77 | 24.21 (19.4–26.54) | |
| 15 | 2/6 | 3.67 ± 0.84 | 55.67 ± 6.44 | ||
| 45 | 5.0 ± 0.0 | 65.83 ± 14.61 | |||
| 75 | 5.0 ± 0.0 | 56.67 ± 6.96 | |||
| 100 | 5.0 ± 0.0 | 57.83 ± 9.27 | |||
| 135 | 5.0 ± 0.0 | 55.17 ± 9.48 | |||
| 27 | 0 | 5.0 ± 0.0 | 56.20 ± 7.70 | ||
| 15 | 4/5 | 1.8 ± 0.97 * | 50.4 ± 3.08 | ||
| 45 | 4.2 ± 0.80 | 80.0 ± 9.42 * | |||
| 75 | 3.4 ± 0.98 | 70.6 ± 27.57 | |||
| 100 | 5.0 ± 0.0 | 62.5 ± 10.71 | |||
| 135 | 5.0 ± 0.0 | 95.0 ± 17.78 * |
| KM-568 (mg/kg) | Time of Test (h) | Seizure Score ± SEM | Seizure Duration (sec) ± SD | Results a | ED50 (mg/kg) |
|---|---|---|---|---|---|
| control | 0.0 | 5.0 ± 0.0 | 156.43 ± 7.31 | 0/7 | 58.69 (38.09–120.15) |
| 20 | 0.25 | 5.0 ± 0.0 | 140 ± 17.55 | 0/7 | |
| control | 0.0 | 5.0 ± 0.0 | 118.86 ± 13.87 | 0/7 | |
| 40 | 0.25 | 3.86 ± 0.74 | 94.71 ± 8.72 | 2/7 | |
| control | 0.0 | 5.0 ± 0.0 | 86.71 ± 9.68 | 0/7 | |
| 80 | 0.25 | 2.86 ± 0.63 * | 56.57 ± 12.34 | 5/7 |
| Time (h) | KM-568 (mg/kg) | Results a | ED50 (mg/kg) | ED97 (mg/kg) |
|---|---|---|---|---|
| 0 | 200 | 8/8 | ||
| 0.5 | 200 | 1/8 | 279.45 (212.61–344.62) | 498.2 (378.3–1526.9) |
| 300 | 5/8 | |||
| 400 | 7/8 |
| Phase | AUC a | ||||
|---|---|---|---|---|---|
| Methylcellulose | KM-568 | % Control | SEM | p Value b | |
| Acute | 241.17 | 184.18 | 76.37 | 13.03 | >0.05 |
| Inflammatory | 819.31 | 556.81 | 67.96 | 13.27 | >0.05 |
| Compound | Concentration (mM) | Salmonella typhimurium | |||
|---|---|---|---|---|---|
| TA98 | TA100 | TA1535 | TA1537 | ||
| KM-568 | 0.1 0.2 0.5 | 0.1 0.9 0.7 | 0.8 0.7 1.0 | 0.7 1.3 1.1 | 1.0 0.7 0.7 |
| PC b | 6.4 | 3.4 | 14.7 | 48.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunia-Krzyżak, A.; Żesławska, E.; Słoczyńska, K.; Żelaszczyk, D.; Sowa, A.; Koczurkiewicz-Adamczyk, P.; Popiół, J.; Nitek, W.; Pękala, E.; Marona, H. S(+)-(2E)-N-(2-Hydroxypropyl)-3-Phenylprop-2-Enamide (KM-568): A Novel Cinnamamide Derivative with Anticonvulsant Activity in Animal Models of Seizures and Epilepsy. Int. J. Mol. Sci. 2020, 21, 4372. https://doi.org/10.3390/ijms21124372
Gunia-Krzyżak A, Żesławska E, Słoczyńska K, Żelaszczyk D, Sowa A, Koczurkiewicz-Adamczyk P, Popiół J, Nitek W, Pękala E, Marona H. S(+)-(2E)-N-(2-Hydroxypropyl)-3-Phenylprop-2-Enamide (KM-568): A Novel Cinnamamide Derivative with Anticonvulsant Activity in Animal Models of Seizures and Epilepsy. International Journal of Molecular Sciences. 2020; 21(12):4372. https://doi.org/10.3390/ijms21124372
Chicago/Turabian StyleGunia-Krzyżak, Agnieszka, Ewa Żesławska, Karolina Słoczyńska, Dorota Żelaszczyk, Aleksandra Sowa, Paulina Koczurkiewicz-Adamczyk, Justyna Popiół, Wojciech Nitek, Elżbieta Pękala, and Henryk Marona. 2020. "S(+)-(2E)-N-(2-Hydroxypropyl)-3-Phenylprop-2-Enamide (KM-568): A Novel Cinnamamide Derivative with Anticonvulsant Activity in Animal Models of Seizures and Epilepsy" International Journal of Molecular Sciences 21, no. 12: 4372. https://doi.org/10.3390/ijms21124372
APA StyleGunia-Krzyżak, A., Żesławska, E., Słoczyńska, K., Żelaszczyk, D., Sowa, A., Koczurkiewicz-Adamczyk, P., Popiół, J., Nitek, W., Pękala, E., & Marona, H. (2020). S(+)-(2E)-N-(2-Hydroxypropyl)-3-Phenylprop-2-Enamide (KM-568): A Novel Cinnamamide Derivative with Anticonvulsant Activity in Animal Models of Seizures and Epilepsy. International Journal of Molecular Sciences, 21(12), 4372. https://doi.org/10.3390/ijms21124372





