UV-Pre-Treated and Protein-Adsorbed Titanium Implants Exhibit Enhanced Osteoconductivity
Abstract
1. Introduction
2. Results
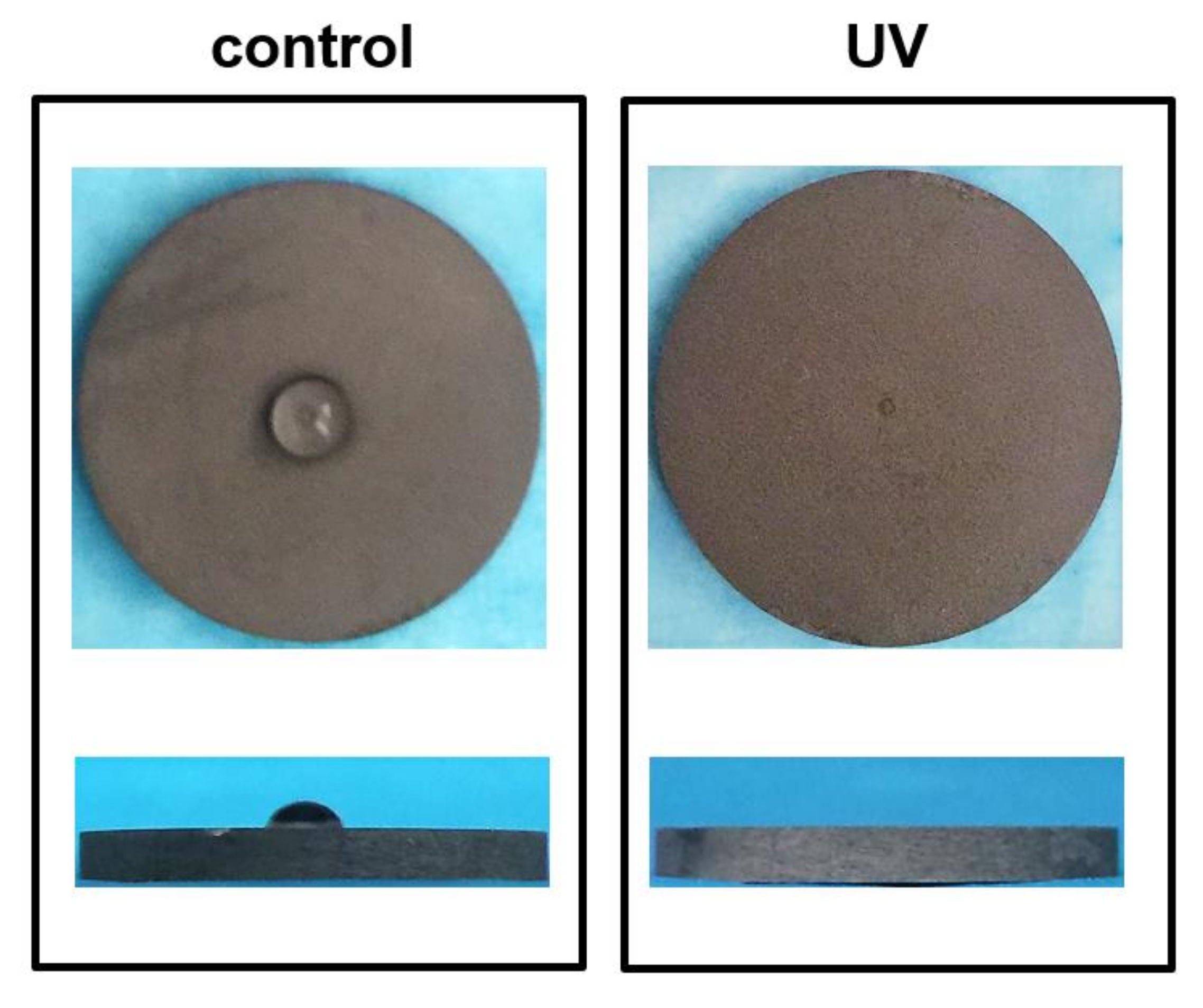
2.1. Characteristics of Titanium Disks Surface with or without UV Treatment
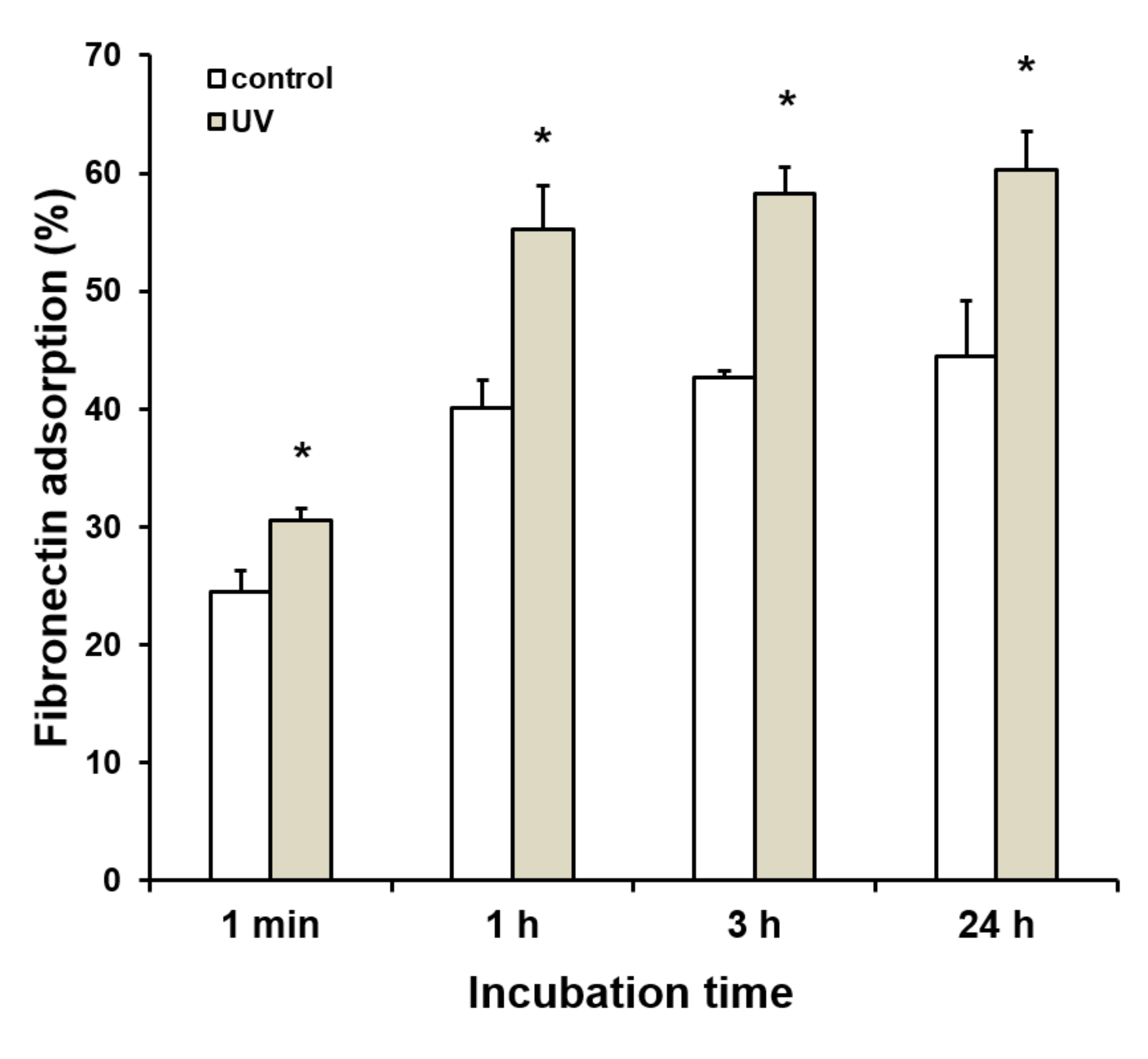
2.2. Enhanced Protein Adsorption on UV-Treated Titanium
2.3. Enhanced Initial Cell Attachment to UV-Treated and Fibronectin-Adsorbed Titanium
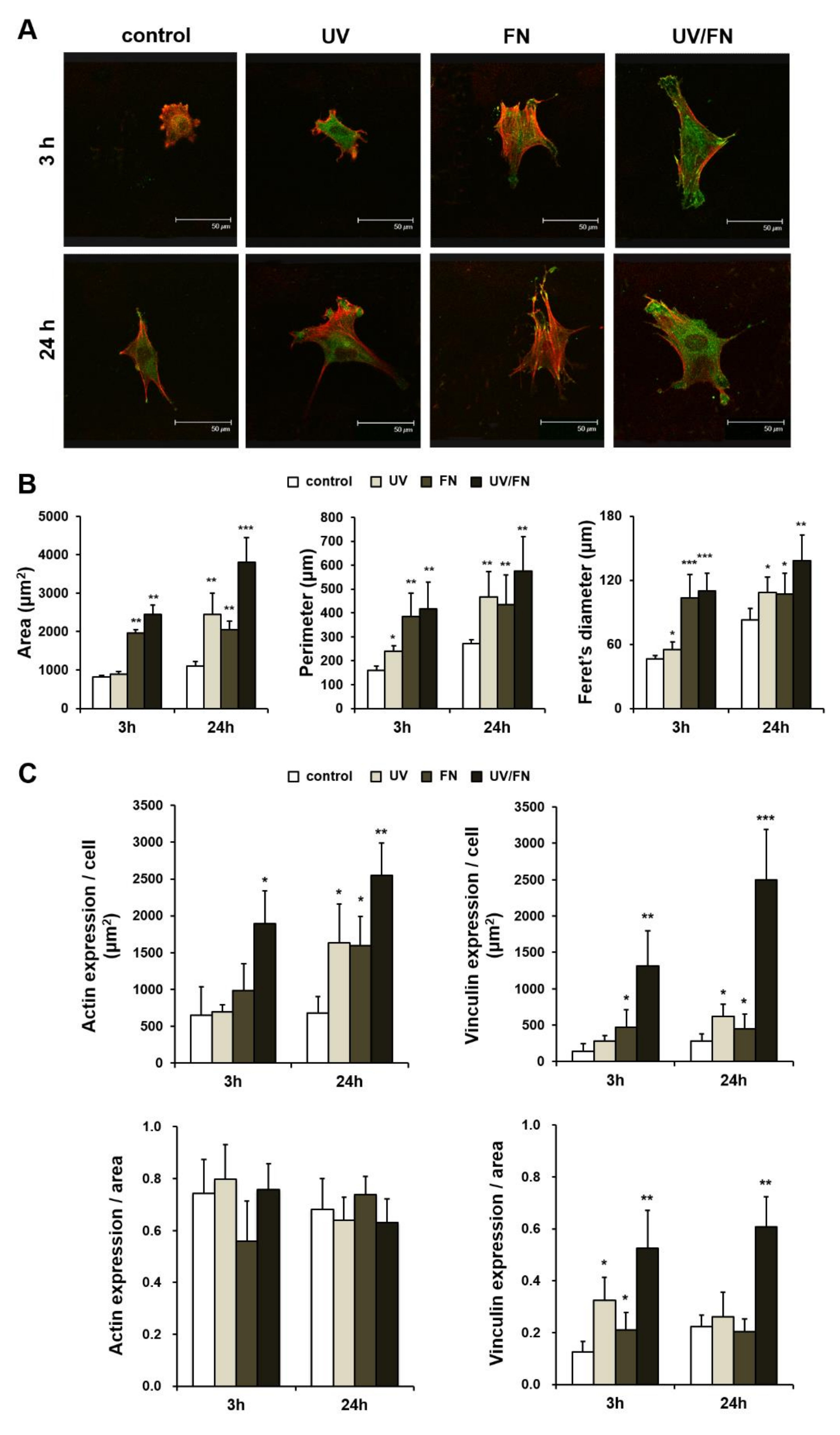
2.4. Enhanced Cell Spreading on UV-Treated and Fibronectin-Adsorbed Titanium Surface
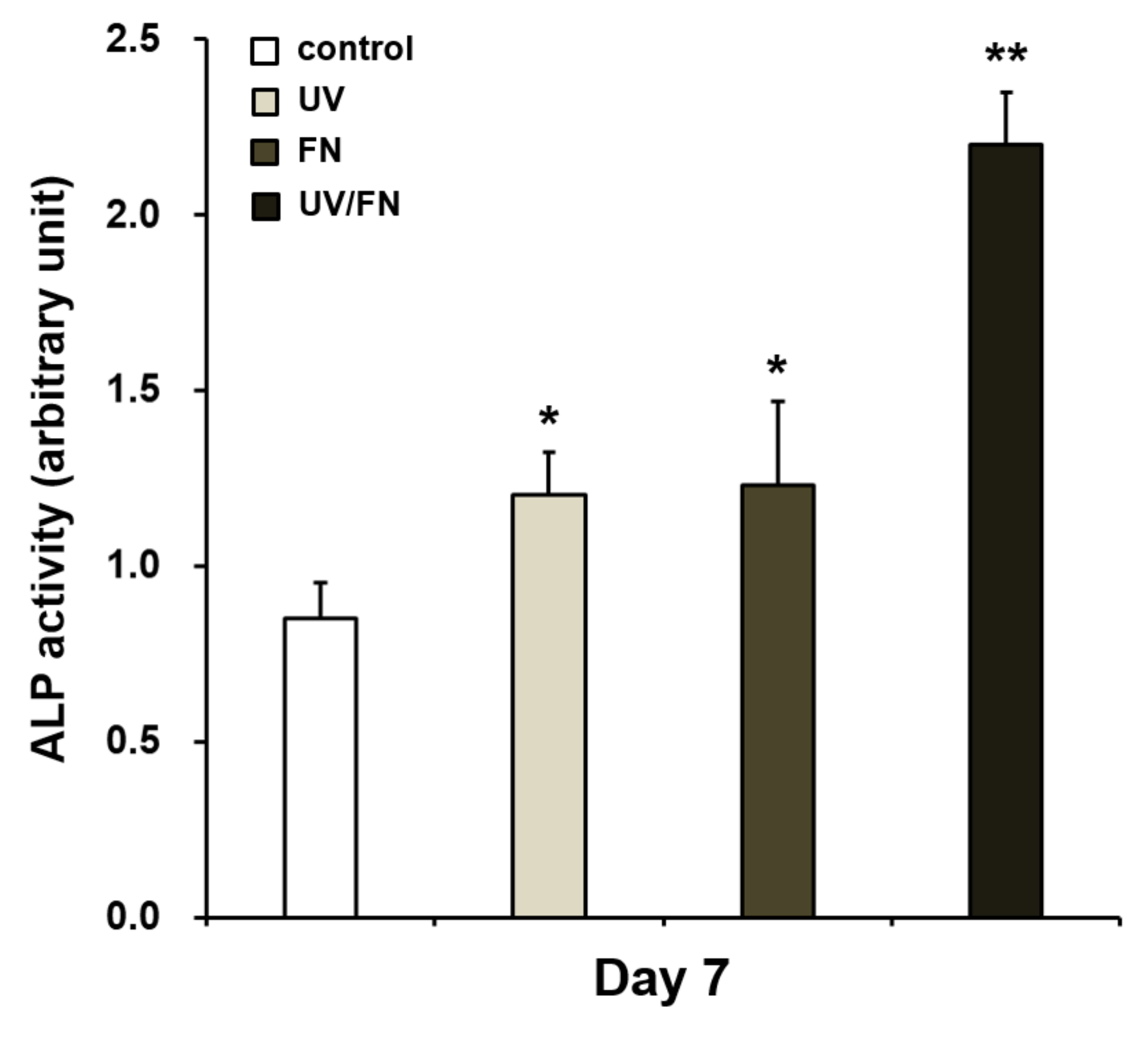
2.5. Enhanced Osteoblastic Phenotype on UV-Treated and Fibronectin-Adsorbed Titanium
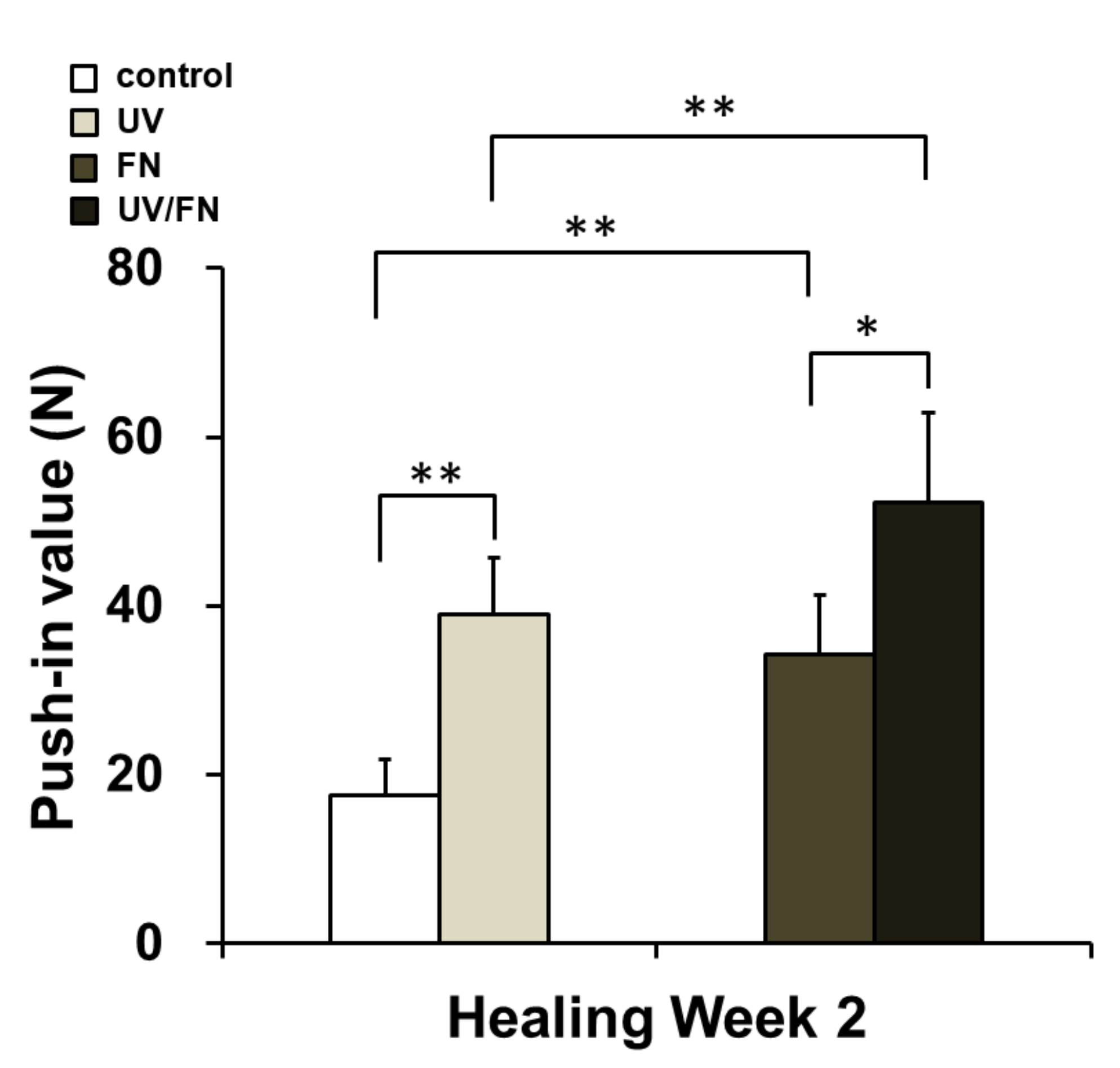
2.6. Combination of UV Treatment and FN Adsorption Enhanced Mechanical Anchorage
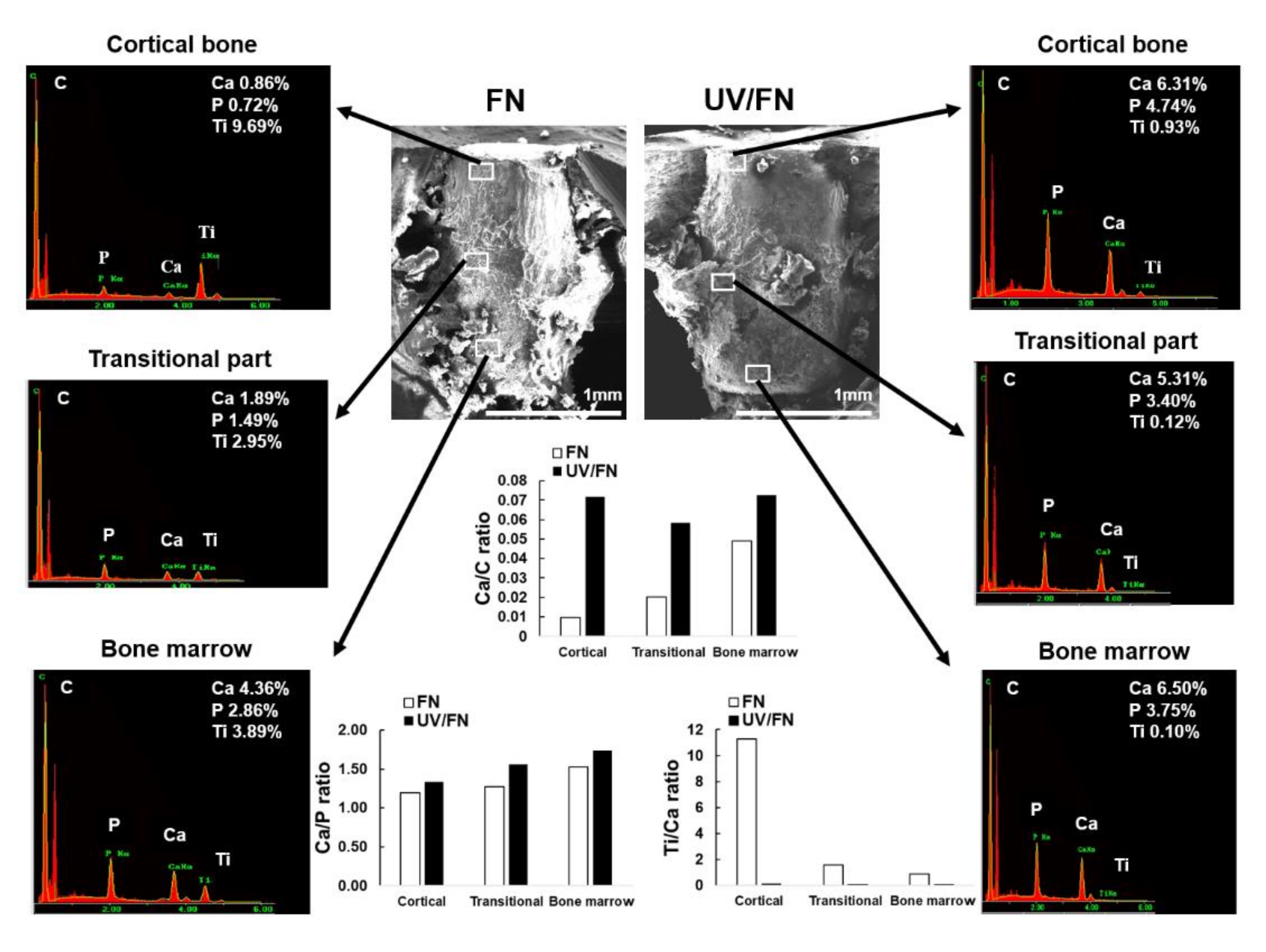
2.7. Tissue Morphology and Chemistry around Implants
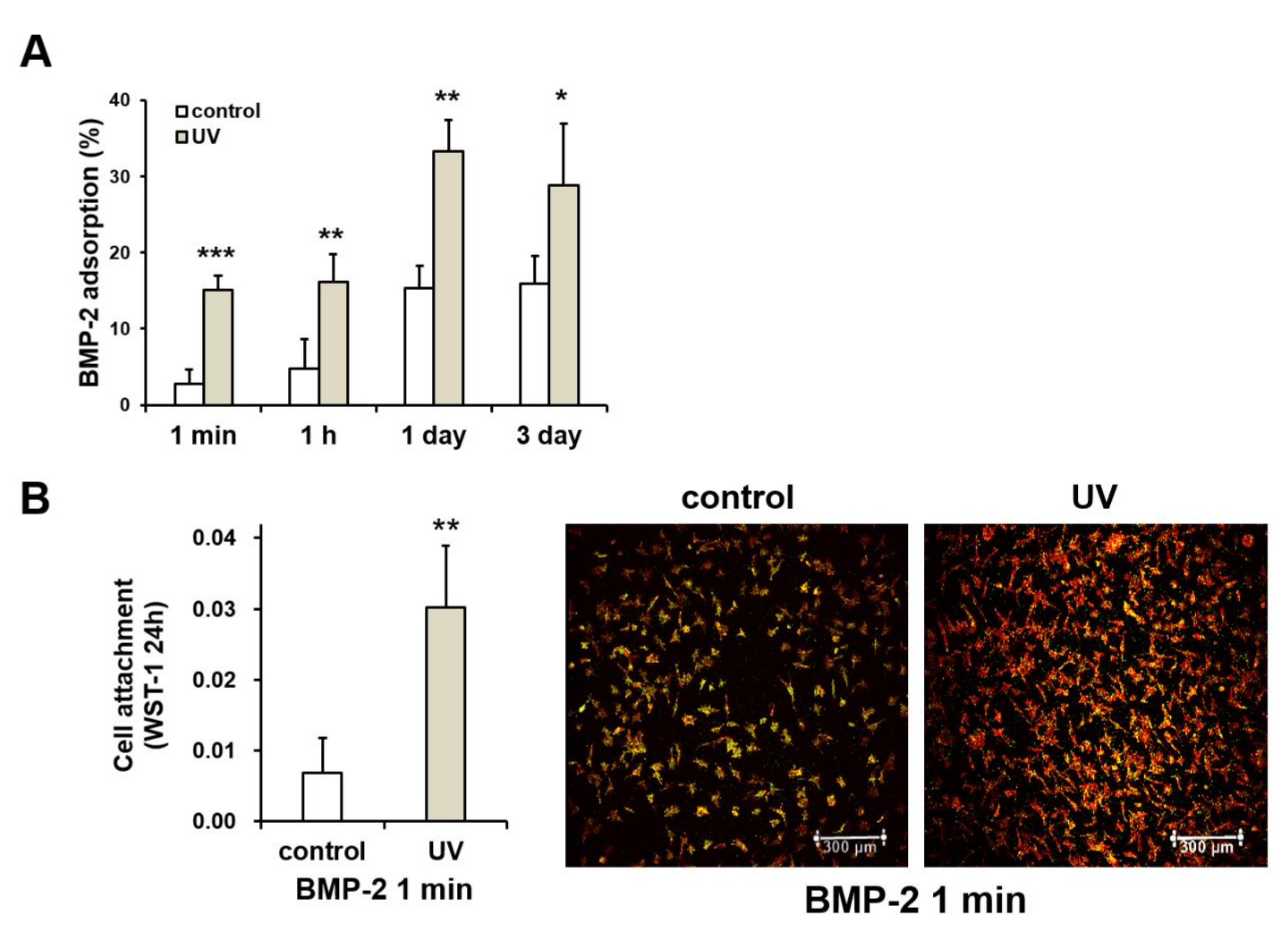
2.8. UV-Enhanced Bone Morphogenetic Protein-2 (BMP-2) Adsorption to Titanium
3. Discussion
4. Materials and Methods
4.1. Titanium Surface Modifications
4.2. Bone Marrow-Derived Stromal Stem Cells Culture
4.3. Protein Adsorption Assay
4.4. Measurement of Initial Cell Attachment to the Titanium Surface
4.5. Analysis of Morphology and Morphometry of Osteoblastic Cells
4.6. Alkaline Phosphatase (ALP) Activity Assay
4.7. Implant Surgery
4.8. Biomechanical Implant Push-In Test
4.9. Morphological and Chemical Profiling of Tissue around Titanium Implants
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| BMP-2 | bone morphogenetic protein-2 |
| CLSM | confocal laser scanning microscopy |
| FN | fibronectin |
| ddH2O | double-distilled H2O |
| EDS | energy-dispersive X-ray spectroscopy |
| RGD | arginine-glycine-aspartate |
| SEM | scanning electron microscopy |
| UV | ultraviolet |
| WST | water-soluble tetrazolium salts |
| XPS | x-ray photoelectron spectroscopy |
References
- Koh, J.; Berger, A.; Benhaim, P. An Overview of Internal Fixation Implant Metallurgy and Galvanic Corrosion Effects. J. Hand Surg. Am. 2015, 40, 1703–1710. [Google Scholar] [CrossRef]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic osseointegration: Implantology and future directions. J. Orthop. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Lu, T.; Wang, Y.; Liang, H.; Gao, Z.; He, X. Anterior Cervical Corpectomy and Fusion and Anterior Cervical Discectomy and Fusion Using Titanium Mesh Cages for Treatment of Degenerative Cervical Pathologies: A Literature Review. Med. Sci. Monit. 2018, 24, 6398–6404. [Google Scholar] [CrossRef] [PubMed]
- Nienkemper, M.; Willmann, J.H.; Drescher, D. Long-term stability behavior of paramedian palatal mini-implants: A repeated cross-sectional study. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 165–171. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Shah, S.A.; Razaq, S.I.A.; Hassan, S.A.; Kadir, M.R.A. Haider, A Arabinoxylan-co-AA/HAp/TiO2 nanocomposite scaffold a potential material for bone tissue engineering: An in vitro study. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Ishijima, M.; Ghassemi, A.; Soltanzadeh, P.; Tanaka, M.; Nakhaei, K.; Park, W.; Hirota, M.; Tsukimura, N.; Ogawa, T. Effect of UV Photofunctionalization on Osseointegration in Aged Rats. Implant Dent. 2016, 25, 744–750. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent degradation of the protein adsorption capacity of titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T. Ultraviolet photofunctionalization of titanium implants. Int. J. Oral Maxillofac. Implants 2014, 29, e95–e102. [Google Scholar] [CrossRef]
- Taniyama, T.; Saruta, J.; Mohammadzadeh Rezaei, N.; Nakhaei, K.; Ghassemi, A.; Hirota, M.; Okubo, T.; Ikeda, T.; Sugita, Y.; Hasegawa, M.; et al. UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model. Int. J. Mol. Sci. 2020, 21, 1235. [Google Scholar] [CrossRef] [PubMed]
- Puisys, A.; Schlee, M.; Linkevicius, T.; Petrakakis, P. Tjaden, A Photo-activated implants: A triple-blinded, split-mouth, randomized controlled clinical trial on the resistance to removal torque at various healing intervals. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef]
- Soltanzadeh, P.; Ghassemi, A.; Ishijima, M.; Tanaka, M.; Park, W.; Iwasaki, C.; Hirota, M.; Ogawa, T. Success rate and strength of osseointegration of immediately loaded UV-photofunctionalized implants in a rat model. J. Prosthet. Dent. 2017, 118, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Funato, A.; Ogawa, T. Photofunctionalized dental implants: A case series in compromised bone. Int. J. Oral Maxillofac. Implants 2013, 28, 1589–1601. [Google Scholar] [CrossRef]
- Funato, A.; Yamada, M.; Ogawa, T. Success rate, healing time, and implant stability of photofunctionalized dental implants. Int. J. Oral Maxillofac. Implants 2013, 28, 1261–1271. [Google Scholar] [CrossRef]
- Tsukimura, N.; Yamada, M.; Iwasa, F.; Minamikawa, H.; Att, W.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials 2011, 32, 4358–4368. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J. Biomed. Mater. Res. A 2014, 102, 3618–3630. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, C.; Hirota, M.; Tanaka, M.; Kitajima, H.; Tabuchi, M.; Ishijima, M.; Park, W.; Sugita, Y.; Miyazawa, K.; Goto, S.; et al. Tuning of Titanium Microfiber Scaffold with UV-Photofunctionalization for Enhanced Osteoblast Affinity and Function. Int. J. Mol. Sci. 2020, 21, 738. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon Deposition Attenuates Osteoblast Activity on Titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed titanium as a bioinert metal-a review. Quintessence Int. 2005, 36, 523–530. [Google Scholar] [CrossRef]
- Park, J.W.; Hanawa, T.; Chung, J.H. The relative effects of Ca and Mg ions on MSC osteogenesis in the surface modification of microrough Ti implants. Int. J. Nanomed. 2019, 14, 5697–5711. [Google Scholar] [CrossRef]
- Hoyos-Nogues, M.; Falgueras-Batlle, E.; Ginebra, M.P.; Manero, J.M.; Gil, J.; Mas-Moruno, C. A Dual Molecular Biointerface Combining RGD and KRSR Sequences Improves Osteoblastic Functions by Synergizing Integrin and Cell-Membrane Proteoglycan Binding. Int. J. Mol. Sci. 2019, 20, 1429. [Google Scholar] [CrossRef]
- Raines, A.L.; Berger, M.B.; Schwartz, Z.; Boyan, B.D. Osteoblasts grown on microroughened titanium surfaces regulate angiogenic growth factor production through specific integrin receptors. Acta Biomater. 2019, 97, 578–586. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Lemons, J.E.; Liu, J.; Raikar, G.N.; Weimer, J.J.; Vohra, Y. Cleaning and heat-treatment effects on unalloyed titanium implant surfaces. Int. J. Oral Maxillofac. Implants 2000, 15, 219–230. [Google Scholar]
- Cordeiro, J.M.; Pantaroto, H.N.; Paschoaleto, E.M.; Rangel, E.C.; Cruz, N.C.d.; Sukotjo, C.; Barão, V.A.R. Synthesis of biofunctional coating for a TiZr alloy: Surface, electrochemical, and biological characterizations. Appl. Surface. Sci. 2018, 452, 268–278. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lotz, E.M.; Schwartz, Z. (*) Roughness and Hydrophilicity as Osteogenic Biomimetic Surface Properties. Tissue Eng. Part. A 2017, 23, 1479–1489. [Google Scholar] [CrossRef]
- Haimov, H.; Yosupov, N.; Pinchasov, G.; Juodzbalys, G. Bone Morphogenetic Protein Coating on Titanium Implant Surface: A Systematic Review. J. Oral Maxillofac. Res. 2017, 8, e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, C.; Moon, B.S.; Kim, H.E.; Jang, T.S. Enhancement of osseointegration by direct coating of rhBMP-2 on target-ion induced plasma sputtering treated SLA surface for dental application. J. Biomater. Appl. 2017, 31, 807–818. [Google Scholar] [CrossRef]
- Huang, S.; Ulloa, A.; Nauman, E.; Stanciu, L. Collagen Coating Effects on Fe-Mn Bioresorbable Alloys. J. Orthop. Res. 2020, 38, 523–535. [Google Scholar] [CrossRef]
- Ishijima, M.; Hirota, M.; Park, W.; Honda, M.J.; Tsukimura, N.; Isokawa, K.; Ishigami, T.; Ogawa, T. Osteogenic cell sheets reinforced with photofunctionalized micro-thin titanium. J. Biomater. Appl. 2015, 29, 1372–1384. [Google Scholar] [CrossRef]
- Jin, C.; Ren, L.F.; Ding, H.Z.; Shi, G.S.; Lin, H.S.; Zhang, F. Enhanced attachment, proliferation, and differentiation of human gingival fibroblasts on titanium surface modified with biomolecules. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2167–2177. [Google Scholar] [CrossRef]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, N.M.; Hasegawa, M.; Ishijima, M.; Nakhaei, K.; Okubo, T.; Taniyama, T.; Ghassemi, A.; Tahsili, T.; Park, W.; Hirota, M.; et al. Biological and osseointegration capabilities of hierarchically (meso-/micro-/nano-scale) roughened zirconia. Int. J. Nanomed. 2018, 13, 3381–3395. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Okubo, T.; Saita, M.; Ishijima, M.; Torii, Y.; Tanaka, M.; Iwasaki, C.; Sekiya, T.; Tabuchi, M.; Mohammadzadeh Rezaei, N.; et al. Novel Osteogenic Behaviors around Hydrophilic and Radical-Free 4-META/MMA-TBB: Implications of an Osseointegrating Bone Cement. Int. J. Mol. Sci. 2020, 21, 2405. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, J.; Ueno, T.; Ogawa, T.; Egusa, H. Cytoprotective Preconditioning of Osteoblast-Like Cells with N-Acetyl-L-Cysteine for Bone Regeneration in Cell Therapy. Int. J. Mol. Sci. 2019, 20, 5199. [Google Scholar] [CrossRef]
- Ikeda, T.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Yamada, M.; Sugita, Y.; Ogawa, T. Effect of photofunctionalization on fluoride-treated nanofeatured titanium. J. Biomater. Appl. 2014, 28, 1200–1212. [Google Scholar] [CrossRef]
- Park, W.; Ishijima, M.; Hirota, M.; Soltanzadeh, P.; Ogawa, T. Engineering bone-implant integration with photofunctionalized titanium microfibers. J. Biomater. Appl. 2016, 30, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Saruta, J.; Sato, N.; Ishijima, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20, 4027. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugita, Y.; Saruta, J.; Taniyama, T.; Kitajima, H.; Hirota, M.; Ikeda, T.; Ogawa, T. UV-Pre-Treated and Protein-Adsorbed Titanium Implants Exhibit Enhanced Osteoconductivity. Int. J. Mol. Sci. 2020, 21, 4194. https://doi.org/10.3390/ijms21124194
Sugita Y, Saruta J, Taniyama T, Kitajima H, Hirota M, Ikeda T, Ogawa T. UV-Pre-Treated and Protein-Adsorbed Titanium Implants Exhibit Enhanced Osteoconductivity. International Journal of Molecular Sciences. 2020; 21(12):4194. https://doi.org/10.3390/ijms21124194
Chicago/Turabian StyleSugita, Yoshihiko, Juri Saruta, Takashi Taniyama, Hiroaki Kitajima, Makoto Hirota, Takayuki Ikeda, and Takahiro Ogawa. 2020. "UV-Pre-Treated and Protein-Adsorbed Titanium Implants Exhibit Enhanced Osteoconductivity" International Journal of Molecular Sciences 21, no. 12: 4194. https://doi.org/10.3390/ijms21124194
APA StyleSugita, Y., Saruta, J., Taniyama, T., Kitajima, H., Hirota, M., Ikeda, T., & Ogawa, T. (2020). UV-Pre-Treated and Protein-Adsorbed Titanium Implants Exhibit Enhanced Osteoconductivity. International Journal of Molecular Sciences, 21(12), 4194. https://doi.org/10.3390/ijms21124194





