Supernumerary B Chromosomes and Plant Genome Changes: A Snapshot of Wild Populations of Aegilops speltoides Tausch (Poaceae, Triticeae)
Abstract
1. Introduction
2. Results
2.1. Rearrangements of B Chromosomes and Ectopic Associations with Standard A Chromosomes During Mitosis and Meiosis
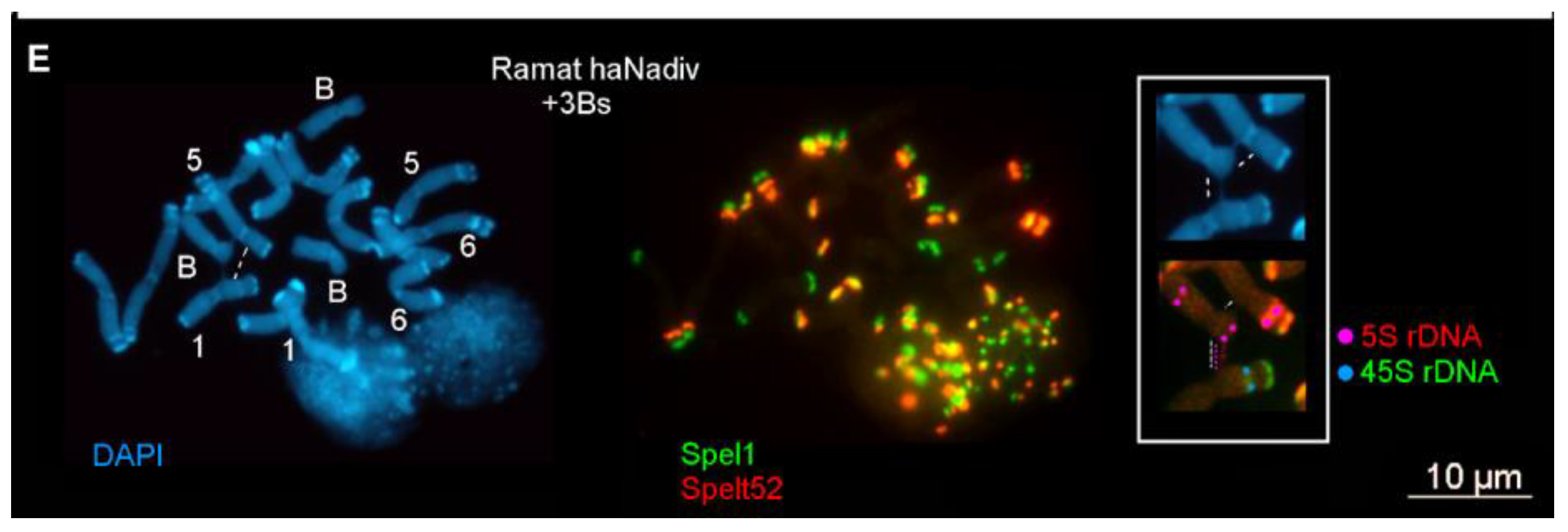
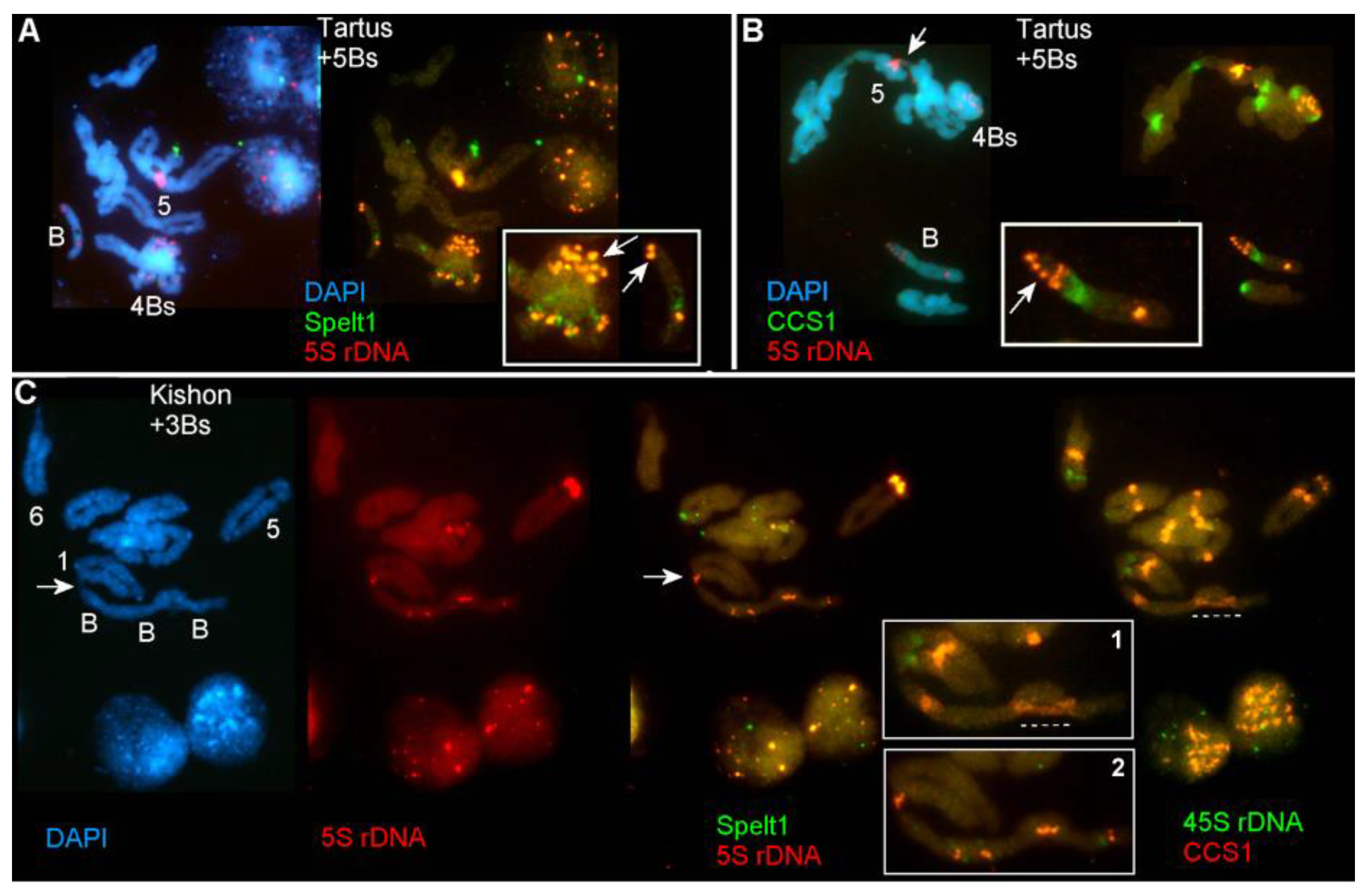
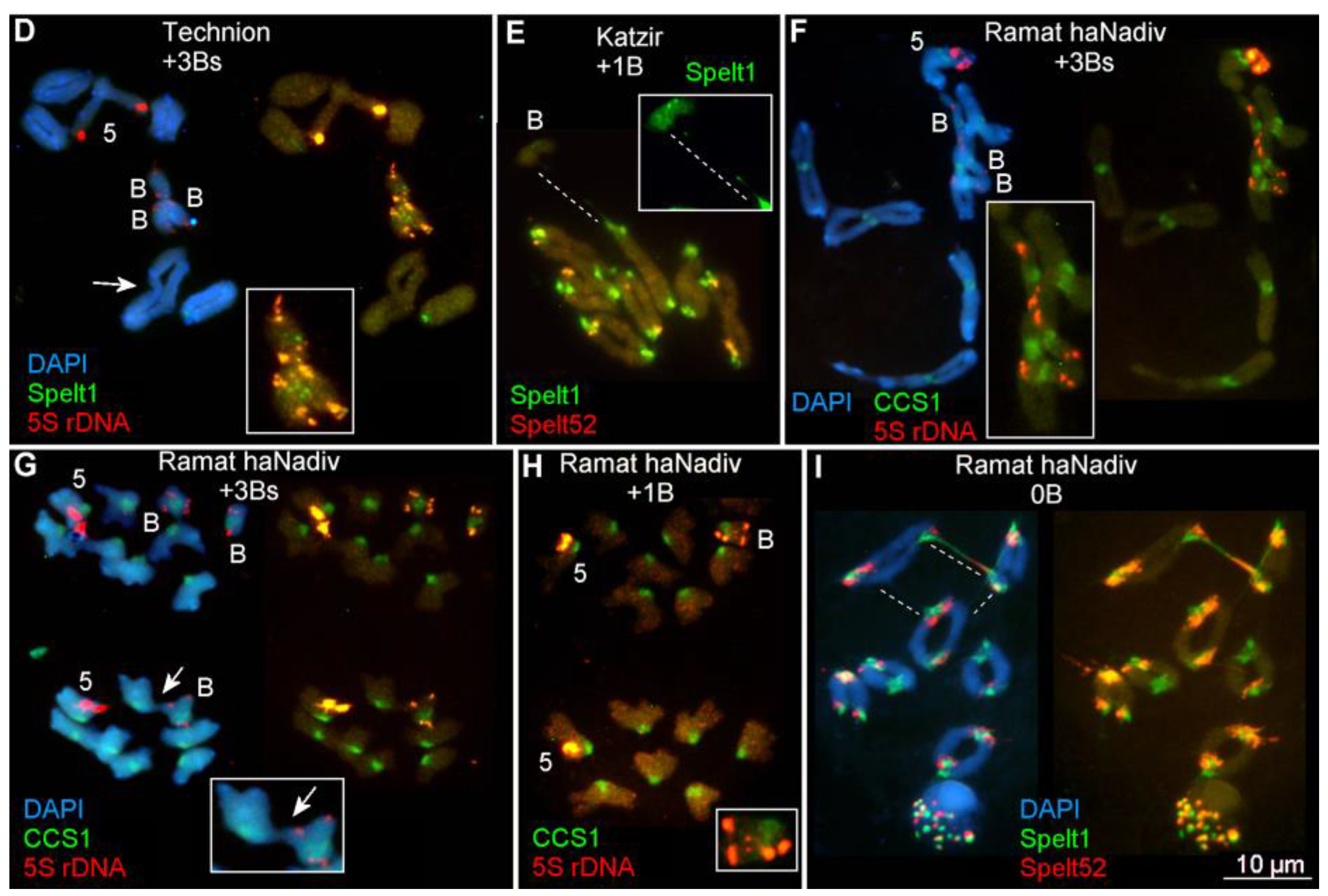
2.1.1. Ectopic B-A Chromosome Associations and Rearrangements of B Chromosomes in Somatic Cells
2.1.2. Ectopic B-A Chromosome Associations and Rearrangements of B Chromosomes in Meiotic Cells
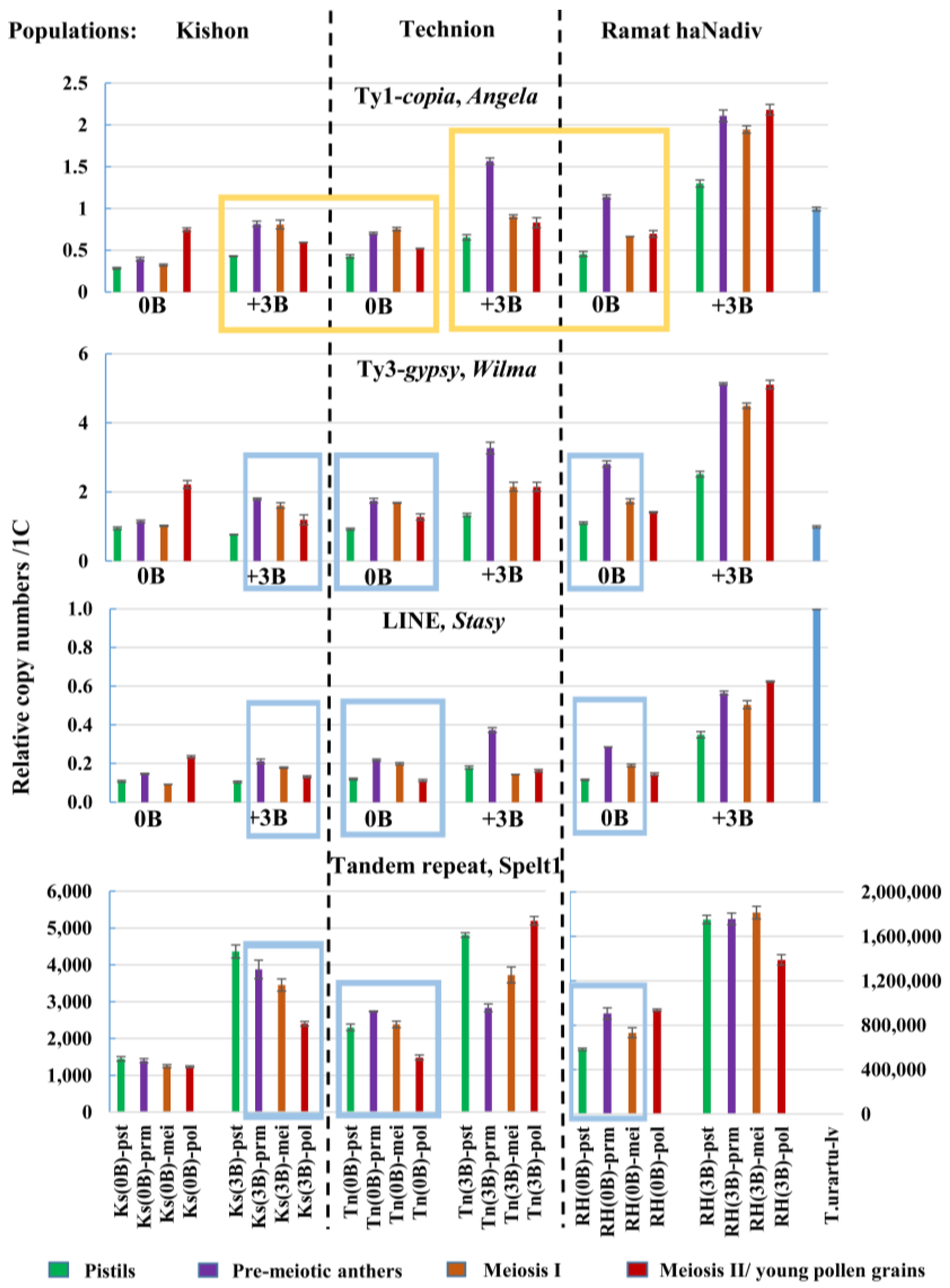
2.2. Variability in the Copy Numbers of the Angela, Wilma, and Stasy Retrotransposons and Spelt1 Tandem Repeat in the Spike Tissues of Individual Plants
2.2.1. Differences Between Individual Genotypes in Abundances of the Angela, Wilma, and Stasy Retrotransposons and Spelt1 Tandem Repeat
2.2.2. Differences in the Copy Numbers of the Angela, Wilma, and Stasy Retrotransposons and Spelt1 Tandem Repeat between Somatic and Generative Tissues of the Same Spike
2.2.3. Inter-Individual and Intra-Organismal Similarities in the Copy Number Dynamics of the Angela, Wilma, and Stasy retrotransposons and Spelt1 Tandem Repeat
3. Discussion
3.1. Rearrangements and Ectopic Recombination in Both Mitosis and Meiosis as Inherent Characteristics of B Chromosomes
3.2. Origin of B Chromosomes in Ae. speltoides
3.3. Role of B Chromosomes in Stabilizing the Genome
4. Materials and Methods
4.1. Plant Material
4.2. Fluorescence In Situ Hybridization Experiments
4.3. DNA Isolation and Cytogenetic Screening
4.4. Real-Time Quantitative PCR, Retrotransposon Sequence Sources, and Primer Design
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | A chromosome |
| B | B chromosome |
| DAPI | 4′,6-diamidino-2-phenylindole |
| FISH | fluorescent in situ hybridization |
| qPCR | real-time quantitative PCR |
| TE | transposable element |
| TR | tandem repeat |
References
- Houben, A.; Jones, N.; Martins, C.; Trifonov, V.A. Evolution, Composition and Regulation of Supernumerary B Chromosomes. Genes 2019, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Martins, C. The Modern View of B Chromosomes Under the Impact of High Scale Omics Analyses. Cells 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.K.; Kour, J.; Kaul, S. Origin, Behaviour, and Transmission of B Chromosome with Special Reference to Plantago lagopus. Genes 2019, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, S.; Miller, D.E.; Eche, S.; Hawley, R.S. Origin, Composition, and Structure of the Supernumerary B Chromosome of Drosophila melanogaster. Genetics 2018, 210, 1197–1212. [Google Scholar] [CrossRef]
- Su, H.; Liu, Y.; Liu, Y.; Birchler, J.A.; Han, F. The Behavior of the Maize B Chromosome and Centromere. Genes 2018, 9, 476. [Google Scholar] [CrossRef]
- Coan, R.; Martins, C. Landscape of Transposable Elements Focusing on the B Chromosome of the Cichlid Fish Astatotilapia latifasciata. Genes 2018, 9, 269. [Google Scholar] [CrossRef]
- Houben, A. B Chromosomes—A Matter of Chromosome Drive. Front. Plant Sci. 2017, 8, 210. [Google Scholar] [CrossRef]
- Banaei-Moghaddam, A.M.; Schubert, V.; Kumke, K.; Weiβ, O.; Klemme, S.; Nagaki, K.; Macas, J.; González-Sánchez, M.; Heredia, V.; Gómez-Revilla, D.; et al. Nondisjunction in Favor of a Chromosome: The Mechanism of Rye B Chromosome Drive during Pollen Mitosis. Plant Cell 2012, 24, 4124–4134. [Google Scholar] [CrossRef]
- Martis, M.M.; Klemme, S.; Banaei-Moghaddam, A.M.; Blattner, F.R.; Macas, J.; Schmutzer, T.; Scholz, U.; Gundlach, H.; Wicker, T.; Šimková, H.; et al. Selfish supernumerary chromosome reveals its origin as a mosaic of host genome and organellar sequences. Proc. Natl. Acad. Sci. USA 2012, 109, 13343–13346. [Google Scholar] [CrossRef]
- Ruban, A.; Schmutzer, T.; Scholz, U.; Houben, A. How Next-Generation Sequencing Has Aided Our Understanding of the Sequence Composition and Origin of B Chromosomes. Genes 2017, 8, 294. [Google Scholar] [CrossRef]
- Kimber, G.; Feldman, M. Wild Wheat, an Introduction; Special Report; College of Agriculture, University of Missouri-Columbia: Columbia, MO, USA, 1987; Volume 353. [Google Scholar]
- Maestra, B.; Naranjo, T. Genome evolution in Triticeae. In Chromosomes Today; Birkhäuser: Basel, Switzerland, 2000; pp. 155–167. [Google Scholar]
- Zohary, D.; Imber, D. Genetic dimorphism in fruit types in Aegilops speltoides. Heredity 1963, 18, 223–231. [Google Scholar] [CrossRef]
- Belyayev, A.; Raskina, O. Chromosome evolution in marginal populations of Aegilops speltoides: Causes and consequences. Ann. Bot. 2013, 111, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, D.; Zohary, D. Behaviour and transmission of supernumerary chromosomes in Aegilops speltoides. Heredity 1972, 29, 329–339. [Google Scholar] [CrossRef]
- Wu, D.; Ruban, A.; Fuchs, J.; Macas, J.; Novak, P.; Vaio, M.; Zhou, Y.; Houben, A. Nondisjunction and unequal spindle organization accompany the drive of Aegilops speltoides B chromosomes. New Phytol. 2019, 223, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Raskina, O.; Brodsky, L.; Belyayev, A. Tandem repeats on an eco-geographical scale: Outcomes from the genome of Aegilops speltoides. Chromosom. Res. 2011, 19, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Jiang, J.; Gill, B. Detection of 5S rDNA and other repeated DNA on supernumerary B chromosomes ofTriticum species (Poaceae). Plant Syst. Evol. 1995, 196, 131–139. [Google Scholar] [CrossRef]
- Ruban, A.; Fuchs, J.; Marques, A.; Schubert, V.; Soloviev, A.; Raskina, O.; Badaeva, E.D.; Houben, A. B Chromosomes of Aegilops speltoides Are Enriched in Organelle Genome-Derived Sequences. PLOS ONE 2014, 9, e90214. [Google Scholar] [CrossRef] [PubMed]
- Raskina, O. Genotype- and Cell-Specific Dynamics of Tandem Repeat Patterns in Aegilops speltoides Tausch (Poaceae, Triticeae). Cytogenet. Genome Res. 2017, 153, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Raskina, O. Transposable Elements in the Organization and Diversification of the Genome of Aegilops speltoides Tausch (Poaceae, Triticeae). Int. J. Genom. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hosid, E.; Brodsky, L.; Kalendar, R.; Raskina, O.; Belyayev, A. Diversity of Long Terminal Repeat Retrotransposon Genome Distribution in Natural Populations of the Wild Diploid Wheat Aegilops speltoides. Genetics 2011, 190, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Raskina, O.; Barber, J.; Nevo, E.; Belyayev, A. Repetitive DNA and chromosomal rearrangements: Speciation-related events in plant genomes. Cytogenet. Genome Res. 2008, 120, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Pollak, Y.; Zelinger, E.; Raskina, O. Repetitive DNA in the Architecture, Repatterning, and Diversification of the Genome of Aegilops speltoides Tausch (Poaceae, Triticeae). Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Schubert, I.; Shaw, P. Organization and dynamics of plant interphase chromosomes. Trends Plant Sci. 2011, 16, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.; Fauser, F.; Puchta, H. DNA recombination in somatic plant cells: Mechanisms and evolutionary consequences. Chromosom. Res. 2014, 22, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Fransz, P.; De Jong, H. From nucleosome to chromosome: A dynamic organization of genetic information. Plant J. 2011, 66, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Hübner, M.R.; Eckersley-Maslin, M.; Spector, D.L. Chromatin organization and transcriptional regulation. Curr. Opin. Genet. Dev. 2012, 23, 89–95. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. ScienceDirect—Molecular Cell: The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2011, 40, 179–204. [Google Scholar] [CrossRef]
- Zeman, M.K.; Cimprich, K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef]
- Zheng, L.; Shen, B. Okazaki fragment maturation: Nucleases take centre stage. J. Mol. Cell Boil. 2011, 3, 23–30. [Google Scholar] [CrossRef]
- Puchta, H. The repair of double-strand breaks in plants: Mechanisms and consequences for genome evolution. J. Exp. Bot. 2004, 56, 1–14. [Google Scholar] [CrossRef]
- Andersen, S.L.; Sekelsky, J. Meiotic versus mitotic recombination: Two different routes for double-strand break repair. BioEssays 2010, 32, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Schubert, V.; Kim, Y.-M.; Berr, A.; Fuchs, J.; Meister, A.; Marschner, S.; Schubert, I. Random homologous pairing and incomplete sister chromatid alignment are common in angiosperm interphase nuclei. Mol. Genet. Genom. 2007, 278, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pecinka, A.; Schubert, V.; Meister, A.; Kreth, G.; Klatte, M.; Lysak, M.A.; Schubert, I.; Fuchs, J. Chromosome territory arrangement and homologous pairing in nuclei of Arabidopsis thaliana are predominantly random except for NOR-bearing chromosomes. Chromosoma 2004, 113, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Schubert, V.; Rudnik, R.; Schubert, I. Chromatin associations in Arabidopsis interphase nuclei. Front. Genet. 2014, 5, 389. [Google Scholar] [CrossRef]
- Anand, R.P.; Tsaponina, O.; Greenwell, P.W.; Lee, C.-S.; Du, W.; Petes, T.D.; Haber, J.E. Chromosome rearrangements via template switching between diverged repeated sequences. Genes Dev. 2014, 28, 2394–2406. [Google Scholar] [CrossRef]
- Gorbunova, V. Non-homologous DNA end joining in plant cells is associated with deletions and filler DNA insertions. Nucleic Acids Res. 1997, 25, 4650–4657. [Google Scholar] [CrossRef]
- Wicker, T.; Buchmann, J.P.; Keller, B. Patching gaps in plant genomes results in gene movement and erosion of colinearity. Genome Res. 2010, 20, 1229–1237. [Google Scholar] [CrossRef]
- Salina, E.A.; Sergeeva, E.; Adonina, I.G.; Shcherban, A.B.; Afonnikov, D.A.; Belcram, H.; Huneau, C.; Chalhoub, B. Isolation and sequence analysis of the wheat B genome subtelomeric DNA. BMC Genom. 2009, 10, 414. [Google Scholar] [CrossRef]
- Salina, E.A.; Sergeeva, E.; Adonina, I.G.; Shcherban, A.B.; Belcram, H.; Huneau, C.; Chalhoub, B. The impact of Ty3-gypsy group LTR retrotransposons Fatima on B-genome specificity of polyploid wheats. BMC Plant Boil. 2011, 11, 99. [Google Scholar] [CrossRef]
- Evtushenko, E.V.; Levitsky, V.G.; Elisafenko, E.A.; Gunbin, K.V.; Belousov, A.I.; Šafář, J.; Doležel, J.; Vershinin, A. The expansion of heterochromatin blocks in rye reflects the co-amplification of tandem repeats and adjacent transposable elements. BMC Genom. 2016, 17, 337. [Google Scholar] [CrossRef]
- Ribeiro, A.T.; Pires, B.; Delgado, M.; Viegas, W.; Jones, N.; Cecilio, L.M. Evidence for ’cross-talk’ between A and B chromosomes of rye. Proc. R. Soc. B Boil. Sci. 2004, 271, S482–S484. [Google Scholar] [CrossRef] [PubMed]
- Borde, V.; De Massy, B. Programmed induction of DNA double strand breaks during meiosis: Setting up communication between DNA and the chromosome structure. Curr. Opin. Genet. Dev. 2013, 23, 147–155. [Google Scholar] [CrossRef] [PubMed]
- González-García, M.; González-Sánchez, M.; Puertas, M. The high variability of subtelomeric heterochromatin and connections between nonhomologous chromosomes, suggest frequent ectopic recombination in rye meiocytes. Cytogenet. Genome Res. 2006, 115, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Zelkowski, M.; Mikhailova, E.I.; Keijzer, C.J.; Houben, A.; Schubert, V. Ultrastructure and Dynamics of Synaptonemal Complex Components During Meiotic Pairing and Synapsis of Standard (A) and Accessory (B) Rye Chromosomes. Front. Plant Sci. 2019, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Jiménez, M.M.; Díez, M. Synaptic patterns of rye B chromosomes. IV. The B isochromosomes. Heredity 1995, 74, 100–107. [Google Scholar] [CrossRef]
- Jiménez, M.; Díez, M.; Santos, J.L. Synaptic patterns of rye B chromosomes. III. The deficient B. Chromosom. Res. 1994, 2, 93–98. [Google Scholar] [CrossRef]
- Santos, J.L.; Jiménez, M.M.; Díez, M. Synaptic patterns of rye B chromosomes. I: The standard type. Chromosom. Res. 1993, 1, 145–152. [Google Scholar] [CrossRef]
- Raskina, O.; Belyayev, A.; Nevo, E. Quantum speciation in Aegilops: Molecular cytogenetic evidence from rDNA cluster variability in natural populations. Proc. Natl. Acad. Sci. USA 2004, 101, 14818–14823. [Google Scholar] [CrossRef]
- Raskina, O.; Belyayev, A.; Nevo, E. Activity of the En/Spm-like transposons in meiosis as a base for chromosome repatterning in a small, isolated, peripheral population of Aegilops speltoides Tausch. Chromosom. Res. 2004, 12, 153–161. [Google Scholar] [CrossRef]
- Belyayev, A.; Kalendar, R.; Brodsky, L.; Nevo, E.; Schulman, A.H.; Raskina, O. Transposable elements in a marginal plant population: Temporal fluctuations provide new insights into genome evolution of wild diploid wheat. Mob. DNA 2010, 1, 6. [Google Scholar] [CrossRef]
- Shams, I.; Raskina, O. Intraspecific and intraorganismal copy number dynamics of retrotransposons and tandem repeat in Aegilops speltoides Tausch (Poaceae, Triticeae). Protoplasma 2018, 255, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, T.; Walbot, V. Hypoxia Triggers Meiotic Fate Acquisition in Maize. Science 2012, 337, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, T.; Walbot, V. Maize germinal cell initials accommodate hypoxia and precociously express meiotic genes. Plant J. 2014, 77, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Z.-Y.; Zhang, W.-J.; Zhao, X.-M.; Li, X.; Zhang, N.; Liu, Q.-Q.; Tang, W.-H. Global gene profiling of laser-captured pollen mother cells indicates molecular pathways and gene subfamilies involved in rice meiosis. Plant Physiol. 2010, 154, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.; Iacuone, S.; Li, S.F.; Dolferus, R.; Parish, R.W. Anther Morphological Development and Stage Determination in Triticum aestivum. Front. Plant Sci. 2018, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Salina, E.A.; Pestsova, E.; Adonina, I.G.; Vershinin, A. Identification of a new family of tandem repeats in Triticeae genomes. Euphytica 1998, 100, 231–237. [Google Scholar] [CrossRef]
- Anamthawat-Jónsson, K.; Heslop-Harrison, P. Isolation and characterization of genome-specific DNA sequences in Triticeae species. Mol. Genet. Genom. 1993, 240, 151–158. [Google Scholar] [CrossRef]
- Bedbrook, J.; Jones, A.M.; O’Dell, M.; Thompson, R.; Flavell, R. A molecular description of telomeric heterochromatin in secale species. Cell 1980, 19, 545–560. [Google Scholar] [CrossRef]
- Taketa, S.; Ando, H.; Takeda, K.; Harrison, G.E.; Heslop-Harrison, P. The distribution, organization and evolution of two abundant and widespread repetitive DNA sequences in the genus Hordeum. Theor. Appl. Genet. 2000, 100, 169–176. [Google Scholar] [CrossRef]
- Baum, B.; Bailey, L.G. The 5S rRNA gene sequence variation in wheats and some polyploid wheat progenitors (Poaceae: Triticeae). Genet. Resour. Crop. Evol. 2001, 48, 35–51. [Google Scholar] [CrossRef]
- Aragón-Alcaide, L.; Miller, T.; Schwarzacher, T.; Reader, S.; Moore, G. A cereal centromeric sequence. Chromosoma 1996, 105, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.V.; Bennett, S.T.; Parokonny, A.S.; Kenton, A.; Callimassia, M.A. Comparison of Plant Telomere Locations using a PCR-generated Synthetic Probe. Ann. Bot. 1993, 72, 239–247. [Google Scholar] [CrossRef]
- Kraitshtein, Z.; Yaakov, B.; Khasdan, V.; Kashkush, K. Genetic and Epigenetic Dynamics of a Retrotransposon After Allopolyploidization of Wheat. Genetics 2010, 186, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| No. | Populations: Origin, Source | Geographical Zone, Elevation, and Coordinates | Population Size, Location | Morphotype |
|---|---|---|---|---|
| 1 | Tartus, Syria 1 PI 487238 | Mediterranean 600 m 35°07′ N, 36°07′ E | N/A; cultivated field * | ssp. aucheri |
| 2 | Kishon, Israel 2 2.22 | Mediterranean 2 m 32°48′ N; 35°02′ E | small; natural habitat endangered | ssp. ligustica ssp. aucheri |
| 3 | Technion, Israel 2 2.36 | Mediterranean 265 m 32°46′ N, 35°00′ E | small; natural abitat; extinct | ssp. ligustica, ssp. aucheri |
| 4 | Ramat haNadiv, Israel 2 2.46 | Mediterranean 100–125 m 32°33′ N, 34°56′ E | big; natural habitat, interrupted area | ssp. ligustica, ssp. aucheri, intermediate |
| 5 | Katzir, Israel 2 2.93 | Mediterranean 233–250 m 32°29′ N, 35°05′ E | big; natural habitat | ssp. aucheri |
| Ty1-copia, Angela | Ty3-gypsy, Wilma | LINE, Stasy | Tandem Repeat, Spelt1 | |
|---|---|---|---|---|
| Genotype | copies/1C | copies/1C | copies/1C | copies/1C |
| Kishon (0B) | ||||
| Ks(0B)-pst | 697 | 172 | 6 | 204 |
| Ks(0B)-prm | 966 | 205 | 8 | 195 |
| Ks(0B)-mei | 796 | 182 | 5 | 175 |
| Ks(0B)-pol | 1,729 | 385 | 12 | 173 |
| average | 1047 | 236 | 8 | 187 |
| max/min ratio * | 2.5 | 2.2 | 2.4 | 1.2 |
| Kishon (3B) | ||||
| Ks(3B)-pst | 1,030 | 140 | 5 | 586 |
| Ks(3B)-prm | 1,847 | 315 | 11 | 521 |
| Ks(3B)-mei | 1,836 | 283 | 9 | 447 |
| Ks(3B)-pol | 1,381 | 213 | 7 | 329 |
| average | 1524 | 238 | 8 | 471 |
| max/min ratio * | 1.8 | 2.3 | 2.2 | 1.8 |
| Technion (0B) | ||||
| Tn(0B)-pst | 1,103 | 164 | 5 | 311 |
| Tn(0B)-prm | 1,531 | 310 | 14 | 377 |
| Tn(0B)-mei | 1,790 | 294 | 10 | 323 |
| Tn(0B)-pol | 1,303 | 225 | 5 | 204 |
| average | 1432 | 248 | 8 | 304 |
| max/min ratio * | 1.6 | 1.9 | 2.8 | 1.8 |
| Technion (3B) | ||||
| Tn(3B)-pst | 1,599 | 235 | 8 | 634 |
| Tn(3B)-prm | 3,377 | 558 | 20 | 383 |
| Tn(3B)-mei | 2,057 | 373 | 7 | 499 |
| Tn(3B)-pol | 1,880 | 334 | 8 | 689 |
| average | 2228 | 375 | 11 | 551 |
| max/min ratio * | 2.1 | 2.4 | 2.9 | 1.8 |
| Ramat haNadiv (0B) | ||||
| RH(0B)-pst | 1,107 | 197 | 6 | 64,533 |
| RH(0B)-prm | 2,419 | 486 | 17 | 99,552 |
| RH(0B)-mei | 1,575 | 301 | 9 | 79,481 |
| RH(0B)-pol | 1,615 | 250 | 7 | 101,945 |
| average | 1679 | 309 | 10 | 86378 |
| max/min ratio * | 2.2 | 2.5 | 2.8 | 1.6 |
| Ramat haNadiv (3B) | ||||
| RH(3B)-pst | 2,913 | 432 | 18 | 184,644 |
| RH(3B)-prm | 4,510 | 856 | 30 | 185,272 |
| RH(3B)-mei | 4,201 | 754 | 26 | 190,825 |
| RH(3B)-pol | 4,686 | 852 | 32 | 147,443 |
| average | 4078 | 723 | 26 | 177046 |
| max/min ratio * | 1.6 | 2.0 | 1.8 | 1.3 |
| T. urartu | ||||
| T. urartu-lv | 3,192 | 713 | 27 | 1 |
| Genotype | TE/TE and TE/TR Correlation | |||
|---|---|---|---|---|
| Wilma | Stasy | Spelt1 | ||
| Kishon (0B) | Angela | 1.00 | 0.97 | −0.62 |
| Wilma | 0.96 | −0.62 | ||
| Stasy | −0.44 | |||
| Kishon (3B) | Angela | 0.99 | 0.96 | −0.16 |
| Wilma | 0.98 | −0.15 | ||
| Stasy | 0.02 | |||
| Technion (0B) | Angela | 0.97 | 0.91 | 0.50 |
| Wilma | 0.90 | 0.45 | ||
| Stasy | 0.80 | |||
| Technion (3B) | Angela | 0.97 | 0.92 | −0.87 |
| Wilma | 0.81 | −0.79 | ||
| Stasy | −0.75 | |||
| Ramat haNadiv (0B) | Angela | 0.97 | 0.96 | 0.73 |
| Wilma | 1.00 | 0.57 | ||
| Stasy | 0.54 | |||
| Ramat haNadiv (3B) | Angela | 1.00 | 0.98 | −0.44 |
| Wilma | 0.97 | −0.38 | ||
| Stasy | −0.60 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shams, I.; Raskina, O. Supernumerary B Chromosomes and Plant Genome Changes: A Snapshot of Wild Populations of Aegilops speltoides Tausch (Poaceae, Triticeae). Int. J. Mol. Sci. 2020, 21, 3768. https://doi.org/10.3390/ijms21113768
Shams I, Raskina O. Supernumerary B Chromosomes and Plant Genome Changes: A Snapshot of Wild Populations of Aegilops speltoides Tausch (Poaceae, Triticeae). International Journal of Molecular Sciences. 2020; 21(11):3768. https://doi.org/10.3390/ijms21113768
Chicago/Turabian StyleShams, Imad, and Olga Raskina. 2020. "Supernumerary B Chromosomes and Plant Genome Changes: A Snapshot of Wild Populations of Aegilops speltoides Tausch (Poaceae, Triticeae)" International Journal of Molecular Sciences 21, no. 11: 3768. https://doi.org/10.3390/ijms21113768
APA StyleShams, I., & Raskina, O. (2020). Supernumerary B Chromosomes and Plant Genome Changes: A Snapshot of Wild Populations of Aegilops speltoides Tausch (Poaceae, Triticeae). International Journal of Molecular Sciences, 21(11), 3768. https://doi.org/10.3390/ijms21113768






