A Guide to the Short, Long and Circular RNAs in Hypertension and Cardiovascular Disease
Abstract
1. Hypertension and Cardiovascular Disease (CVD)
2. Hypertension Genetics
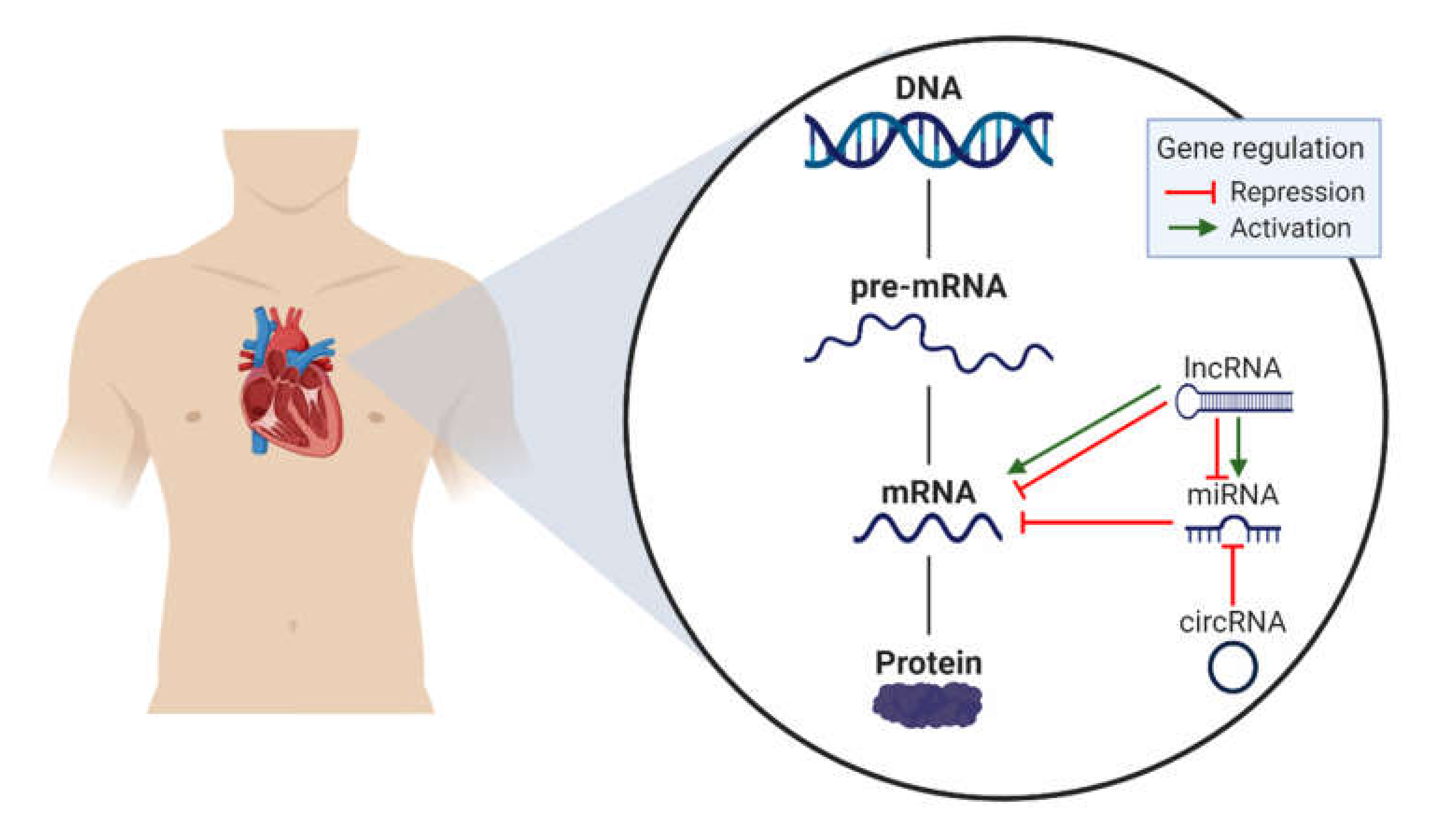
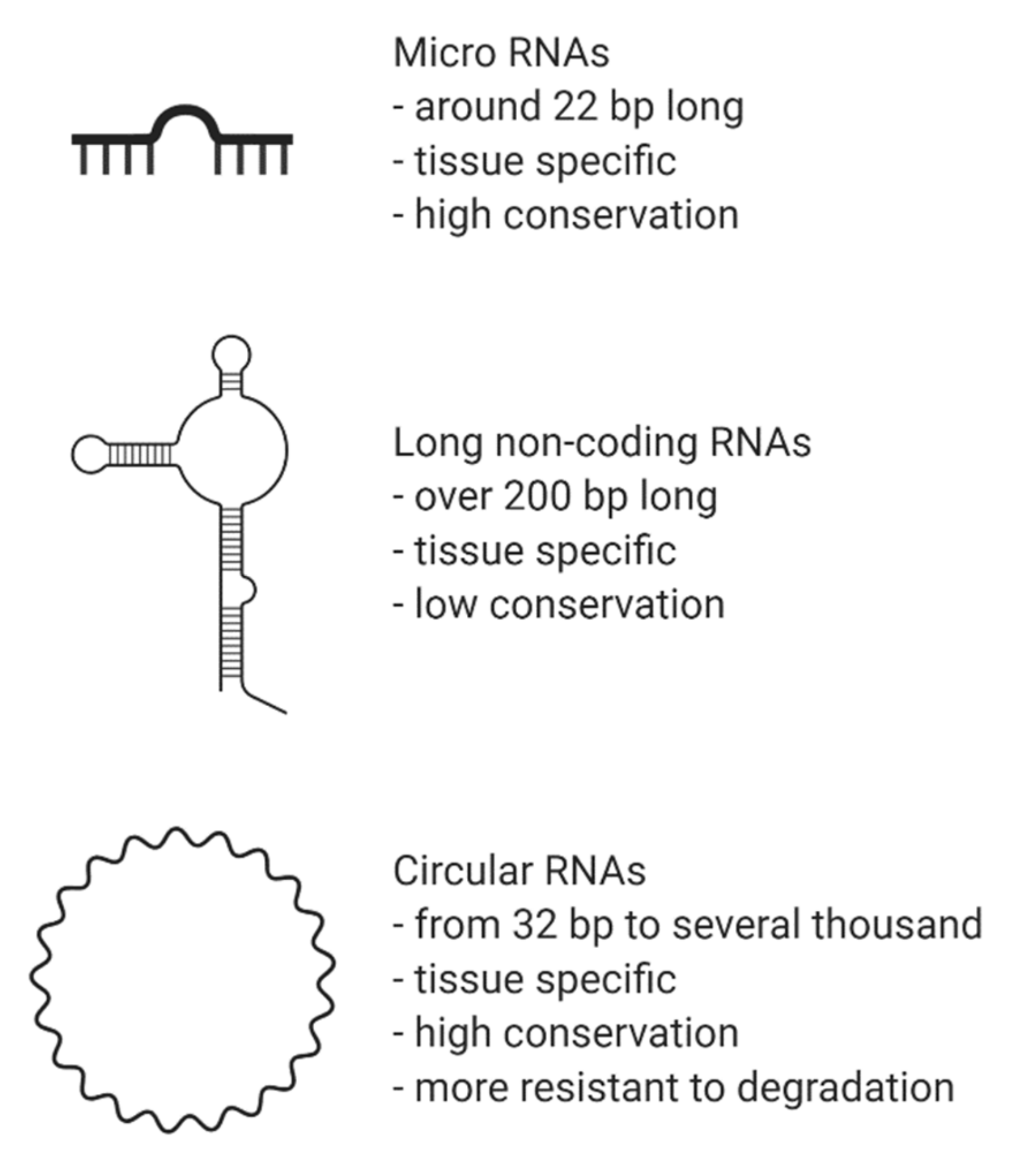
3. Non-Coding RNAs (ncRNA)
3.1. MicroRNAs in CVD and Hypertension
3.2. Long Non-Coding RNAs and Their Involvement in CVD and Hypertension
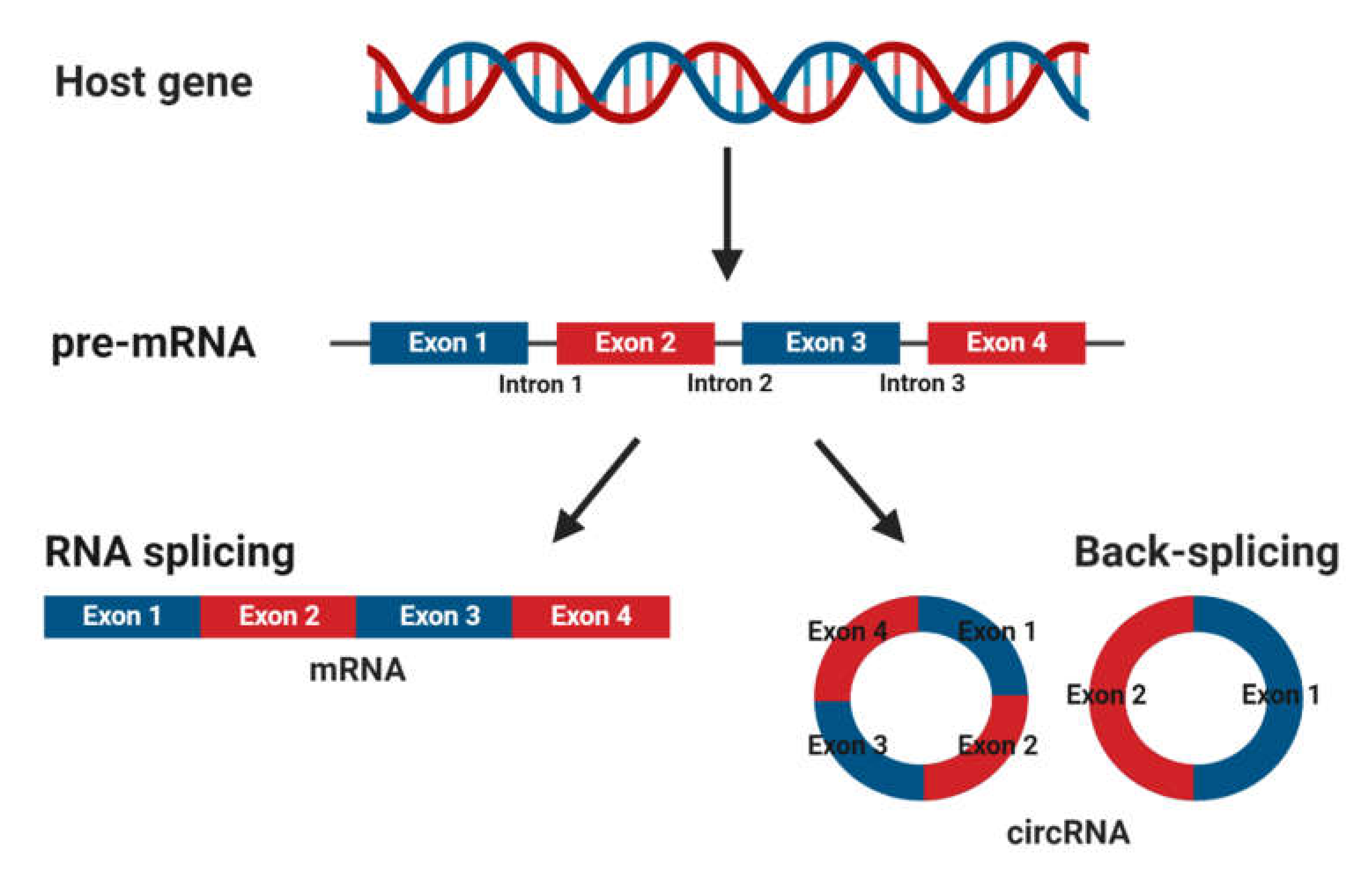
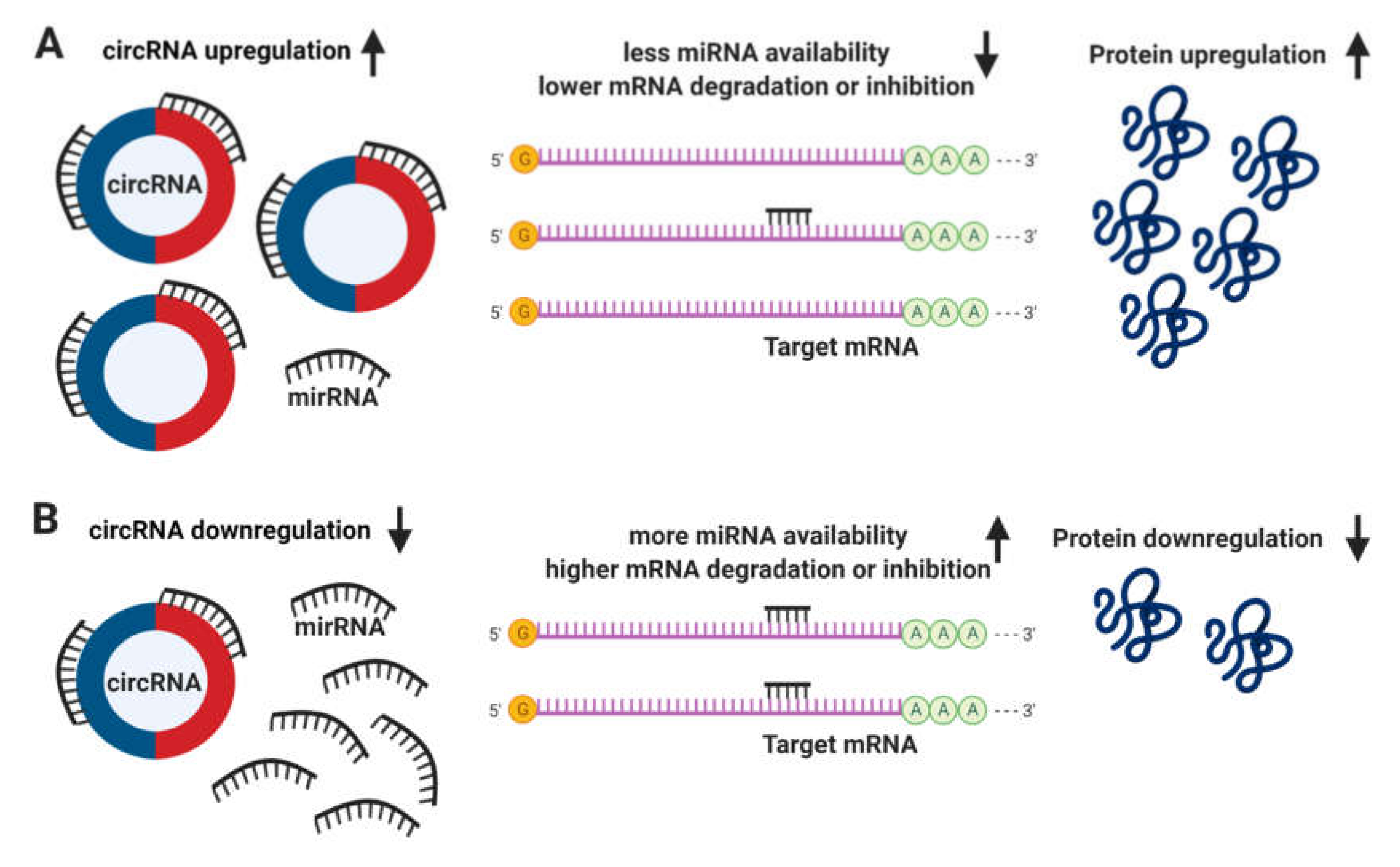
3.3. Circular RNAs: What Are They and How Do They Function?
3.4. Circular RNAs and Their Involvement in CVD
3.4.1. Circular RNAs and Hypertension
3.4.2. Circular RNAs, Myocardial Infarction and Heart Failure
3.4.3. Circular RNAs, Atherosclerosis and CAD
3.4.4. Circular RNAs and Cardiomyopathy
4. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| Ang II | angiotensin II |
| ANRIL | antisense noncoding RNA in the INK4 locus |
| ARC | activity-regulated cytoskeleton-associated protein |
| BP | blood pressure |
| CAD | coronary artery disease |
| Carl | cardiac apoptosis-related lncRNA |
| Chaer | cardiac hypertrophy associated epigenetic regulator |
| CHAST | cardiac hypertrophy associated transcript |
| circRNA | circular RNA |
| COVID-19 | coronavirus |
| CVD | cardiovascular disease |
| EC | endothelial cells |
| GAS5 | growth arrest-specific 5 |
| HF | heart failure |
| hiPSC | human induced pluripotent stem cells |
| hiPSC-CM | hiPSC-derived cardiomyocytes |
| HRCR | heart-related circRNA |
| HT | hypertension |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| lncRNA | long non-coding RNA |
| Mdrl | mitochondrial dynamic related lncRNA |
| Mhrt | myosin heavy chain associated RNA transcript |
| MI | myocardial infarction |
| miRNA | microRNA |
| mRNA | messenger RNA |
| ncRNA | non-coding RNA |
| PRC2 | polycomb repressor complex 2 |
| RAAS | renin-angiotensin-aldosterone system |
| TAC | transverse aortic surgery |
| TGFβ | transforming growth factor beta |
| VSMC | vascular smooth muscle cells |
References
- O’Shea, P.; Griffin, M.D.; FitzGibbon, M. Hypertension: The role of biochemistry in the diagnosis and management. Clin. Chim. Acta 2017, 465, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Zaman, M.A.; Calhoun, D.A. Pathogenesis of hypertension. Ann. Intern. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Carretero, O.A.; Oparil, S. Essential hypertension. Part I: Definition and etiology. Circulation 2000, 101, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, E.B.; Heincelman, M.; Bishu, K.G.; Schumann, S.O.; Schreiner, A.; Axon, R.N.; Mauldin, P.D.; Moran, W.P. Trends in Healthcare Expenditures Among US Adults with Hypertension: National Estimates, 2003–2014. J. Am. Hear. Assoc. 2018, 7, e008731. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Biino, G.; Parati, G.; Concas, M.P.; Adamo, M.; Angius, A.; Vaccargiu, S.; Pirastu, M. Environmental and Genetic Contribution to Hypertension Prevalence: Data from an Epidemiological Survey on Sardinian Genetic Isolates. PLoS ONE 2013, 8, e59612. [Google Scholar] [CrossRef]
- Evangelou, E.; Program, T.M.V.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Feng, W.; Dell’Italia, L.J.; Sanders, P.W. Novel Paradigms of Salt and Hypertension. J. Am. Soc. Nephrol. 2017, 28, 1362–1369. [Google Scholar] [CrossRef]
- Nakao, E.; Adachi, H.; Enomoto, M.; Fukami, A.; Kumagai, E.; Nakamura, S.; Nohara, Y.; Kono, S.; Sakaue, A.; Morikawa, N.; et al. Elevated Plasma Transforming Growth Factor beta1 Levels Predict the Development of Hypertension in Normotensives: The 14-Year Follow-Up Study. Am. J. Hypertens. 2017, 30, 808–814. [Google Scholar] [CrossRef][Green Version]
- Jeunemaître, X.; Soubrier, F.; Kotelevtsev, Y.V.; Lifton, R.P.; Williams, C.S.; Charru, A.; Hunt, S.C.; Hopkins, P.N.; Williams, R.R.; Lalouel, J.-M.; et al. Molecular basis of human hypertension: Role of angiotensinogen. Cell 1992, 71, 169–180. [Google Scholar] [CrossRef]
- Zeller, T.; Schurmann, C.; Schramm, K.; Müller, C.; Kwon, S.; Wild, P.S.; Teumer, A.; Herrington, D.; Schillert, A.; Iacoviello, L.; et al. Transcriptome-Wide Analysis Identifies Novel Associations with Blood Pressure. Hypertension 2017, 70, 743–750. [Google Scholar] [CrossRef]
- Marques, F.Z.; A Booth, S.; Charchar, F. The emerging role of non-coding RNA in essential hypertension and blood pressure regulation. J. Hum. Hypertens. 2014, 29, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M. Circular RNAs in hypertension: Challenges and clinical promise. Hypertens. Res. 2019, 42, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Sadayappan, S.; Becker, R.C.; Martin, L.J.; Urbina, E.M. Epigenetic modification: A regulatory mechanism in essential hypertension. Hypertens. Res. 2019, 42, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Charchar, F. microRNAs in Essential Hypertension and Blood Pressure Regulation. Adv. Exp. Med. Biol. 2015, 888, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef] [PubMed]
- Hocine, S.; Singer, R.H.; Grünwald, D. RNA Processing and Export. Cold Spring Harb. Perspect. Boil. 2010, 2, a000752. [Google Scholar] [CrossRef]
- Krchnakova, Z.; Thakur, P.K.; Krausová, M.; Bieberstein, N.; Haberman, N.; Müller-McNicoll, M.; Staněk, D. Splicing of long non-coding RNAs primarily depends on polypyrimidine tract and 5’ splice-site sequences due to weak interactions with SR proteins. Nucleic Acids Res. 2019, 47, 911–928. [Google Scholar] [CrossRef]
- Hobuß, L.; Bär, C.; Thum, T. Long Non-coding RNAs: At the Heart of Cardiac Dysfunction? Front. Physiol. 2019, 10, 30. [Google Scholar] [CrossRef]
- Pisarello, M.J.L.; Loarca, L.; Ivanics, T.; Morton, L.; LaRusso, N.F. MicroRNAs in the Cholangiopathies: Pathogenesis, Diagnosis, and Treatment. J. Clin. Med. 2015, 4, 1688–1712. [Google Scholar] [CrossRef]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Quiat, D.; Olson, E. MicroRNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J. Clin. Investig. 2013, 123, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-S.; Jin, J.-P.; Wang, J.-Q.; Zhang, Z.-G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Hear. J. 2017, 39, 2704–2716. [Google Scholar] [CrossRef]
- Jusic, A.; Devaux, Y.; Action, E.U.-C.C. Noncoding RNAs in Hypertension. Hypertension 2019, 74, 477–492. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Wang, Z. microRNA-21 and hypertension. Hypertens. Res. 2018, 41, 649–661. [Google Scholar] [CrossRef]
- Kontaraki, J.E.; Marketou, M.E.; Zacharis, E.A.; Parthenakis, F.I.; Vardas, P.E. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: Novel targets in essential hypertension. J. Hum. Hypertens. 2013, 28, 510–516. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, H.; Ge, D.; Xu, Y.; Xu, H.; Yang, Y.; Gu, M.; Zhou, Y.; Zhu, J.; Ge, T.; et al. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell. Physiol. Biochem. 2017, 42, 2207–2219. [Google Scholar] [CrossRef]
- Wang, G.-K.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Hear. J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- Gao, F.; Kataoka, M.; Liu, N.; Liang, T.; Huang, Z.-P.; Gu, F.; Ding, J.; Liu, J.; Zhang, F.; Ma, Q.; et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 2019, 10, 1802. [Google Scholar] [CrossRef]
- Roncarati, R.; Anselmi, C.V.; Losi, M.A.; Papa, L.; Cavarretta, E.; Martins, P.D.C.; Contaldi, C.; Jotti, G.S.; Franzone, A.; Galastri, L.; et al. Circulating miR-29a, Among Other Up-Regulated MicroRNAs, Is the Only Biomarker for Both Hypertrophy and Fibrosis in Patients With Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Nazari-Jahantigh, M.; Neth, P.; Weber, C.; Schober, A. MicroRNA-126, -145, and -155: A therapeutic triad in atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2013, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, N.; Steer, B.M.; Ingram, A.J.; Gupta, M.; Al-Omran, M.; et al. MicroRNA-145 Targeted Therapy Reduces Atherosclerosis. Circulation 2012, 126, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-Dos-Santos, A.J.; Da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 1–8. [Google Scholar] [CrossRef]
- Liu, Q.; Du, J.; Yu, X.; Xu, J.; Huang, F.; Li, X.; Zhang, C.; Li, X.; Chang, J.; Shang, D.; et al. miRNA-200c-3p is crucial in acute respiratory distress syndrome. Cell Discov. 2017, 3, 17021. [Google Scholar] [CrossRef]
- Zhang, R.; Su, H.; Ma, X.; Xu, X.; Liang, L.; Ma, G.; Shi, L. MiRNA let-7b promotes the development of hypoxic pulmonary hypertension by targeting ACE2. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L547–L557. [Google Scholar] [CrossRef]
- Trojanowicz, B.; Imdahl, T.; Ulrich, C.; Fiedler, R.; Girndt, M. Circulating miR-421 Targeting Leucocytic Angiotensin Converting Enzyme 2 Is Elevated in Patients with Chronic Kidney Disease. Nephron 2018, 141, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Zhang, Y.; Liu, C.-X.; Huang, J.; Ding, G.-H. microRNA-125b contributes to high glucose-induced reactive oxygen species generation and apoptosis in HK-2 renal tubular epithelial cells by targeting angiotensin-converting enzyme 2. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4055–4062. [Google Scholar] [PubMed]
- Li, W.; Wang, R.; Ma, J.-Y.; Wang, M.; Cui, J.; Wu, W.-B.; Liu, R.-M.; Zhang, C.-X.; Wang, S.-M. A Human Long Non-Coding RNA ALT1 Controls the Cell Cycle of Vascular Endothelial Cells Via ACE2 and Cyclin D1 Pathway. Cell. Physiol. Biochem. 2017, 43, 1152–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.-Z.; Shan, K.; Yao, M.-D.; Yao, J.; Wang, J.-J.; Li, X.; Liu, B.; Zhang, Y.-Y.; Ji, Y.; Jiang, Q.; et al. Long Noncoding RNA-GAS5Novelty and Significance. Hypertension 2016, 68, 736–748. [Google Scholar] [CrossRef]
- Hou, L.; Lin, Z.; Ni, Y.; Chen, D.; Hu, H.; Wu, Y.; Song, L.; Huang, X.; Yang, D. Microarray expression profiling and gene ontology analysis of long non-coding RNAs in spontaneously hypertensive rats and their potential roles in the pathogenesis of hypertension. Mol. Med. Rep. 2015, 13, 295–300. [Google Scholar] [CrossRef]
- Han, P.; Li, W.; Lin, C.-H.; Yang, J.; Shang, C.; Nurnberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.-Y.; Lin, C.-J.; et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 2014, 514, 102–106. [Google Scholar] [CrossRef]
- Viereck, J.; Kumarswamy, R.; Foinquinos, A.; Xiao, K.; Avramopoulos, P.; Kunz, M.; Dittrich, M.; Maetzig, T.; Zimmer, K.; Remke, J.; et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci. Transl. Med. 2016, 8, 326ra22. [Google Scholar] [CrossRef]
- Qu, X.; Du, Y.; Shu, Y.; Gao, M.; Sun, F.; Luo, S.; Yang, T.; Zhan, L.; Yuan, Y.; Chu, W.; et al. MIAT Is a Pro-fibrotic Long Non-coding RNA Governing Cardiac Fibrosis in Post-infarct Myocardium. Sci. Rep. 2017, 7, 42657. [Google Scholar] [CrossRef]
- Verjans, J.W.; Van De Borne, S.W.; Hofstra, L.; Narula, J. Molecular Imaging of Myocardial Remodeling After Infarction. Breast Cancer 2010, 680, 227–235. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Yuan, Y.-X.; Rao, S.-L.; Wang, P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3653–3660. [Google Scholar]
- Li, Y.; Wang, J.; Sun, L.; Zhu, S. LncRNA myocardial infarction-associated transcript (MIAT) contributed to cardiac hypertrophy by regulating TLR4 via miR-93. Eur. J. Pharmacol. 2018, 818, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Long, B.; Zhou, L.-Y.; Liu, F.; Zhou, Q.-Y.; Liu, C.-Y.; Fan, Y.-Y.; Li, P.-F. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat. Commun. 2014, 5, 3596. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, T.; Li, N.; Wang, Y.; Wang, J.-X.; Zhou, L.-Y.; Long, B.; Liu, C.-Y.; Liu, F.; Li, P.-F. MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361. PLoS Genet. 2014, 10, e1004467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.-J.; Ji, Y.-X.; Zhang, P.; Deng, K.-Q.; Gong, J.; Ren, S.; Wang, X.; Chen, I.; Wang, H.; et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat. Med. 2016, 22, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Black, D.L. Mechanisms of Alternative Pre-Messenger RNA Splicing. Annu. Rev. Biochem. 2003, 72, 291–336. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar] [CrossRef]
- Starke, S.; Jost, I.; Rossbach, O.; Schneider, T.; Schreiner, S.; Hung, L.-H.; Bindereif, A. Exon Circularization Requires Canonical Splice Signals. Cell Rep. 2015, 10, 103–111. [Google Scholar] [CrossRef]
- Salzman, J. Circular RNA Expression: Its Potential Regulation and Function. Trends Genet. 2016, 32, 309–316. [Google Scholar] [CrossRef]
- Chen, N.; Lu, X.; Yang, F.; Xing, N. Circular RNA circHIPK3 promotes cell proliferation and invasion of prostate cancer by sponging miR-193a-3p and regulating MCL1 expression. Cancer Manag. Res. 2019, 11, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, W.; Sun, T.; Tariq, M.A.; Xu, T.; Li, P.; Wang, J. Biogenesis of circular RNA s and their roles in cardiovascular development and pathology. FEBS J. 2017, 285, 220–232. [Google Scholar] [CrossRef]
- Fan, X.; Weng, X.; Zhao, Y.; Chen, W.; Gan, T.; Xu, D. Circular RNAs in Cardiovascular Disease: An Overview. BioMed. Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Lim, B.T.; Anene-Nzelu, C.G.; Ackers-Johnson, M.; Dashi, A.; See, K.; Tiang, Z.; Lee, D.P.; Chua, W.W.; Luu, T.D.; et al. A landscape of circular RNA expression in the human heart. Cardiovasc. Res. 2017, 113, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Werfel, S.; Nothjunge, S.; Schwarzmayr, T.; Strom, T.-M.; Meitinger, T.; Engelhardt, S. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell. Cardiol. 2016, 98, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Jin, L.; Cai, J. Profiling and bioinformatics analyses reveal differential circular RNA expression in hypertensive patients. Clin. Exp. Hypertens. 2017, 39, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; He, X.; Zheng, S.; Sun, J.; Luo, Y.; Tan, R.; Zhao, J.; Zhong, F.; Zhang, L.-N. Up-regulation of circular RNA hsa_circ_0037909 promotes essential hypertension. J. Clin. Lab. Anal. 2019, 33, e22853. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zheng, S.; Mao, S.; Gu, T.; Liu, S.; Sun, J.; Zhang, L.-N. A potential risk factor of essential hypertension in case-control study: Circular RNA hsa_circ_0037911. Biochem. Biophys. Res. Commun. 2018, 498, 789–794. [Google Scholar] [CrossRef]
- Liu, L.; Gu, T.; Bao, X.; Zheng, S.; Zhao, J.; Zhang, L. Microarray Profiling of Circular RNA Identifies hsa_circ_0126991 as a Potential Risk Factor for Essential Hypertension. Cytogenet. Genome Res. 2019, 157, 203–212. [Google Scholar] [CrossRef]
- Zheng, S.; Gu, T.; Bao, X.; Sun, J.; Zhao, J.; Zhang, T.; Zhang, L.-N. Circular RNA hsa_circ_0014243 may serve as a diagnostic biomarker for essential hypertension. Exp. Ther. Med. 2018, 17, 1728–1736. [Google Scholar] [CrossRef]
- Geng, H.-H.; Li, R.; Su, Y.-M.; Xiao, J.; Pan, M.; Cai, X.-X.; Ji, X.-P. The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of miR-7a on Its Target Genes Expression. PLoS ONE 2016, 11, e0151753. [Google Scholar] [CrossRef]
- Cai, L.-D.; Qi, B.; Wu, X.; Peng, S.; Zhou, G.; Wei, Y.; Xu, J.; Chen, S.; Liu, S. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR-15b. J. Mol. Cell. Cardiol. 2019, 130, 10–22. [Google Scholar] [CrossRef]
- Salgado-Somoza, A.; Zhang, L.; Vausort, M.; Devaux, Y. The circular RNA MICRA for risk stratification after myocardial infarction. IJC Hear. Vasc. 2017, 17, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hu, Y.; Lou, J.; Yin, S.; Wang, W.; Wang, Y.; Xia, Y.; Wu, W. CircRNA-0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR-107. Mol. Med. Rep. 2019, 19, 3923–3932. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, R.; Zhang, X.; Wu, Y.; Li, X.; Zhang, S.; Hou, W.; Ding, Y.; Tian, J.; Sun, L.; et al. Comprehensive analysis of circRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of atherosclerosis in rabbits. Aging 2018, 10, 2266–2283. [Google Scholar] [CrossRef]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef]
- Yang, L.; Yang, F.; Zhao, H.; Wang, M.; Zhang, Y. Circular RNA circCHFR Facilitates the Proliferation and Migration of Vascular Smooth Muscle via miR-370/FOXO1/Cyclin D1 Pathway. Mol. Ther. Nucleic Acids 2019, 16, 434–441. [Google Scholar] [CrossRef]
- Shang, L.; Quan, A.; Sun, H.; Xu, Y.; Sun, G.; Cao, P. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene 2019, 711, 143948. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Wang, Y.; Zou, T.; Zhu, H.; Lu, X.; Li, L.; Yang, B.; Chen, J.; Chen, S.; et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis 2019, 286, 88–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Gao, C.; Jian, D.; Hao, P.; Rao, L.; Li, M. Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci. Rep. 2017, 7, 39918. [Google Scholar] [CrossRef]
- Lin, F.; Zhao, G.; Chen, Z.; Wang, X.; Lv, F.; Zhang, Y.; Yang, X.; Liang, W.; Cai, R.; Li, J.; et al. circRNA-miRNA association for coronary heart disease. Mol. Med. Rep. 2019, 19, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Long, B.; Liu, F.; Wang, J.-X.; Liu, C.-Y.; Zhao, B.; Zhou, L.-Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Hear. J. 2016, 37, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, A.; Qin, Y.; Che, H.; Wang, Y.; Lv, J.; Li, Y.; Li, H.; Yue, E.; Ding, X.; et al. A Novel Circular RNA Mediates Pyroptosis of Diabetic Cardiomyopathy by Functioning as a Competing Endogenous RNA. Mol. Ther. Nucleic Acids 2019, 17, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, J.-D.; Fang, X.-H.; Zhu, J.-N.; Yang, J.; Pan, R.; Yuan, S.-J.; Zeng, N.; Yang, Z.-Z.; Yang, H.; et al. Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR26b-5p and miR-140-3p binding to Gata4. Cardiovasc. Res. 2019. [Google Scholar] [CrossRef]
- Lim, T.B.; Aliwarga, E.; Luu, T.D.A.; Li, Y.P.; Ng, S.L.; Annadoray, L.; Sian, S.; Ackers-Johnson, M.A.; Foo, R. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc. Res. 2019, 115, 1998–2007. [Google Scholar] [CrossRef]
- Kim, Y.; Hooten, N.N.; Dluzen, D.F.; Martindale, J.L.; Gorospe, M.; Evans, M.K. Posttranscriptional Regulation of the Inflammatory Marker C-Reactive Protein by the RNA-Binding Protein HuR and MicroRNA 637. Mol. Cell. Boil. 2015, 35, 4212–4221. [Google Scholar] [CrossRef]
- Fang, Y.; Shi, C.; Manduchi, E.; Civelek, M.; Davies, P.F. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 13450–13455. [Google Scholar] [CrossRef]
- Wu, H.-J.; Zhang, C.-Y.; Zhang, S.; Chang, M.; Wang, H.-Y. Microarray Expression Profile of Circular RNAs in Heart Tissue of Mice with Myocardial Infarction-Induced Heart Failure. Cell. Physiol. Biochem. 2016, 39, 205–216. [Google Scholar] [CrossRef]
- Schulte, C.; Barwari, T.; Joshi, A.; Theofilatos, K.; Zampetaki, A.; Barallobre-Barreiro, J.; Singh, B.; Sörensen, N.A.; Neumann, J.T.; Zeller, T.; et al. Comparative Analysis of Circulating Noncoding RNAs Versus Protein Biomarkers in the Detection of Myocardial Injury. Circ. Res. 2019, 125, 328–340. [Google Scholar] [CrossRef]
- Vausort, M.; Salgado-Somoza, A.; Zhang, L.; Leszek, P.; Scholz, M.; Teren, A.; Burkhardt, R.; Thiery, J.; Wagner, D.R.; Devaux, Y. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016, 68, 1247–1248. [Google Scholar] [CrossRef]
- Holdt, L.M.; Teupser, D. Recent Studies of the Human Chromosome 9p21 Locus, Which Is Associated With Atherosclerosis in Human Populations. Arter. Thromb. Vasc. Boil. 2012, 32, 196–206. [Google Scholar] [CrossRef]
- Holdt, L.M.; Teupser, D. Long Noncoding RNA ANRIL: Lnc-ing Genetic Variation at the Chromosome 9p21 Locus to Molecular Mechanisms of Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Burd, C.E.; Jeck, W.R.; Liu, Y.; Sanoff, H.K.; Wang, Z.; Sharpless, N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010, 6, e1001233. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Ma, L.; Yu, B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed. Pharmacother. 2017, 95, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-Y.; Zhao, C.-H.; Yuan, J.-X.; Zhang, Y.-J.; Jin, J.-L.; Gu, M.-F.; Mao, Z.-Y.; Sun, H.-J.; Jia, Q.-W.; Ji, M.-Y.; et al. Circular RNA profile in coronary artery disease. Am. J. Transl. Res. 2019, 11, 7115–7125. [Google Scholar] [PubMed]
- Yu, F.; Tie, Y.; Zhang, Y.; Wang, Z.; Yu, L.; Zhong, L.; Zhang, C. Circular RNA expression profiles and bioinformatic analysis in coronary heart disease. Epigenomics 2020, 12, 439–454. [Google Scholar] [CrossRef]
- Vilades, D.; Martínez-Camblor, P.; Ferrero-Gregori, A.; Bär, C.; Lu, D.; Xiao, K.; Vea, A.; Nasarre, L.; Vega, J.S.; Leta, R.; et al. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: Performance as a biomarker. FASEB J. 2020, 34, 4403–4414. [Google Scholar] [CrossRef]
- Khan, M.; Reckman, Y.J.; Aufiero, S.; Hoogenhof, M.V.D.; Van Der Made, I.; Beqqali, A.; Koolbergen, D.R.; Rasmussen, T.B.; Van Der Velden, J.; Creemers, E.E.; et al. RBM20 Regulates Circular RNA Production from the Titin Gene. Circ. Res. 2016, 119, 996–1003. [Google Scholar] [CrossRef]
- Siede, D.; Rapti, K.; Gorska, A.; Katus, H.; Altmüller, J.; Boeckel, J.; Meder, B.; Maack, C.; Völkers, M.; Müller, O.; et al. Identification of circular RNAs with host gene-independent expression in human model systems for cardiac differentiation and disease. J. Mol. Cell. Cardiol. 2017, 109, 48–56. [Google Scholar] [CrossRef]
- Lei, W.; Feng, T.; Fang, X.; Yu, Y.; Yang, J.; Zhao, Z.A.; Liu, J.; Shen, Z.; Deng, W.; Hu, S. Signature of circular RNAs in human induced pluripotent stem cells and derived cardiomyocytes. Stem. Cell Res. Ther. 2018, 9, 56. [Google Scholar] [CrossRef]
| Disease | Circular RNA | Species | Sample | Expression | References |
|---|---|---|---|---|---|
| Hypertension | hsa_circ_0005870 | Human | Blood | Down | [64] |
| hsa_circ_0037909 | Human | Blood | Up | [65] | |
| hsa_circ_0037911 | Human | Blood | Up | [66] | |
| hsa_circ_0126991 | Human | Blood | Up | [67] | |
| hsa_circ_0014243 | Human | Blood | Up | [68] | |
| Myocardial infarction | mmu_circ_0001878 | Mouse | Cardiomyocytes | Up | [69] |
| circTtc3 | Rats | Cardiomyocytes | Up | [70] | |
| MICRA | Human | Blood | Down | [71] | |
| circNfix | Mouse | Cardiomyocytes | Up | [72] | |
| circFndc3b | Mouse Human | Cardiomyocytes | Down | [73] | |
| Atherosclerosis | circRNA-0044073 | Human | Blood | Up | [74] |
| ocu-ciR-novel-18038 | Rabbit | Blood | Down | [75] | |
| ocu-ciR-novel-18298 | Rabbit | Blood | Up | ||
| ocu-ciR-novel-15993 | Rabbit | Blood | Up | ||
| ocu-ciR-novel-17934 | Rabbit | Blood | Down | ||
| ocu-ciR-novel-17879 | Rabbit | Blood | Up | ||
| ocu-ciR-novel-18036 | Rabbit | Blood | Up | ||
| ocu-ciR-novel-14389 | Rabbit | Blood | Up | ||
| circANRIL | Human | Blood | Up | [76] | |
| circCHFR | Human | VSMC | Up | [77] | |
| circRNA-0003575 | Mouse | Endothelial cells | Up | [78] | |
| Coronary artery disease | hsa_circ_0001879 | Human | Blood | Up | [79] |
| hsa_circ_0004104 | Human | Blood | Up | ||
| hsa_circ_0124644 | Human | Blood | Up | [80] | |
| hsa_circ_0098964 | Human | Blood | Up | ||
| hsa_circ_0030769 | Human | Blood | Up | [81] | |
| hsa_circ_0079828 | Human | Blood | Up | ||
| hsa_circ_15486-161 | Human | Blood | Up | ||
| hsa_circ_0122274 | Human | Blood | Up | ||
| hsa_circ_16316-13 | Human | Blood | Up | ||
| hsa_circ_0140538 | Human | Blood | Up | ||
| Cardiomyopathy | mmu_circ_0000254 | Mouse | Cardiomyocytes | Down | [82] |
| hsa_circ_0076631 | Human | Cardiomyocytes and Serum | Up | [83] | |
| circRNA_000203 | Mouse | Ventricular Cardiomyocytes | Up | [84] | |
| circSlc8a1 | Mouse | Cardiomyocytes | Up | [85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prestes, P.R.; Maier, M.C.; Woods, B.A.; Charchar, F.J. A Guide to the Short, Long and Circular RNAs in Hypertension and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 3666. https://doi.org/10.3390/ijms21103666
Prestes PR, Maier MC, Woods BA, Charchar FJ. A Guide to the Short, Long and Circular RNAs in Hypertension and Cardiovascular Disease. International Journal of Molecular Sciences. 2020; 21(10):3666. https://doi.org/10.3390/ijms21103666
Chicago/Turabian StylePrestes, Priscilla R., Michelle C. Maier, Bradley A. Woods, and Fadi J. Charchar. 2020. "A Guide to the Short, Long and Circular RNAs in Hypertension and Cardiovascular Disease" International Journal of Molecular Sciences 21, no. 10: 3666. https://doi.org/10.3390/ijms21103666
APA StylePrestes, P. R., Maier, M. C., Woods, B. A., & Charchar, F. J. (2020). A Guide to the Short, Long and Circular RNAs in Hypertension and Cardiovascular Disease. International Journal of Molecular Sciences, 21(10), 3666. https://doi.org/10.3390/ijms21103666





