Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps
Abstract
1. Introduction
2. Results
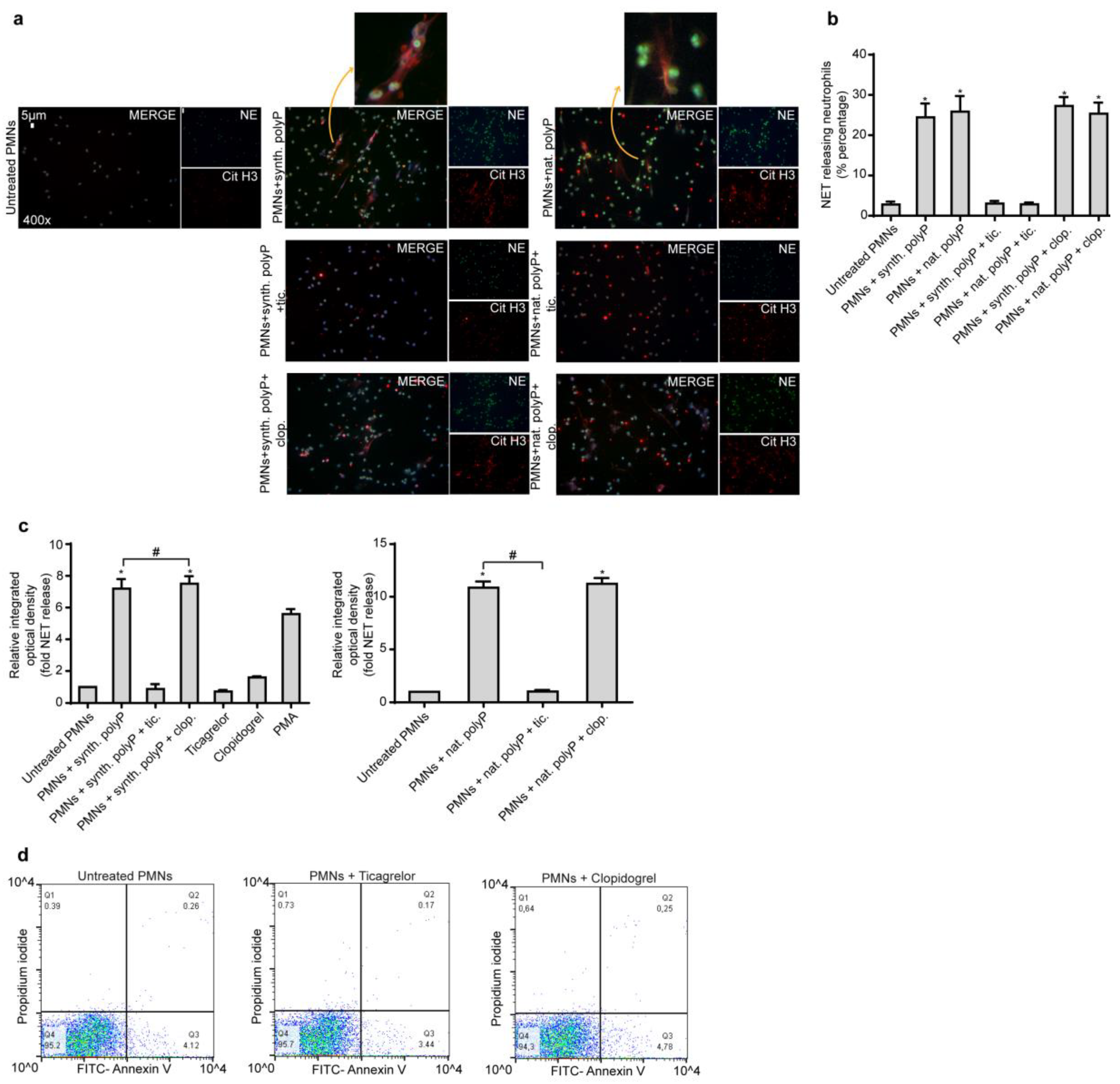
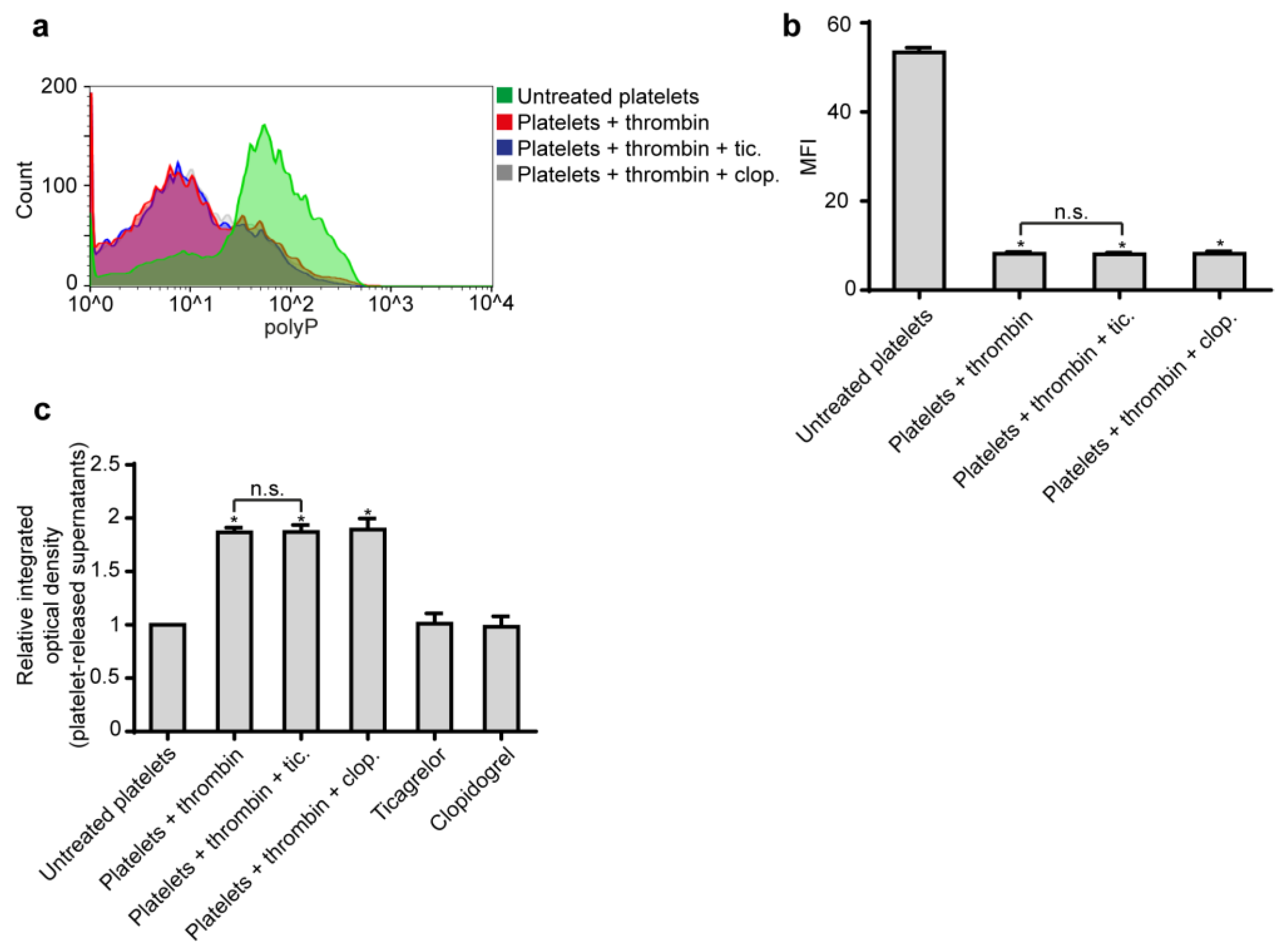
2.1. Ticagrelor Attenuates polyP-Induced NETs without Affecting polyP Secretion from Platelets
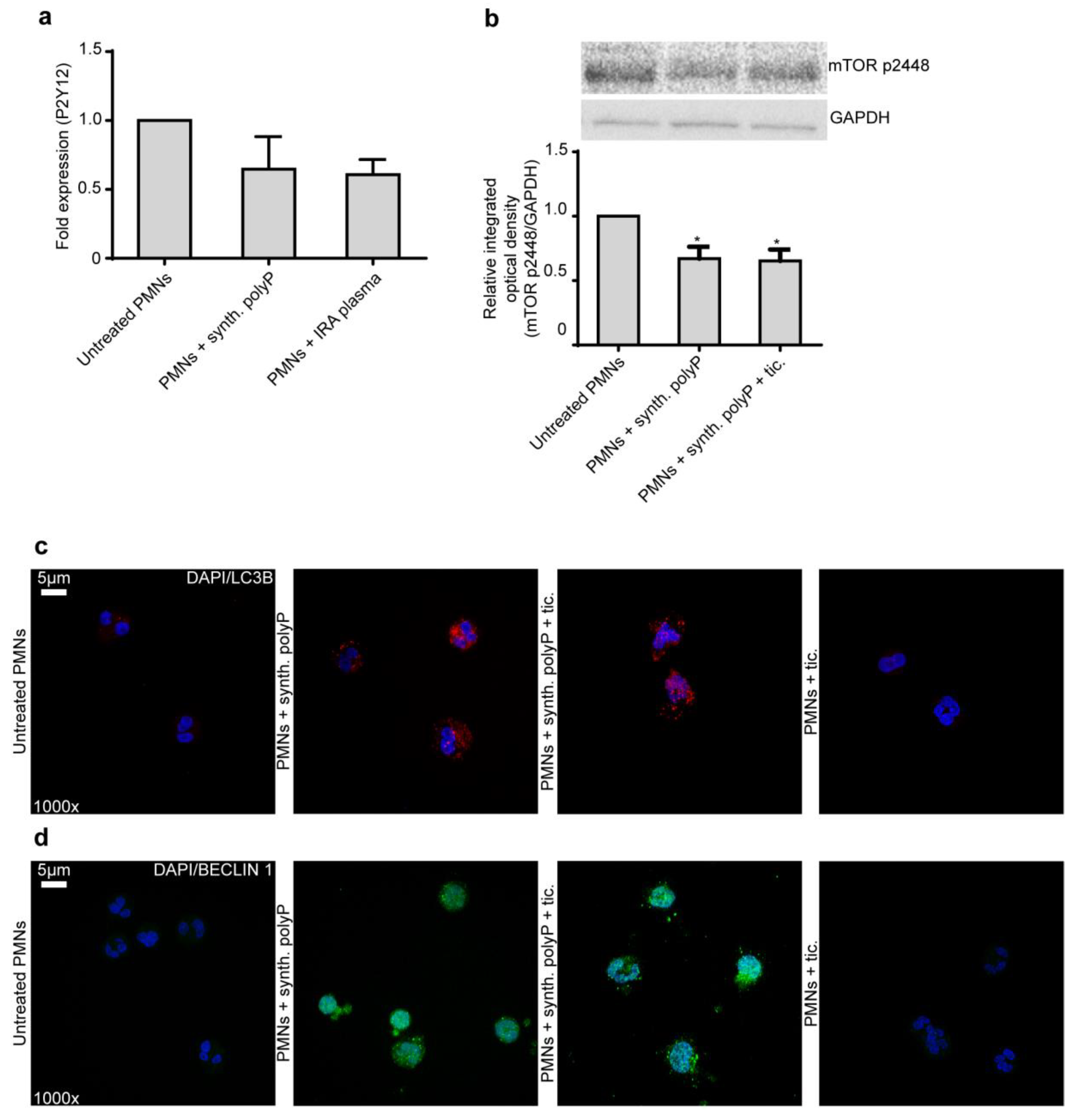
2.2. Ticagrelor Effect on Neutrophils Does not Rely on P2Y12 Receptor and Autophagy
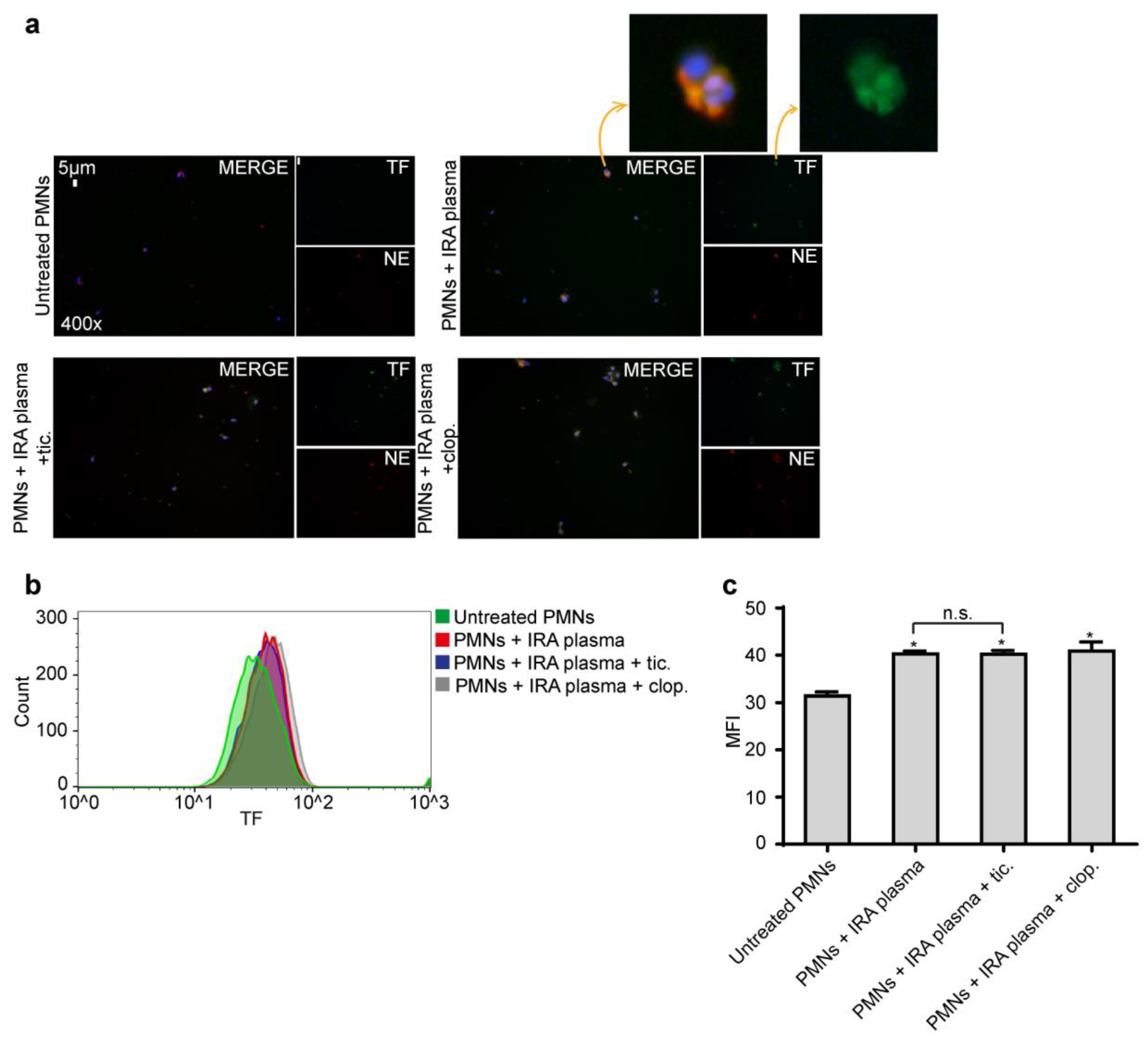
2.3. Ticagrelor Has no Effect on Intracellular Expression of TF
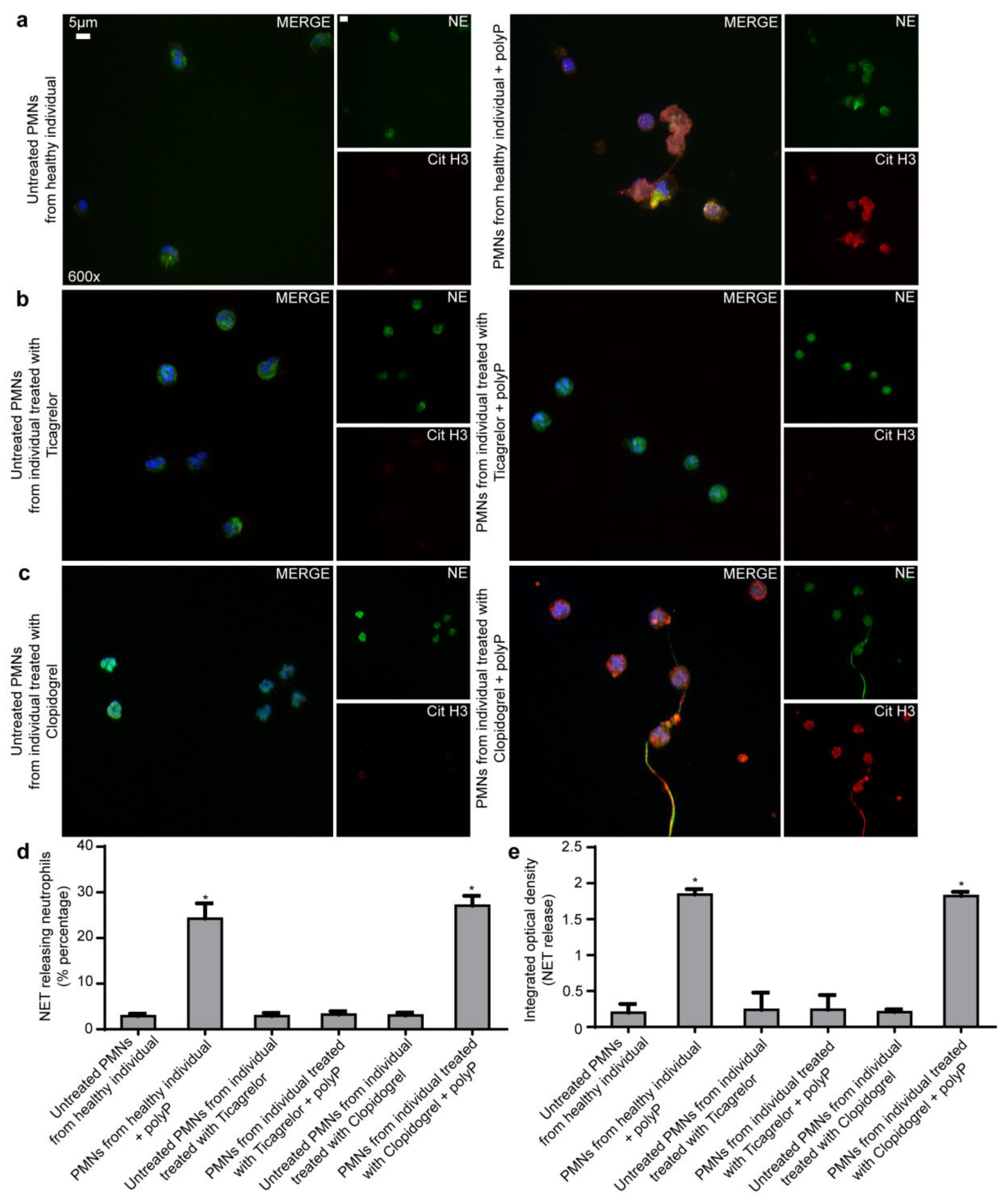
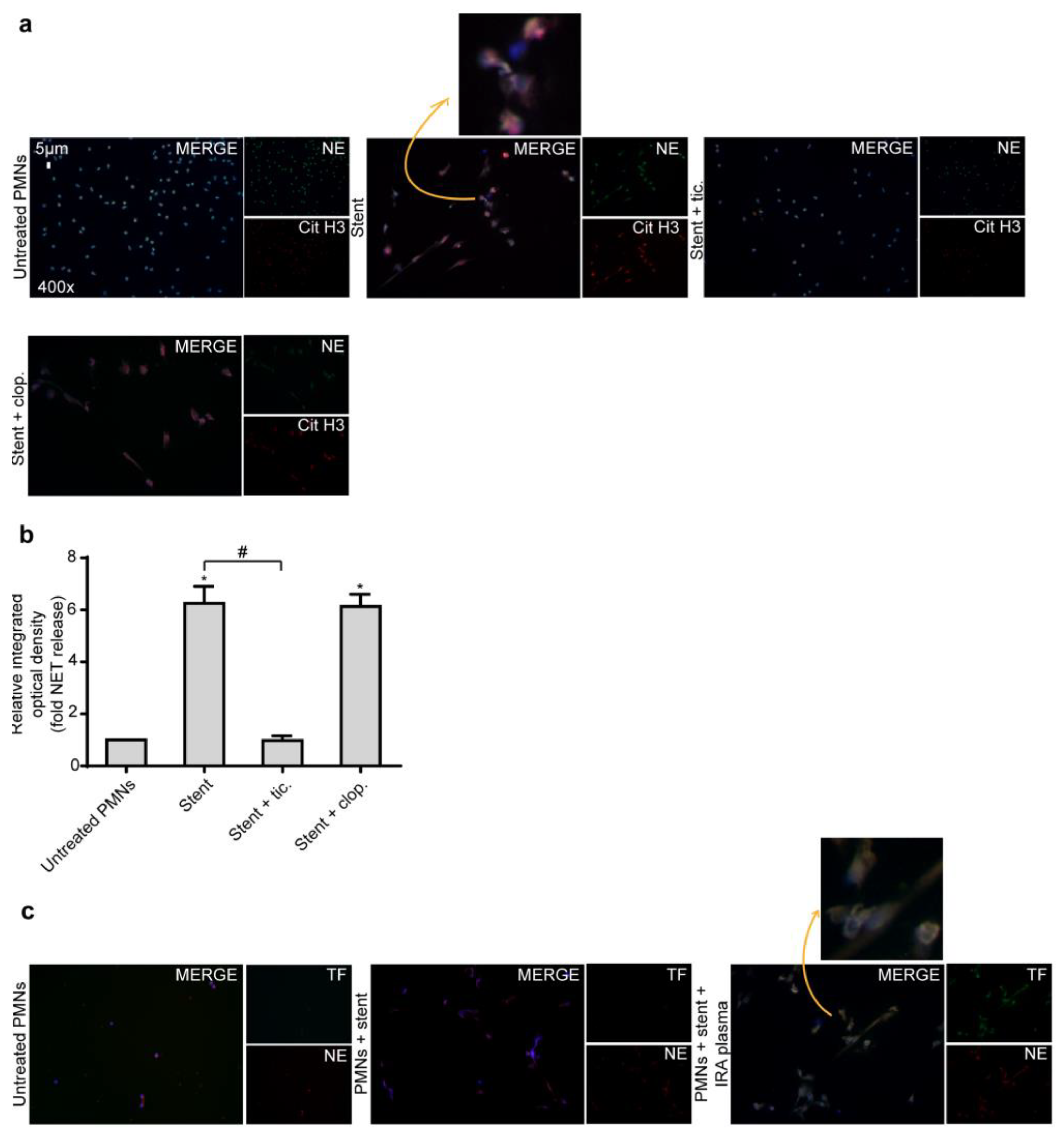
2.4. Ticagrelor Inhibits Thrombotic NET Release Induced by Drug Eluting Coronary Stents
3. Discussion
4. Material and Methods
4.1. Patients and Sample Collection
4.2. Stimulation and Inhibition Studies
4.3. PolyP Detection Using Fluorescent Probes
4.4. Other Experimental Procedures
4.4.1. Immunofluorescence Staining
4.4.2. NET Structures Isolation
4.4.3. MPO/DNA Complex ELISA
4.4.4. Flow Cytometry Analysis
4.4.5. RNA Isolation, cDNA Synthesis, and qRT-PCR
4.4.6. Western Blot Analysis
4.5. Statistical Analysis
4.6. Ethical Approval
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.B.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Investig. 2012, 122, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Kambas, K.; Stakos, D.; Mitroulis, I.; Mitsios, A.; Vidali, V.; Angelidou, I.; Bochenek, M.; Arelaki, S.; Arampatzioglou, A.; et al. Interferon lambda1/IL-29 and inorganic polyphosphate are novel regulators of neutrophil-driven thromboinflammation. J. Pathol. 2017, 243, 111–122. [Google Scholar] [CrossRef]
- Ruiz, F.A.; Lea, C.R.; Oldfield, E.; Docampo, R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J. Biol. Chem. 2004, 279, 44250–44257. [Google Scholar] [CrossRef]
- Müller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renné, T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef]
- Choi, S.H.; Smith, S.A.; Morrissey, J.H. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood 2011, 118, 6963–6970. [Google Scholar] [CrossRef]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Markiewski, M.M.; Doumas, M.; Rafail, S.; Kambas, K.; Mitroulis, I.; Panagoutsos, S.; Passadakis, P.; Vargemezis, V.; Magotti, P.; et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood 2010, 116, 631–639. [Google Scholar] [CrossRef]
- Ollivier, V.; Roques, C.; Receveur, N.; Gratz, M.; Feldman, L.; Letourneur, D.; Gachet, C.; Mangin, P.H.; Jandrot-Perrus, M. Bioreactivity of stent material: Activation of platelets, coagulation, leukocytes and endothelial cell dysfunction in vitro. Platelets 2017, 28, 529–539. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Neutrophil extracellular trap formation upon exposure of hydrophobic materials to human whole blood causes thrombogenic reactions. Biomater. Sci. 2017, 5, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Limeres, J.; Lip, G.Y.H.; del Blanco, B.G.; Ferreira-González, I.; Mutuberria, M.; Alfonso, F.; Bueno, H.; Cequier, A.; Prendergast, B.; Zueco, J.; et al. Safety of drug-eluting stents compared to bare metal stents in patients with an indication for long-term oral anticoagulation: A propensity score matched analysis. Thromb. Res. 2019, 177, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Werner, M. Drug eluting stents and modern stent technologies for in-stent restenosis. J. Cardiovasc. Surg. 2017, 58, 497–500. [Google Scholar] [CrossRef]
- Byrne, R.A.; Joner, M.; Kastrati, A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur. Heart J. 2015, 36, 3320–3331. [Google Scholar] [CrossRef]
- Olivier, C.B.; Diehl, P.; Schnabel, K.; Weik, P.; Zhou, Q.; Bode, C.; Moser, M. Third generation P2Y12 antagonists inhibit platelet aggregation more effectively than clopidogrel in a myocardial infarction registry. Thromb. Haemost. 2014, 111, 266–272. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. Effect of P2Y12 inhibitors on inflammation and immunity. Thromb. Haemost. 2015, 114, 490–497. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Kral, J.B.; Badrnya, S.; Assinger, A. Aspirin and P2Y12 Inhibitors in platelet-mediated activation of neutrophils and monocytes. Thromb. Haemost. 2015, 114, 478–489. [Google Scholar] [CrossRef]
- Varenhorst, C.; Alström, U.; Scirica, B.M.; Hogue, C.W.; Åsenblad, N.; Storey, R.F.; Steg, P.G.; Horrow, J.; Mahaffey, K.W.; Becker, R.C.; et al. Factors contributing to the lower mortality with ticagrelor compared with clopidogrel in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 2012, 60, 1623–1630. [Google Scholar] [CrossRef]
- Thomas, M.R.; Outteridge, S.N.; Ajjan, R.A.; Phoenix, F.; Sangha, G.K.; Faulkner, R.E.; Ecob, R.; Judge, H.M.; Khan, H.; West, L.E.; et al. Platelet P2Y12 inhibitors reduce systemic inflammation and its prothrombotic effects in an experimental human model. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2562–2570. [Google Scholar] [CrossRef]
- Rahman, M.; Gustafsson, D.; Wang, Y.; Thorlacius, H.; Braun, O.Ö. Ticagrelor reduces neutrophil recruitment and lung damage in abdominal sepsis. Platelets 2014, 25, 257–263. [Google Scholar] [CrossRef]
- Alsharif, K.F.; Thomas, M.R.; Judge, H.M.; Khan, H.; Prince, L.R.; Sabroe, I.; Ridger, V.C.; Storey, R.F. Ticagrelor potentiates adenosine-induced stimulation of neutrophil chemotaxis and phagocytosis. Vascul. Pharmacol. 2015, 71, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Liverani, E.; Rico, M.C.; Garcia, A.E.; Kilpatrick, L.E.; Kunapuli, S.P. Prasugrel metabolites inhibit neutrophil functions. J. Pharmacol. Exp. Ther. 2013, 344, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Byrne, R.A.; Joner, M.; Chandraratne, S.; Gershlick, A.H.; Ten Berg, J.M.; Adriaenssens, T.; Guagliumi, G.; Godschalk, T.C.; Neumann, F.-J.; et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: A report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur. Heart J. 2016, 37, 1538–1549. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. JTH 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Von Brühl, M.-L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Darbousset, R.; Thomas, G.M.; Mezouar, S.; Frère, C.; Bonier, R.; Mackman, N.; Renné, T.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 2012, 120, 2133–2143. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Massberg, S.; Grahl, L.; von Bruehl, M.-L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef]
- Ruf, W.; Ruggeri, Z.M. Neutrophils release brakes of coagulation. Nat. Med. 2010, 16, 851–852. [Google Scholar] [CrossRef]
- Jiménez-Alcázar, M.; Rangaswamy, C.; Panda, R.; Bitterling, J.; Simsek, Y.J.; Long, A.T.; Bilyy, R.; Krenn, V.; Renné, C.; Renné, T.; et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017, 358, 1202–1206. [Google Scholar] [CrossRef]
- Mitsios, A.; Arampatzioglou, A.; Arelaki, S.; Mitroulis, I.; Ritis, K. NETopathies? Unraveling the Dark Side of Old Diseases through Neutrophils. Front. Immunol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Storey, R.F.; James, S.K.; Siegbahn, A.; Varenhorst, C.; Held, C.; Ycas, J.; Husted, S.E.; Cannon, C.P.; Becker, R.C.; Steg, P.G.; et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets 2014, 25, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sexton, T.R.; Zhang, G.; Macaulay, T.E.; Callahan, L.A.; Charnigo, R.; Vsevolozhskaya, O.A.; Li, Z.; Smyth, S. Ticagrelor Reduces Thromboinflammatory Markers in Patients with Pneumonia. JACC Basic Transl. Sci. 2018, 3, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Musumeci, L.; Jacques, N.; Servais, L.; Goffin, E.; Pirotte, B.; Oury, C. Antibacterial Activity of Ticagrelor in Conventional Antiplatelet Dosages Against Antibiotic-Resistant Gram-Positive Bacteria. JAMA Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, C.; Varjú, I.; Sótonyi, P.; Szabó, L.; Krumrey, M.; Hoell, A.; Bóta, A.; Varga, Z.; Komorowicz, E.; Kolev, K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 2013, 288, 6946–6956. [Google Scholar] [CrossRef]
- Varjú, I.; Longstaff, C.; Szabó, L.; Farkas, Á.Z.; Varga-Szabó, V.J.; Tanka-Salamon, A.; Machovich, R.; Kolev, K. DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb. Haemost. 2015, 113, 1289–1298. [Google Scholar] [CrossRef]
- Rao, L.V.M.; Kothari, H.; Pendurthi, U.R. Tissue Factor encryption and decryption: Facts and controversies. Thromb. Res. 2012, 129, S13–S17. [Google Scholar] [CrossRef]
- Kambas, K.; Mitroulis, I.; Apostolidou, E.; Girod, A.; Chrysanthopoulou, A.; Pneumatikos, I.; Skendros, P.; Kourtzelis, I.; Koffa, M.; Kotsianidis, I.; et al. Autophagy Mediates the Delivery of Thrombogenic Tissue Factor to Neutrophil Extracellular Traps in Human Sepsis. PLoS ONE 2012, 7, e45427. [Google Scholar] [CrossRef]
- Kambas, K.; Markiewski, M.M.; Pneumatikos, I.A.; Rafail, S.S.; Theodorou, V.; Konstantonis, D.; Kourtzelis, I.; Doumas, M.N.; Magotti, P.; Deangelis, R.A.; et al. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J. Immunol. Baltim. Md 1950 2008, 180, 7368–7375. [Google Scholar]
- Boeltz, S.; Amini, P.; Anders, H.-J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kambas, K.; Chrysanthopoulou, A.; Skendros, P.; Apostolidou, E.; Kourtzelis, I.; Drosos, G.I.; Boumpas, D.T.; Ritis, K. Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS ONE 2011, 6, e29318. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Ding, Z. Role of P2Y12 Receptor in Thrombosis. Adv. Exp. Med. Biol. 2017, 906, 307–324. [Google Scholar] [CrossRef]
- Shishikura, K.; Horiuchi, T.; Sakata, N.; Trinh, D.; Shirakawa, R.; Kimura, T.; Asada, Y.; Horiuchi, H. Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br. J. Pharmacol. 2016, 173, 319–331. [Google Scholar] [CrossRef]
- Ali, R.A.; Gandhi, A.A.; Meng, H.; Yalavarthi, S.; Vreede, A.P.; Estes, S.K.; Palmer, O.R.; Bockenstedt, P.L.; Pinsky, D.J.; Greve, J.M.; et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Hahn, J.-Y.; Song, Y.B.; Oh, J.-H.; Chun, W.J.; Park, Y.H.; Jang, W.J.; Im, E.-S.; Jeong, J.-O.; Cho, B.R.; Oh, S.K.; et al. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA 2019, 321, 2428–2437. [Google Scholar] [CrossRef]
- Syberg, S.; Brandao-Burch, A.; Patel, J.J.; Hajjawi, M.; Arnett, T.R.; Schwarz, P.; Jorgensen, N.R.; Orriss, I.R. Clopidogrel (Plavix), a P2Y12 receptor antagonist, inhibits bone cell function in vitro and decreases trabecular bone in vivo. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 2373–2386. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, F.; Asztély, A.-K.; Jeppsson, A.; Nylander, S.; Berggren, A.; Nelander, K.; Castellheim, A.; Romlin, B.S. In vitro anti-platelet potency of ticagrelor in blood samples from infants and children. Thromb. Res. 2015, 136, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.A.; Reimann, S.; Schrör, K. Specific inhibition of ADP-induced platelet aggregation by clopidogrel in vitro. Br. J. Pharmacol. 1999, 126, 415–420. [Google Scholar] [CrossRef]
- Calmette, L.; Martin, A.-C.; Le Bonniec, B.; Zlotnik, D.; Gouin-Thibault, I.; Bachelot-Loza, C.; Gaussem, P.; Godier, A. Ticagrelor reversal: In vitro assessment of four haemostatic agents. J. Clin. Pathol. 2017, 70, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Agrawalla, B.K.; Elustondo, P.A.; Gordon, J.; Shiba, T.; Abramov, A.Y.; Chang, Y.-T.; Pavlov, E.V. In situ investigation of mammalian inorganic polyphosphate localization using novel selective fluorescent probes JC-D7 and JC-D8. ACS Chem. Biol. 2014, 9, 2101–2110. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef]
- Moreno-Sanchez, D.; Hernandez-Ruiz, L.; Ruiz, F.A.; Docampo, R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J. Biol. Chem. 2012, 287, 28435–28444. [Google Scholar] [CrossRef]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef]
- Konstantinidis, T.; Kambas, K.; Mitsios, A.; Panopoulou, M.; Tsironidou, V.; Dellaporta, E.; Kouklakis, G.; Arampatzioglou, A.; Angelidou, I.; Mitroulis, I.; et al. Immunomodulatory Role of Clarithromycin in Acinetobacter baumannii Infection via Formation of Neutrophil Extracellular Traps. Antimicrob. Agents Chemother. 2016, 60, 1040–1048. [Google Scholar] [CrossRef]
- Jimenez-Nuñez, M.D.; Moreno-Sanchez, D.; Hernandez-Ruiz, L.; Benítez-Rondán, A.; Ramos-Amaya, A.; Rodríguez-Bayona, B.; Medina, F.; Brieva, J.A.; Ruiz, F.A. Myeloma cells contain high levels of inorganic polyphosphate which is associated with nucleolar transcription. Haematologica 2012, 97, 1264–1271. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsios, A.; Chrysanthopoulou, A.; Arampatzioglou, A.; Angelidou, I.; Vidali, V.; Ritis, K.; Skendros, P.; Stakos, D. Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps. Int. J. Mol. Sci. 2020, 21, 3625. https://doi.org/10.3390/ijms21103625
Mitsios A, Chrysanthopoulou A, Arampatzioglou A, Angelidou I, Vidali V, Ritis K, Skendros P, Stakos D. Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps. International Journal of Molecular Sciences. 2020; 21(10):3625. https://doi.org/10.3390/ijms21103625
Chicago/Turabian StyleMitsios, Alexandros, Akrivi Chrysanthopoulou, Athanasios Arampatzioglou, Iliana Angelidou, Veroniki Vidali, Konstantinos Ritis, Panagiotis Skendros, and Dimitrios Stakos. 2020. "Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps" International Journal of Molecular Sciences 21, no. 10: 3625. https://doi.org/10.3390/ijms21103625
APA StyleMitsios, A., Chrysanthopoulou, A., Arampatzioglou, A., Angelidou, I., Vidali, V., Ritis, K., Skendros, P., & Stakos, D. (2020). Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps. International Journal of Molecular Sciences, 21(10), 3625. https://doi.org/10.3390/ijms21103625




