Methanol-Essential Growth of Corynebacterium glutamicum: Adaptive Laboratory Evolution Overcomes Limitation due to Methanethiol Assimilation Pathway
Abstract
1. Introduction
2. Results and Discussion
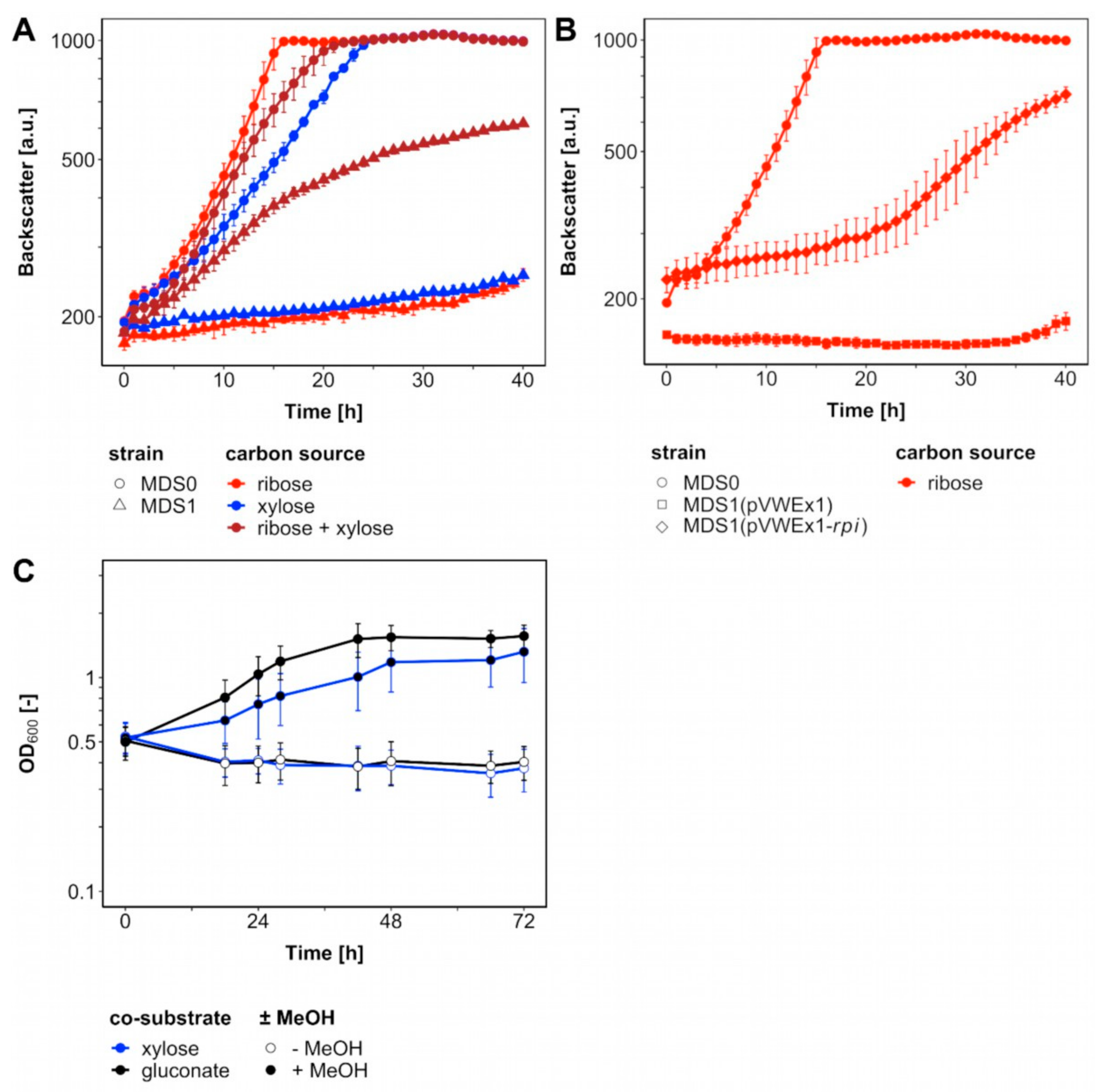
2.1. Methanol-Dependent Complementation of a Ribose 5-Phosphate Isomerase Mutant
2.2. Adaptive Laboratory Evolution for Accelerated Methanol-Dependent Complementation of a Ribulose 5-Phosphate Epimerase Mutant
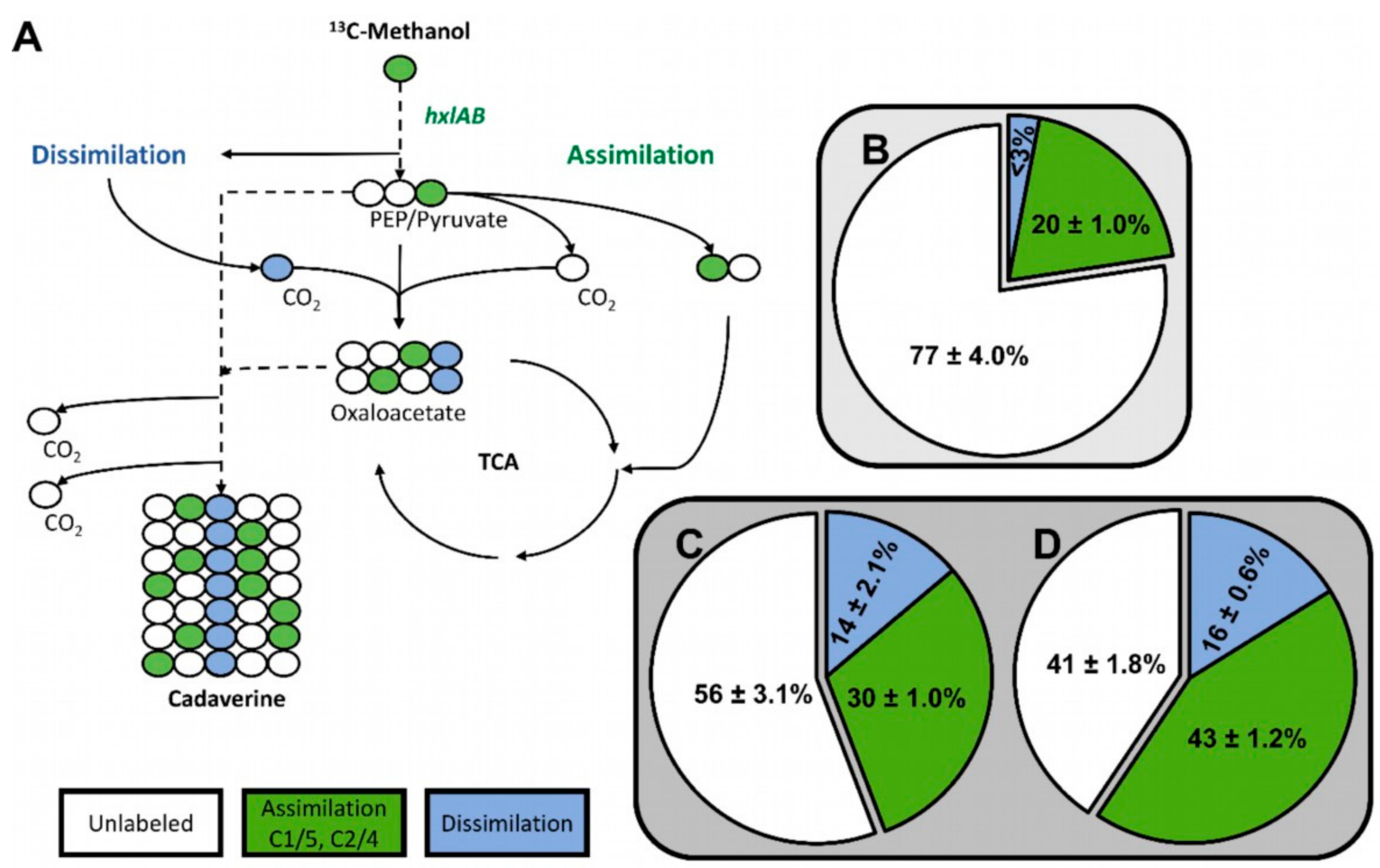
2.3. Genome Sequencing of ALE Strains Revealed Candidate Mutations that May Accelerate Methanol-Dependent Biomass Formation
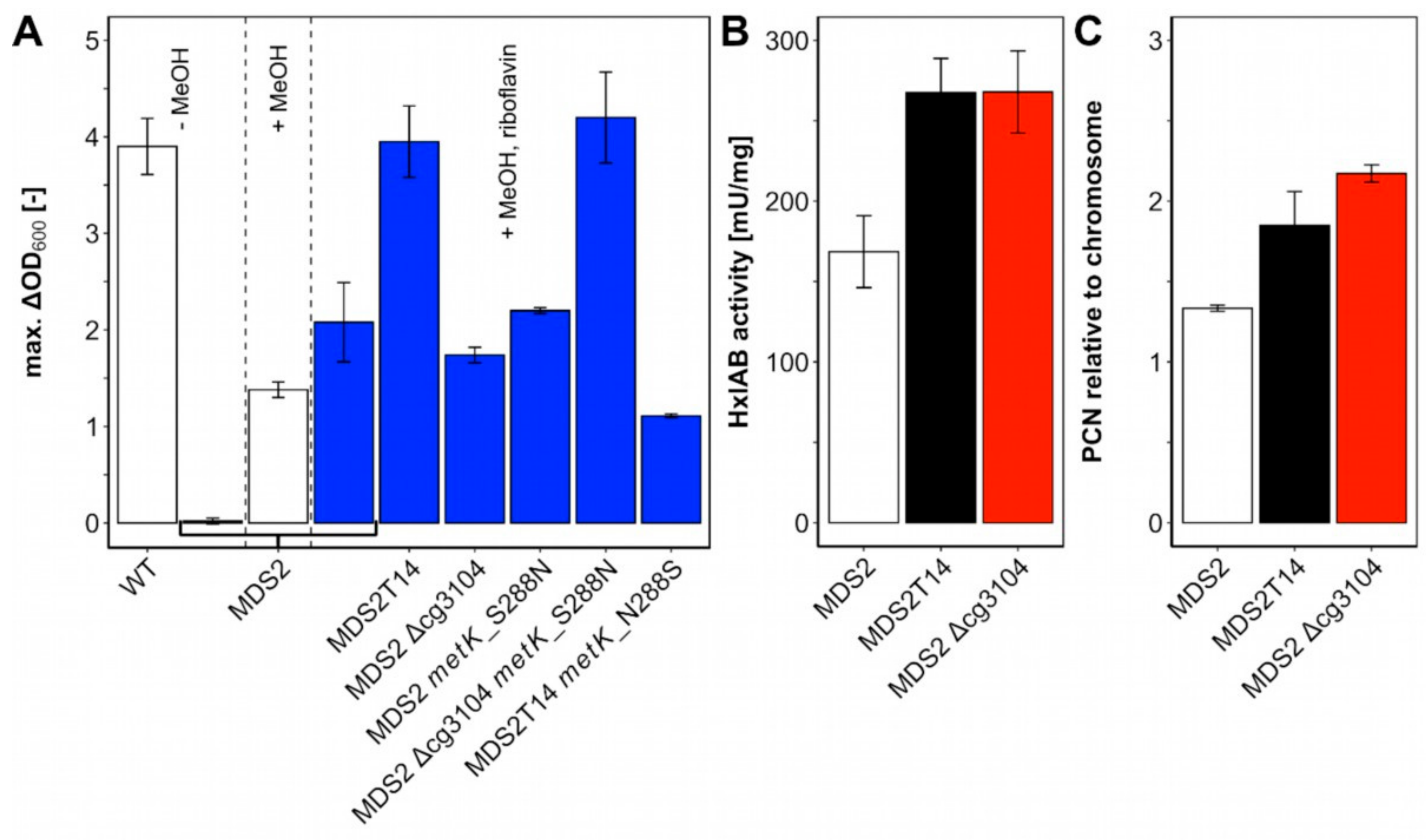
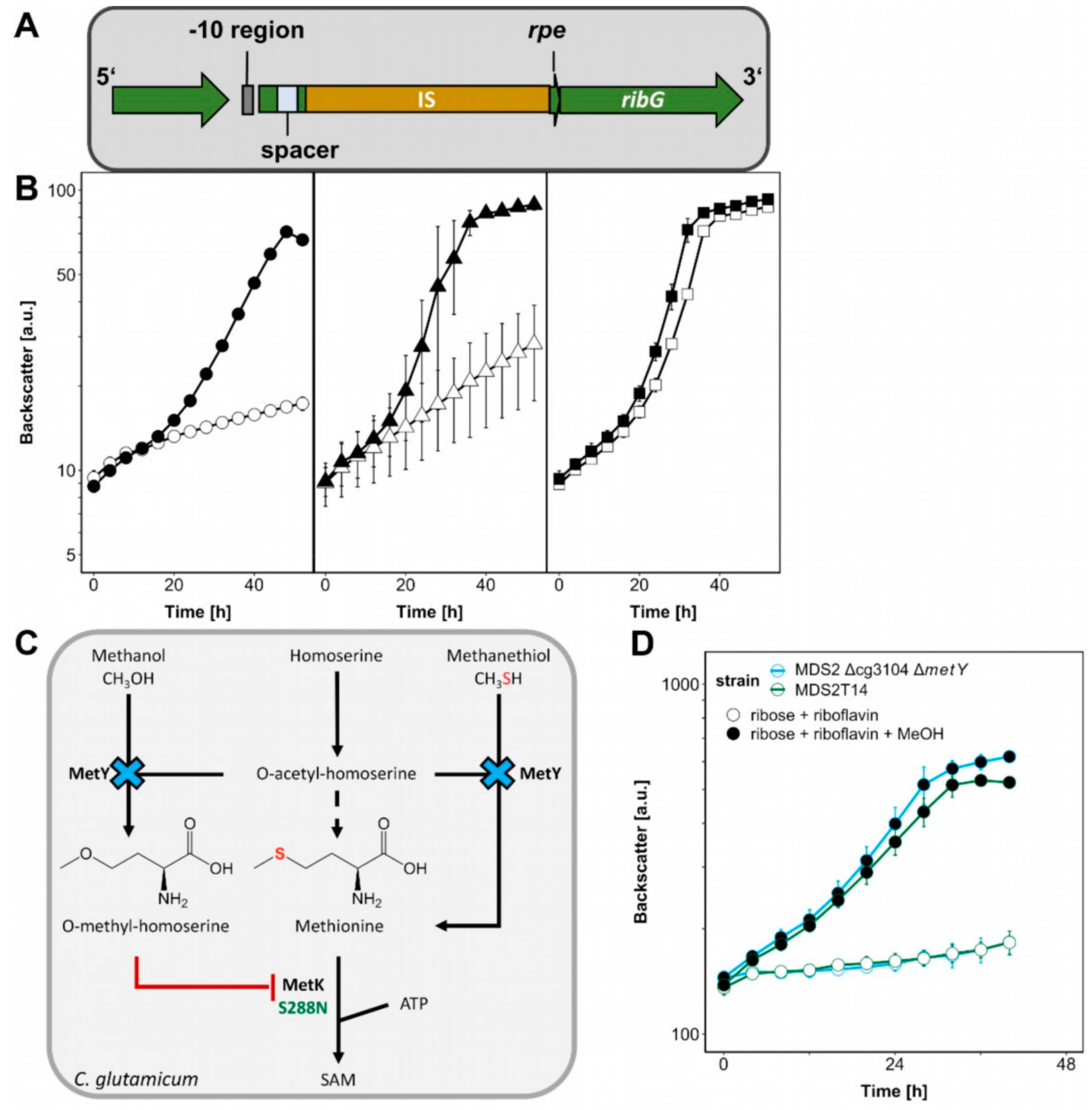
2.4. Identification of Mutations Causal for Methanol-Essential Growth
3. Materials and Methods
3.1. Microorganisms and Cultivation Conditions
3.2. ALE Experiments
3.3. Molecular Biology Methods
3.4. Coupled in vitro Activity of HxlA and HxlB
3.5. Whole-Genome Sequencing
3.6. Quantitative PCR
3.7. Quantification of 13C-Enrichment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olah, G.A. Towards oil independence through renewable methanol chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Waterhouse, G.I.N.; Shi, R.; Zhao, J.; Li, Z.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. From Solar Energy to Fuels: Recent Advances in Light-Driven C1 Chemistry. Angew. Chem. Int. Ed. 2019, 58, 17528–17551. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, R.S.; Lade, H.; Paul, D. Methylotrophic bacteria in sustainable agriculture. World J. Microbiol. Biotechnol. 2016, 32, 120. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.E.N.; Heggeset, T.M.B.; Wendisch, V.F.; Vorholt, J.A.; Brautaset, T. Methylotrophy in the thermophilic Bacillus methanolicus, basic insights and application for commodity production from methanol. Appl. Microbiol. Biotechnol. 2015, 99, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, A.M.; Sonntag, F.; Buchhaupt, M.; Schrader, J.; Vorholt, J.A. Methylobacterium extorquens: Methylotrophy and biotechnological applications. Appl. Microbiol. Biotechnol. 2014, 99, 517–534. [Google Scholar] [CrossRef]
- Erb, T.J.; Berg, I.A.; Brecht, V.; Muller, M.; Fuchs, G.; Alber, B.E. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: The ethylmalonyl-CoA pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 10631–10636. [Google Scholar] [CrossRef]
- Anthony, C. Methanol oxidation and growth yields in methylotrophic bacteria: A review. Acta Biotechnol. 1983, 3, 261–268. [Google Scholar] [CrossRef]
- Cotton, C.A.; Claassens, N.J.; Benito-Vaquerizo, S.; Bar-Even, A. Renewable methanol and formate as microbial feedstocks. Curr. Opin. Biotechnol. 2020, 62, 168–180. [Google Scholar] [CrossRef]
- Pfeifenschneider, J.; Brautaset, T.; Wendisch, V.F. Methanol as carbon substrate in the bio-economy: Metabolic engineering of aerobic methylotrophic bacteria for production of value-added chemicals. Biofuels Bioprod. Biorefin. 2017, 11, 719–731. [Google Scholar] [CrossRef]
- Brautaset, T.; Jakobsen, Ø.M.; Degnes, K.F.; Netzer, R.; Nærdal, I.; Krog, A.; Dillingham, R.; Flickinger, M.C.; Ellingsen, T.E. Bacillus methanolicus pyruvate carboxylase and homoserine dehydrogenase I and II and their roles for L-lysine production from methanol at 50 °C. Appl. Microbiol. Biotechnol. 2010, 87, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Drejer, E.B.; Chan, D.T.C.; Haupka, C.; Wendisch, V.F.; Brautaset, T.; Irla, M. Methanol-based acetoin production by genetically engineered Bacillus methanolicus. Green Chem. 2020. [Google Scholar] [CrossRef]
- Irla, M.; Nærdal, I.; Brautaset, T.; Wendisch, V.F. Methanol-based γ-aminobutyric acid (GABA) production by genetically engineered Bacillus methanolicus strains. Ind. Crops Prod. 2017, 106, 12–20. [Google Scholar] [CrossRef]
- Nærdal, I.; Pfeifenschneider, J.; Brautaset, T.; Wendisch, V.F. Methanol-based cadaverine production by genetically engineered Bacillus methanolicus strains. Microb. Biotechnol. 2015, 8, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, M.; Vieira, G.; Brautaset, T.; Portais, J.C.; Heux, S. Quantitative metabolomics of the thermophilic methylotroph Bacillus methanolicus. Microb. Cell Factories 2016, 15, s12934-s016. [Google Scholar] [CrossRef] [PubMed]
- Nærdal, I.; Netzer, R.; Irla, M.; Krog, A.; Heggeset, T.M.B.; Wendisch, V.F.; Brautaset, T. L-lysine production by Bacillus methanolicus: Genome-based mutational analysis and L-lysine secretion engineering. J. Biotech. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schultenkämper, K.; Brito, L.F.; López, M.G.; Brautaset, T.; Wendisch, V.F. Establishment and application of CRISPR interference to affect sporulation, hydrogen peroxide detoxification, and mannitol catabolism in the methylotrophic thermophile Bacillus methanolicus. Appl. Microbiol. Biotechnol. 2019, 103, 5879–5889. [Google Scholar] [CrossRef]
- Irla, M.; Heggeset, T.M.B.; Nærdal, I.; Paul, L.; Haugen, T.; Le, S.B.; Brautaset, T.; Wendisch, V.F. Genome-based genetic tool development for Bacillus methanolicus: Theta-and rolling circle-replicating plasmids for inducible gene expression and application to methanol-based cadaverine production. Front. Microbiol. 2016, 7, 1481. [Google Scholar] [CrossRef]
- Zhang, W.; Song, M.; Yang, Q.; Dai, Z.; Zhang, S.; Xin, F.; Dong, W.; Ma, J.; Jiang, M. Current advance in bioconversion of methanol to chemicals. Biotechnol. Biofuels 2018, 11, 260-260. [Google Scholar] [CrossRef]
- Leßmeier, L.; Pfeifenschneider, J.; Carnicer, M.; Heux, S.; Portais, J.C.; Wendisch, V.F. Production of carbon-13-labeled cadaverine by engineered Corynebacterium glutamicum using carbon-13-labeled methanol as co-substrate. Appl. Microbiol. Biotechnol. 2015, 99, 10163–10176. [Google Scholar] [CrossRef]
- Müller, J.E.N.; Meyer, F.; Litsanov, B.; Kiefer, P.; Potthoff, E.; Heux, S.; Quax, W.J.; Wendisch, V.F.; Brautaset, T.; Portais, J.C.; et al. Engineering Escherichia coli for methanol conversion. Metab. Eng. 2015, 28, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Witthoff, S.; Schmitz, K.; Niedenführ, S.; Nöh, K.; Noack, S.; Bott, M.; Marienhagen, J. Metabolic engineering of Corynebacterium glutamicum for methanol metabolism. Appl. Environ. Microbiol. 2015, 81, 2215–2225. [Google Scholar] [CrossRef]
- Chen, C.-T.; Chen, F.Y.-H.; Bogorad, I.W.; Wu, T.-Y.; Zhang, R.; Lee, A.S.; Liao, J.C. Synthetic methanol auxotrophy of Escherichia coli for methanol-dependent growth and production. Metab. Eng. 2018, 49, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Keller, P.; Hartl, J.; Gröninger, O.G.; Kiefer, P.; Vorholt, J.A. Methanol-essential growth of Escherichia coli. Nat. Commun. 2018, 9, 1508-1508. [Google Scholar] [CrossRef] [PubMed]
- Tuyishime, P.; Wang, Y.; Fan, L.; Zhang, Q.; Li, Q.; Zheng, P.; Sun, J.; Ma, Y. Engineering Corynebacterium glutamicum for methanol-dependent growth and glutamate production. Metab. Eng. 2018, 49, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lindner, S.N.; Aslan, S.; Yishai, O.; Wenk, S.; Schann, K.; Bar-Even, A. Growth of E. coli on formate and methanol via the reductive glycine pathway. Nat. Chem. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Irla, M.; Neshat, A.; Winkler, A.; Albersmeier, A.; Heggeset, T.M.B.; Brautaset, T.; Kalinowski, J.; Wendisch, V.F.; Rückert, C. Complete genome sequence of Bacillus methanolicus MGA3, a thermotolerant amino acid producing methylotroph. J. Biotechnol. 2014, 188, 110–111. [Google Scholar] [CrossRef]
- Frunzke, J.; Gätgens, C.; Brocker, M.; Bott, M. Control of heme homeostasis in Corynebacterium glutamicum by the two-component system HrrSA. J. Bacteriol. 2011, 193, 1212–1221. [Google Scholar] [CrossRef]
- Connelly, J.C.; de Leau, E.S.; Okely, E.A.; Leach, D.R.F. Overexpression, Purification, and Characterization of the SbcCD protein from Escherichia coli. J. Biol. Chem. 1997, 272, 19819–19826. [Google Scholar] [CrossRef]
- Pan, X.; Leach, D.R.F. The roles of mutS, sbcCD and recA in the propagation of TGG repeats in Escherichia coli. Nucleic Acids Res. 2000, 28, 3178–3184. [Google Scholar] [CrossRef][Green Version]
- Kalinowski, J.; Bathe, B.; Bartels, D.; Bischoff, N.; Bott, M.; Burkovski, A.; Dusch, N.; Eggeling, L.; Eikmanns, B.J.; Gaigalat, L.; et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 2003, 104, 5–25. [Google Scholar] [CrossRef]
- Takemoto, N.; Tanaka, Y.; Inui, M.; Yukawa, H. The physiological role of riboflavin transporter and involvement of FMN-riboswitch in its gene expression in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2014, 98, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer-Sancar, K.; Mentz, A.; Rückert, C.; Kalinowski, J. Comprehensive analysis of the Corynebacterium glutamicum transcriptome using an improved RNAseq technique. BMC Genom. 2013, 14, 888. [Google Scholar] [CrossRef] [PubMed]
- Vogl, C.; Grill, S.; Schilling, O.; Stulke, J.; Mack, M.; Stolz, J. Characterization of riboflavin (vitamin B2) transport proteins from Bacillus subtilis and Corynebacterium glutamicum. J. Bacteriol. 2007, 189, 7367–7375. [Google Scholar] [CrossRef] [PubMed]
- Price, J.V.; Chen, L.; Whitaker, W.B.; Papoutsakis, E.T.; Chen, W. Scaffoldless engineered enzyme assembly for enhanced methanol utilization. Proc. Natl. Acad. Sci. USA 2016, 113, 12691–12696. [Google Scholar] [CrossRef] [PubMed]
- Woolston, B.M.; King, J.R.; Reiter, M.; Van Hove, B.; Stephanopoulos, G. Improving formaldehyde consumption drives methanol assimilation in engineered E. coli. Nat. Commun. 2018, 9, 2387-2387. [Google Scholar] [CrossRef]
- Sekowska, A.; Kung, H.-F.; Danchin, A. Sulfur Metabolism in Escherichia coli and Related Bacteria: Facts and Fiction. J. Microb. Biotechnol. 2000, 2, 145–177. [Google Scholar]
- Großmann, K.; Herbster, K.; Mack, M. Rapid cloning of metK encoding methionine adenosyltransferase from Corynebacterium glutamicum by screening a genomic library on a high density colony-array. FEMS Microbiol. Lett. 2000, 193, 99–103. [Google Scholar] [CrossRef]
- Roblin, R.O.; Lampen, J.O.; English, J.P.; Cole, Q.P.; Vaughan, J.R. Studies in Chemotherapy. VIII. Methionine and Purine Antagonists and their Relation to the Sulfonamides. J. Am. Chem. Soc. 1945, 67, 290–294. [Google Scholar] [CrossRef]
- Murooka, Y.; Kakihara, K.; Miwa, T.; Seto, K.; Harada, T. O-alkylhomoserine synthesis catalyzed by O-acetylhomoserine sulfhydrylase in microorganisms. J. Bacteriol. 1977, 130, 62–73. [Google Scholar] [CrossRef]
- Leßmeier, L.; Hoefener, M.; Wendisch, V.F. Formaldehyde degradation in Corynebacterium glutamicum involves acetaldehyde dehydrogenase and mycothiol-dependent formaldehyde dehydrogenase. Microbiology 2013, 159, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, L.; Tuyishime, P.; Liu, J.; Zhang, K.; Gao, N.; Zhang, Z.; Ni, X.; Feng, J.; Yuan, Q.; et al. Adaptive laboratory evolution enhances methanol tolerance and conversion in engineered Corynebacterium glutamicum. Commun. Biol. 2020, 3, 217. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Techniques for Transformation of E. coli. DNA Cloning: A Practical Approach; Glover, D.M., Ed.; IRL Press: Oxford, UK, 1985; Volume 1, pp. 109–135. [Google Scholar]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Eggeling, L.; Bott, M. Experiments. In Handbook of Corynebacterium glutamicum; Eggeling, L., Bott, M., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 530–531, pp. 421–422. ISBN 0-8493-1821-1. [Google Scholar]
- Simon, R.; Priefer, U.; Pühler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in Gram negative bacteria. Bio/Technology 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Kirchner, O.; Tauch, A. Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J. Biotechnol. 2003, 104, 287–299. [Google Scholar] [CrossRef]
- Stansen, C.; Uy, D.; Delaunay, S.; Eggeling, L.; Goergen, J.L.; Wendisch, V.F. Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl. Environ. Microbiol. 2005, 71, 5920–5928. [Google Scholar] [CrossRef]
- Meiswinkel, T.M.; Gopinath, V.; Lindner, S.N.; Nampoothiri, K.M.; Wendisch, V.F. Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microb. Biotechnol. 2013, 6, 131–140. [Google Scholar] [CrossRef]
- Peters-Wendisch, P.; Schiel, B.; Wendisch, V.F.; Katsoulidis, E.; Möckel, B.; Sahm, H.; Eikmanns, B.J. Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 2001, 3, 295–300. [Google Scholar]
- Eikmanns, B.J.; Thum-Schmitz, N.; Eggeling, L.; Lüdtke, K.U.; Sahm, H. Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 1994, 140, 1817–1828. [Google Scholar] [CrossRef]
- López, M.G.; Irla, M.; Brito, L.F.; Wendisch, V.F. Characterization of D-Arabitol as newly discovered carbon source of Bacillus methanolicus. Front. Microbiol. 2019, 10, 1725. [Google Scholar] [CrossRef]
- Kibret, M.; Guerrero-Garzón, J.F.; Urban, E.; Zehl, M.; Wronski, V.-K.; Rückert, C.; Busche, T.; Kalinowski, J.; Rollinger, J.M.; Abate, D.; et al. Streptomyces spp. from Ethiopia producing antimicrobial compounds: Characterization via bioassays, genome analyses, and mass spectrometry. Front. Microbiol. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]

| Locus | Gene Name | Strain | Gene Product | Mutation/Effect |
|---|---|---|---|---|
| cg0387 | fadH | MDS0 | mycothiol-dependent formaldehyde dehydrogenase | V9F |
| cg0414 | wzz | MDS0 | cell surface polysaccharide biosynthesis/chain length determinant protein | E363D |
| cg0822 | - | MDS0 | unknown | V23A |
| cg1245 | - | MDS1 | putative membrane protein | L328S |
| cg2204 | hrtA | MDS1 | ABC-type transport system, involved in lipoprotein release, ATPase component | D67H |
| cg3096 | ald | MDS1 | acetaldehyde dehydrogenase | T2S |
| cg3096 | ald | MDS1 | acetaldehyde dehydrogenase | L494M |
| cg3104 | - | MDS2T8 | putative ATPase involved in DNA repair | 15 bp deletion within coding sequence (CDS) |
| cg1806 | metK | MDS2T8 | S-adenosylmethionine synthetase | S288N |
| cg1929 | res | MDS2T8 | Site-specific recombinase | R91H |
| cg1801 | rpe | MDS2T14 | Ribulose-5-phosphate epimerase | Insertion of a 1.4 kb transposon |
| Strain | Relevant Characteristics | Reference |
|---|---|---|
| E. coli | ||
| DH5α | ∆lacU169 (φ80lacZ ∆M15), supE44, hsdR17, recA1, endA1, gyrA96, thi-1, relA1 | [43] |
| S17-1 | recA, pro, hsdR, RP4- 2Tc∷Mu Km∷Tn7 integrated into the chromosome | [46] |
| C. glutamicum | ||
| WT | ATCC13032 | ATCC |
| MDS0 | ATCC13032 Δald ΔfadH | [41] |
| MDS1 | ATCC13032 Δald ΔfadH Δrpi (pEKEx3-xylAB) | This study |
| MDS2 | ATCC13032 Δald ΔfadH Δrpe (pEKEx3-mdh-hxlAB) | This study |
| MDS2T8 | strain evolved from MDS2; after 8 transfers | This study |
| MDS2T14 | strain evolved from MDS2; after 14 transfers | This study |
| MDS2 Δcg3104 | MDS2 with deletion Δcg3104 | This study |
| MDS2 Δcg3104 ΔmetY | MDS2 Δcg3104 with deletion ΔmetY | This study |
| MDS2 metK_S288N | MDS2 with SNP in metK resulting in amino acid exchange S288N | This study |
| MDS2 Δcg3104 metK_S288N | MDS2 with deletion Δcg3104 and SNP in metK, resulting in amino acid exchange S288N | This study |
| MDS2T14 metK_N288S | MDS2T14 with reversion of metK to the WT sequence | This study |
| Plasmid | Relevant Characteristics | Reference |
|---|---|---|
| pEC-XT99A | TetR, C. glutamicum/E. coli shuttle vector (Ptrc lacIq, pGA1, oriVE.c.) | [47] |
| pEC-XT99A-mdh-hxlAB | pEC-XT99A expressing mdh from B. methanolicus and hxlA and hxlB from B. subtilis | This study |
| pEC-XT99A-lysCfbr-ldcC | pEC-XT99A expressing feedback-resistant lysC from C. glutamicum and ldcC from E. coli | This study |
| pEKEx3 | SpecR, C. glutamicum/E. coli shuttle vector (Ptac lacIq pBL1, oriVEc) | [48] |
| pEKEx3-mdh-hxlAB | pEKEx3, expressing mdh from B. methanolicus and hxlA and hxlB from B. subtilis | [20] |
| pEKEx3-xylAB | pEKEx3, expressing xylA from X. campestris and xylB from C. glutamicum | [49] |
| pK19mobsacB | KanR, mobilizable E. coli vector mutagenesis (oriV, sacB) | [44] |
| pK19mobsacB-∆rpi | pK19mobsacB with a deletion construct rpi | This study |
| pK19mobsacB-∆rpe | pK19mobsacB with a deletion construct rpe | This study |
| pK19mobsacB-Δcg3104 | pK19mobsacB with a deletion construct cg3104 | This study |
| pK19mobsacB-metK_S288N | pK19mobsacB with an amino acid exchange metK_S288N from C. glutamicum | This study |
| pK19mobsacB-metK_N288S | pK19mobsacB with WT metK for reversion of amino acid exchange S288N in MDS2T14 | This study |
| pVWEx1 | KanR, C. glutamicum/E. coli shuttle vector (Ptac, lacIq) | [50] |
| pVWEx1-mdh-hxlAB | pVWEx1 expressing mdh from B. methanolicus and hxlA and hxlB from B. subtilis | This study |
| pVWEx1-rpi | pVWEx1 expressing rpi from C. glutamicum | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennig, G.; Haupka, C.; Brito, L.F.; Rückert, C.; Cahoreau, E.; Heux, S.; Wendisch, V.F. Methanol-Essential Growth of Corynebacterium glutamicum: Adaptive Laboratory Evolution Overcomes Limitation due to Methanethiol Assimilation Pathway. Int. J. Mol. Sci. 2020, 21, 3617. https://doi.org/10.3390/ijms21103617
Hennig G, Haupka C, Brito LF, Rückert C, Cahoreau E, Heux S, Wendisch VF. Methanol-Essential Growth of Corynebacterium glutamicum: Adaptive Laboratory Evolution Overcomes Limitation due to Methanethiol Assimilation Pathway. International Journal of Molecular Sciences. 2020; 21(10):3617. https://doi.org/10.3390/ijms21103617
Chicago/Turabian StyleHennig, Guido, Carsten Haupka, Luciana F. Brito, Christian Rückert, Edern Cahoreau, Stéphanie Heux, and Volker F. Wendisch. 2020. "Methanol-Essential Growth of Corynebacterium glutamicum: Adaptive Laboratory Evolution Overcomes Limitation due to Methanethiol Assimilation Pathway" International Journal of Molecular Sciences 21, no. 10: 3617. https://doi.org/10.3390/ijms21103617
APA StyleHennig, G., Haupka, C., Brito, L. F., Rückert, C., Cahoreau, E., Heux, S., & Wendisch, V. F. (2020). Methanol-Essential Growth of Corynebacterium glutamicum: Adaptive Laboratory Evolution Overcomes Limitation due to Methanethiol Assimilation Pathway. International Journal of Molecular Sciences, 21(10), 3617. https://doi.org/10.3390/ijms21103617







