Decreased Blood Level of MFSD2a as a Potential Biomarker of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics of Subjects
2.2. Reduced Level of Blood MFSD2a in Alzheimer’s Disease Patients and Impact on Fatty Acid Profile in Serum
2.3. Levels of MFSD2a Expression in Brain Stay Unaltered in Alzheimer’s Disease Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Western Blotting Analyses
4.3. Antibodies
4.4. Analysis of Fatty Acids
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MFSD2a | Major Facilitator Superfamily Domain containing 2A |
| AD | Alzheimer’s disease |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| GDS | Global Deterioration Scale |
| LC-PUFA | Long-chain polyunsaturated fatty acids |
| FA | Fatty acid |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| GADPH | D-Glyceraldehyde-3-Phosphate Dehydrogenase |
References
- Maresova, P.; Mohelska, H.; Dolejs, J.; Kuca, K. Socio-economic Aspects of Alzheimer’s Disease. Curr Alzheimer Res. 2015, 12, 903–911. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement 2013, 9, 208–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Hasegawa, Y.; Takane, K.; Koibuchi, N.; Cao, C.; Kim-Mitsuyama, S. High-Fat-Diet Intake Enhances Cerebral Amyloid Angiopathy and Cognitive Impairment in a Mouse Model of Alzheimer’s Disease, Independently of Metabolic Disorders. J. Am. Heart Assoc. 2016, 5, e003154. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 Polyunsaturated Fatty Acids and Oxylipins in Neuroinflammation and Management of Alzheimer Disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Kotani, S.; Sakaguchi, E.; Warashina, S.; Matsukawa, N.; Ishikura, Y.; Kiso, Y.; Sakakibara, M.; Yoshimoto, T.; Guo, J.; Yamashima, T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci. Res. 2006, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimers Dement (N. Y.) 2017, 3, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Yoder, E.; Murphy, S.; Dutton, G.R.; Spector, A.A. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6). J. Neurochem. 1991, 56, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot Essent Fat. Acids 2018, 136, 35–45. [Google Scholar] [CrossRef]
- Eser Ocak, P.; Ocak, U.; Sherchan, P.; Zhang, J.H.; Tang, J. Insights into major facilitator superfamily domain-containing protein-2a (Mfsd2a) in physiology and pathophysiology. What do we know so far? J. Neurosci. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Wong, B.H.; Chan, J.P.; Cazenave-Gassiot, A.; Poh, R.W.; Foo, J.C.; Galam, D.L.; Ghosh, S.; Nguyen, L.N.; Barathi, V.A.; Yeo, S.W.; et al. Mfsd2a Is a Transporter for the Essential omega-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem. 2016, 291, 10501–10514. [Google Scholar] [CrossRef]
- Quek, D.Q.; Nguyen, L.N.; Fan, H.; Silver, D.L. Structural Insights into the Transport Mechanism of the Human Sodium-dependent Lysophosphatidylcholine Transporter MFSD2A. J. Biol. Chem. 2016, 291, 9383–9394. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Guemez-Gamboa, A.; Nguyen, L.N.; Yang, H.; Zaki, M.S.; Kara, M.; Ben-Omran, T.; Akizu, N.; Rosti, R.O.; Rosti, B.; Scott, E.; et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 2015, 47, 809–813. [Google Scholar] [CrossRef]
- Alakbarzade, V.; Hameed, A.; Quek, D.Q.; Chioza, B.A.; Baple, E.L.; Cazenave-Gassiot, A.; Nguyen, L.N.; Wenk, M.R.; Ahmad, A.Q.; Sreekantan-Nair, A.; et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat. Genet. 2015, 47, 814–817. [Google Scholar] [CrossRef]
- Harel, T.; Quek, D.Q.Y.; Wong, B.H.; Cazenave-Gassiot, A.; Wenk, M.R.; Fan, H.; Berger, I.; Shmueli, D.; Shaag, A.; Silver, D.L.; et al. Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics 2018, 19, 227–235. [Google Scholar] [CrossRef]
- Chan, J.P.; Wong, B.H.; Chin, C.F.; Galam, D.L.A.; Foo, J.C.; Wong, L.C.; Ghosh, S.; Wenk, M.R.; Cazenave-Gassiot, A.; Silver, D.L. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol. 2018, 16, e2006443. [Google Scholar] [CrossRef]
- Milanovic, D.; Petrovic, S.; Brkic, M.; Avramovic, V.; Perovic, M.; Ivkovic, S.; Glibetic, M.; Kanazir, S. Short-Term Fish Oil Treatment Changes the Composition of Phospholipids While Not Affecting the Expression of Mfsd2a Omega-3 Transporter in the Brain and Liver of the 5xFAD Mouse Model of Alzheimer’s Disease. Nutrients 2018, 10, 1250. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement 2014, 10, 485–502. [Google Scholar] [CrossRef]
- Martins, I.J.; Hone, E.; Foster, J.K.; Sunram-Lea, S.I.; Gnjec, A.; Fuller, S.J.; Nolan, D.; Gandy, S.E.; Martins, R.N. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol. Psychiatry 2006, 11, 721–736. [Google Scholar] [CrossRef]
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 2000, 35, 1305–1312. [Google Scholar] [CrossRef]
- Cherubini, A.; Andres-Lacueva, C.; Martin, A.; Lauretani, F.; Iorio, A.D.; Bartali, B.; Corsi, A.; Bandinelli, S.; Mattson, M.P.; Ferrucci, L. Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1120–1126. [Google Scholar] [CrossRef]
- Husted, K.S.; Bouzinova, E.V. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina (Kaunas) 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Wang, D.C.; Sun, C.H.; Liu, L.Y.; Sun, X.H.; Jin, X.W.; Song, W.L.; Liu, X.Q.; Wan, X.L. Serum fatty acid profiles using GC-MS and multivariate statistical analysis: potential biomarkers of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1057–1066. [Google Scholar] [CrossRef]
- Giunta, M.; Rigamonti, A.E.; Scarpini, E.; Galimberti, D.; Bonomo, S.M.; Venturelli, E.; Muller, E.E.; Cella, S.G. The leukocyte expression of CD36 is low in patients with Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2007, 28, 515–518. [Google Scholar] [CrossRef]
- Ricciarelli, R.; D’Abramo, C.; Zingg, J.M.; Giliberto, L.; Markesbery, W.; Azzi, A.; Marinari, U.M.; Pronzato, M.A.; Tabaton, M. CD36 overexpression in human brain correlates with beta-amyloid deposition but not with Alzheimer’s disease. Free Radic. Biol. Med. 2004, 36, 1018–1024. [Google Scholar] [CrossRef]
- Berger, J.H.; Charron, M.J.; Silver, D.L. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS ONE 2012, 7, e50629. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Wooten, J.S.; Harris, M.P.; Schaller, M.L.; Umbaugh, D.S.; Witt, K.A. Mfsd2a and Glut1 Brain Nutrient Transporters Expression Increase with 32-Week Low and High Lard Compared with Fish-Oil Dietary Treatment in C57Bl/6 Mice. Curr. Dev. Nutr. 2018, 2, nzy065. [Google Scholar] [CrossRef]
- Xu, M.X.; Tan, B.C.; Zhou, W.; Wei, T.; Lai, W.H.; Tan, J.W.; Dong, J.H. Resolvin D1, an endogenous lipid mediator for inactivation of inflammation-related signaling pathways in microglial cells, prevents lipopolysaccharide-induced inflammatory responses. CNS Neurosci. Ther. 2013, 19, 235–243. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.C.; Serhan, C.N.; Schultzberg, M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Abeta42 Phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef]
- Freund Levi, Y.; Vedin, I.; Cederholm, T.; Basun, H.; Faxen Irving, G.; Eriksdotter, M.; Hjorth, E.; Schultzberg, M.; Vessby, B.; Wahlund, L.O.; et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: the OmegAD study. J. Intern. Med. 2014, 275, 428–436. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay-Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 691–697. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Liu, H.K.; Cuthbertson, D.J.; McManus, E.J.; Akhtar, S.; Lipina, C.; Morris, A.D.; Petrie, J.R.; Hundal, H.S.; Sutherland, C. A novel regulation of IRS1 (insulin receptor substrate-1) expression following short term insulin administration. Biochem. J. 2005, 392, 345–352. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Prieto-Sanchez, M.T.; Ruiz-Palacios, M.; Blanco-Carnero, J.E.; Pagan, A.; Hellmuth, C.; Uhl, O.; Peissner, W.; Ruiz-Alcaraz, A.J.; Parrilla, J.J.; Koletzko, B.; et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin. Nutr. 2017, 36, 513–521. [Google Scholar] [CrossRef]
- Ruiz-Palacios, M.; Ruiz-Alcaraz, A.J.; Sanchez-Campillo, M.; Larque, E. Role of Insulin in Placental Transport of Nutrients in Gestational Diabetes Mellitus. Ann. Nutr. Metab. 2017, 70, 16–25. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Stoffel, W.; Chu, F.; Ahrens, E. Analysis of long-chain fatty acids by gas liquid chromatography. Micromethod for preparation of methyl esters. Anal. Chem. 1959, 31, 307–308. [Google Scholar] [CrossRef]
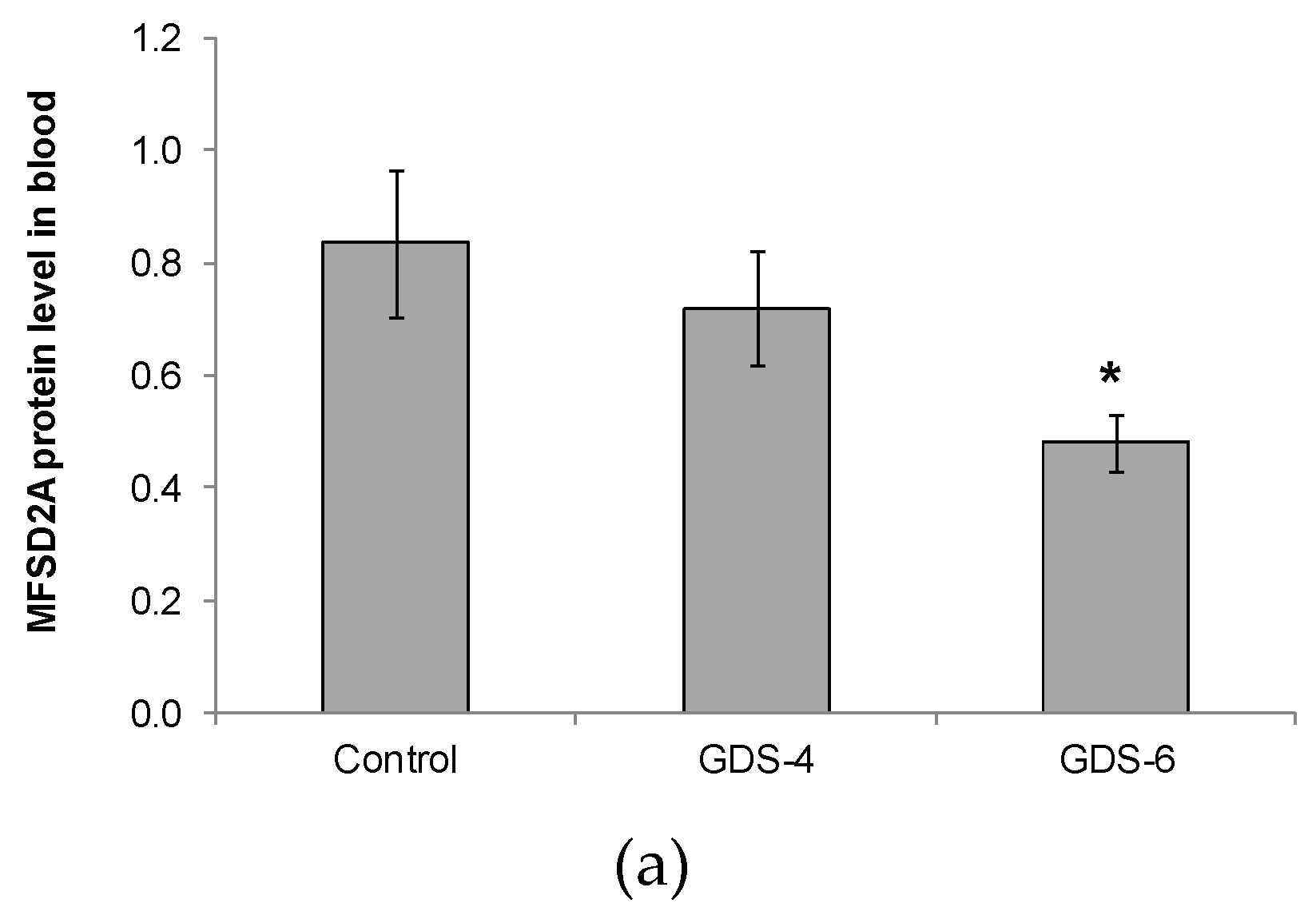
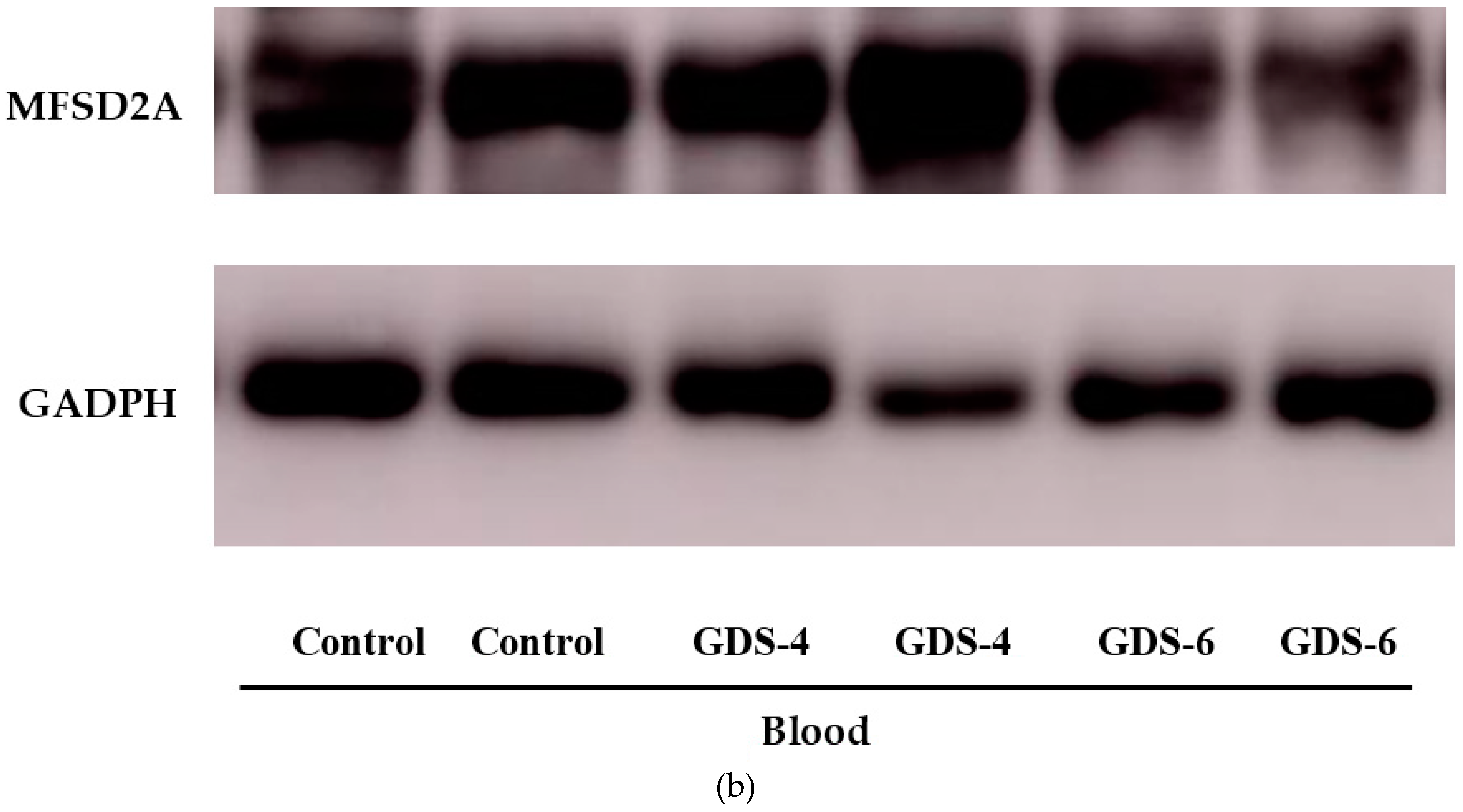
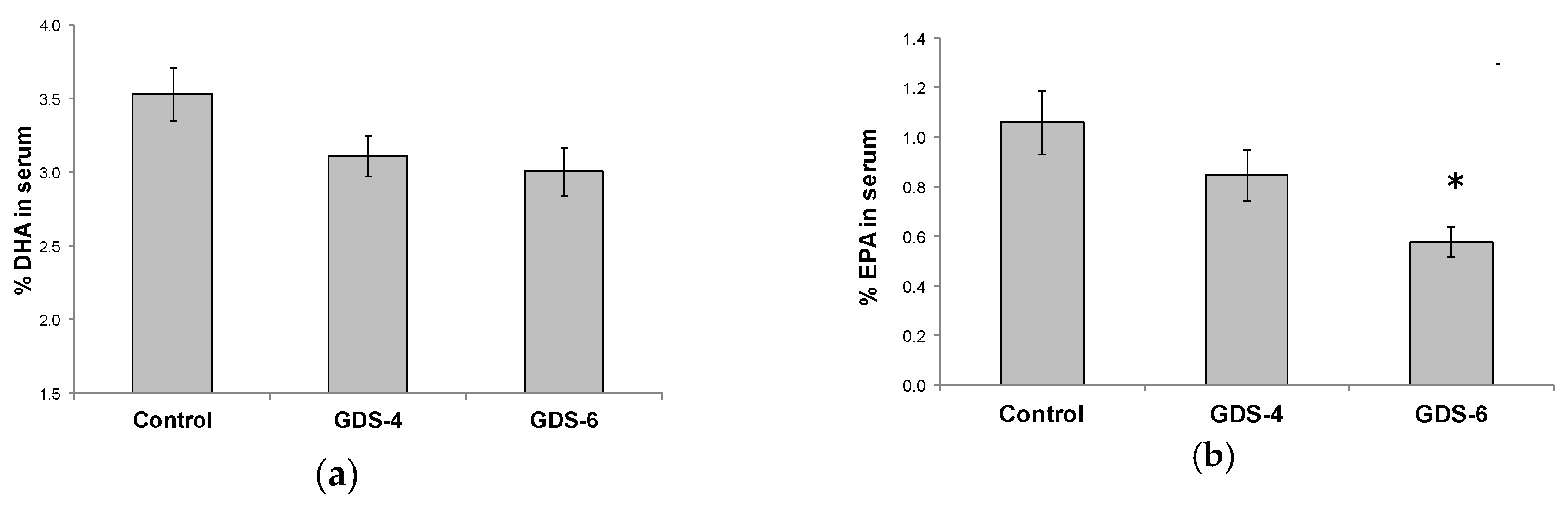
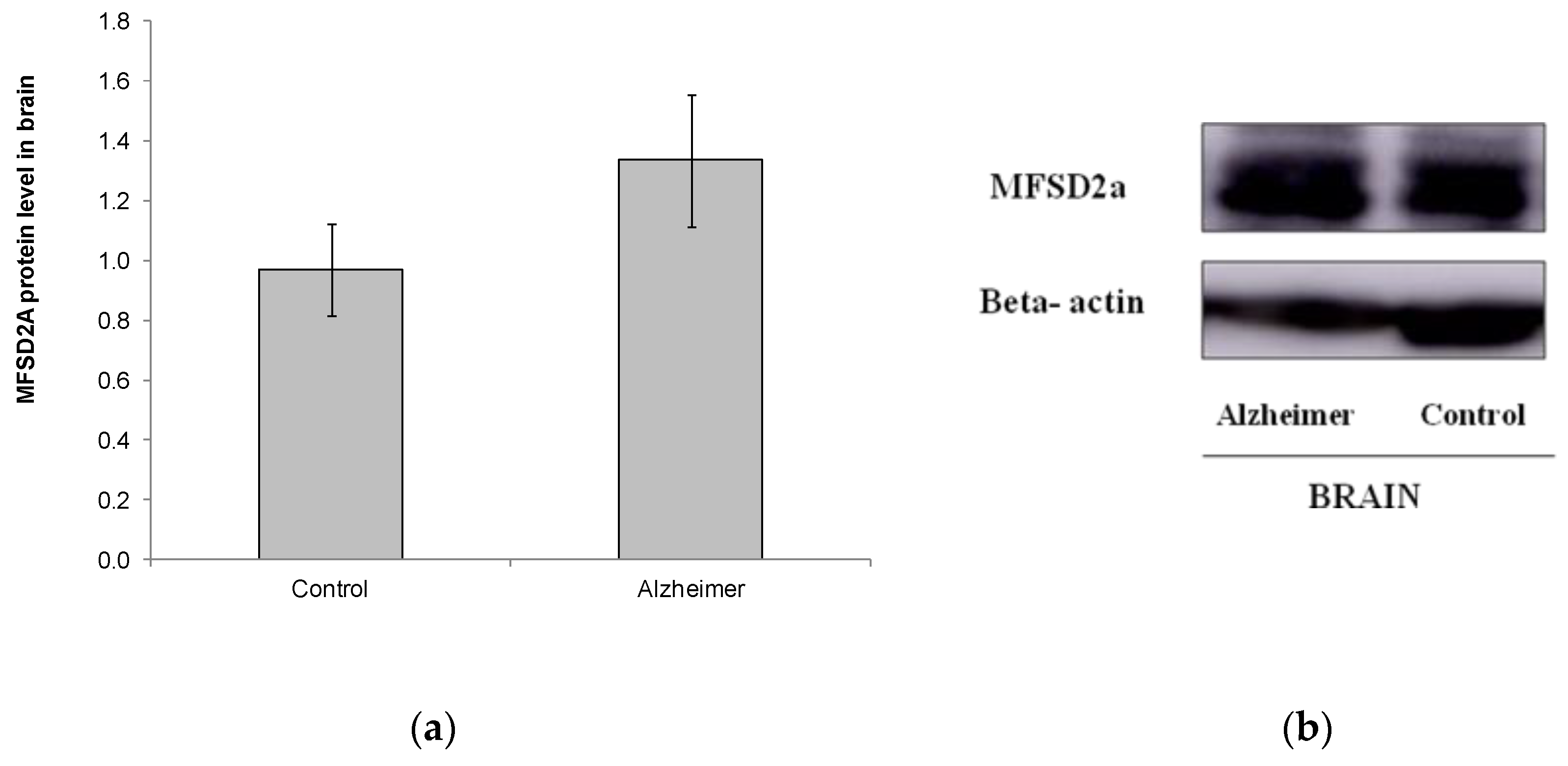
| Control n = 38 | GDS4 n = 48 | GDS6 n = 47 | p | |
|---|---|---|---|---|
| Sex (n) | 10♂, 28♀ | 13♂, 35♀ | 18♂, 29♀ | 0.385 |
| Age | 67 ± 2 | 74 ± 1* | 76 ± 1* | <0.001 |
| BMI (kg/m2) | 27.4 ± 0.8 | 26.2 ± 1.8 | 28.9 ± 1.4 | 0.300 |
| Hypertension (%) | n/a | 50.0 | 51.1 | 0.917 |
| Diabetes (%) | n/a | 27.1 | 26.1 | 0.913 |
| Smoking (%) | n/a | 14.6 | 25.5 | 0.182 |
| Dementia family history (%) | n/a | 73.9 | 68.3 | 0.563 |
| MMSE score | n/a | 18.7 ± 0.7 | 8.3 ± 0.8 † | <0.001 |
| Fatty Acid (%) with Respect to Total Fatty Acid Content | Control (n = 38) | GDS4 (n = 48) | GDS6 (n = 45) | p |
|---|---|---|---|---|
| Lauric acid (12:0) | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.14 ± 0.02 | 0.843 |
| Myristic acid (14:0) | 1.49 ± 0.19 | 1.51 ± 0.10 | 1.54 ± 0.15 | 0.971 |
| Palmitic acid (16:0) | 21.79 ± 0.30 | 21.74 ± 0.29 | 22.33 ± 0.28 | 0.282 |
| Hexadecanoic acid (16:1 n-9) | 0.29 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.338 |
| Palmitoleic acid (16:1 n-7) | 1.37 ± 0.12 | 1.45 ± 0.09 | 1.63 ± 0.12 | 0.240 |
| Margaric acid (17:0) | 0.18 ± 0.02 | 0.22 ± 0.01 | 0.23 ± 0.01* | 0.040 |
| Stearic acid (18:0) | 8.39 ± 0.21 | 7.62 ± 0.18* | 7.72 ± 0.18* | 0.012 |
| Oleic acid (18:1 n-9) | 20.19 ± 0.63 | 21.10 ± 0.50 | 20.62 ± 0.56 | 0.525 |
| Cis-vaccenic acid (18:1 n-7) | 1.59 ± 0.05 | 1.67 ± 0.04 | 1.75 ± 0.06 | 0.073 |
| Linoleic acid (18:2 n-6) | 26.64 ± 0.76 | 26.94 ± 0.76 | 26.55 ± 0.77 | 0.931 |
| γ-linolenic acid (18:3 n-6) | 0.40 ± 0.03 | 0.42 ± 0.02 | 0.43 ± 0.03 | 0.755 |
| α-linolenic acid (18:3 n-3) | 0.16 ± 0.02 | 0.19 ± 0.01 | 0.16 ± 0.01 | 0.319 |
| Arachidic acid (20:0) | 0.30 ± 0.02 | 0.31 ± 0.03 | 0.31 ± 0.02 | 0.971 |
| Gondoic acid (20:1 n-9) | 0.23 ± 0.03 | 0.29 ± 0.03 | 0.27 ± 0.03 | 0.392 |
| Eicosadienoic acid (20:2 n-6) | 0.21 ± 0.03 | 0.40 ± 0.03* | 0.32 ± 0.04* | <0.001 |
| Dihomo-γ-linolenic acid (20:3 n-6) | 1.63 ± 0.06 | 1.65 ± 0.05 | 1.59 ± 0.06 | 0.738 |
| Arachidonic acid (20:4 n-6, AA) | 7.29 ± 0.29 | 7.20 ± 0.28 | 7.71 ± 0.33 | 0.445 |
| Heneicosylic acid (21:0) | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.073 |
| Behenic acid (22:0) | 0.73 ± 0.04 | 0.65 ± 0.03 | 0.63 ± 0.02* | 0.046 |
| Adrenic acid (22:4 n-6) | 0.08 ± 0.02 | 0.13 ± 0.02 | 0.12 ± 0.03 | 0.401 |
| Docosapentaenoic acid (22:5 n-3) | 0.32 ± 0.02 | 0.28 ± 0.01 | 0.28 ± 0.02 | 0.102 |
| Tricosylic acid (23:0) | 0.33 ± 0.02 | 0.26 ± 0.01* | 0.24 ± 0.01* | <0.001 |
| Lignoceric acid (24:0) | 0.64 ± 0.04 | 0.58 ± 0.03 | 0.57 ± 0.02 | 0.297 |
| Nervonic acid (24:1 n-9) | 0.98 ± 0.06 | 0.94 ± 0.06 | 0.91 ± 0.06 | 0.779 |
| SFA | 34.02 ± 0.50 | 33.07 ± 0.43 | 33.74 ± 0.33 | 0.264 |
| MUFA | 24.65 ± 0.69 | 25.76 ± 0.55 | 25.50 ± 0.65 | 0.451 |
| PUFA | 41.33 ± 0.77 | 41.17 ± 0.73 | 40.76 ± 0.77 | 0.863 |
| PUFA n-6 | 36.24 ± 0.79 | 36.74 ± 0.72 | 36.73 ± 0.78 | 0.880 |
| PUFA n-3 | 5.09 ± 0.3 | 4.43 ± 0.23 | 4.03 ± 0.23* | 0.018 |
| n-6/n-3 | 7.98 ± 0.46 | 9.35 ± 0.50 | 10.23 ± 0.56* | 0.013 |
| LC-PUFA n-6 | 9.20 ± 0.31 | 9.39 ± 0.29 | 9.74 ± 0.34 | 0.481 |
| LC-PUFA n-3 | 4.92 ± 0.30 | 4.24 ± 0.23 | 3.87 ± 0.23* | 0.017 |
| Total Fatty acid concentration (mg/dL) | 384.90 ± 17.11 | 494.31 ± 28.83* | 488.50 ± 34.15* | 0.016 |
| Fatty Acid (%) Respect to Total Fatty Acid Content | Control (n = 11) | GDS6 (n = 11) | p |
|---|---|---|---|
| Lauric acid (12:0) | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.159 |
| Palmitic acid (16:0) | 20.00 ± 0.55 | 20.19 ± 1.00 | 0.864 |
| Hexadecanoic acid (16:1 n-9) | 0.52 ± 0.03 | 0.59 ± 0.06 | 0.342 |
| Palmitoleic acid (16:1 n-7) | 0.65 ± 0.04 | 0.56 ± 0.04 | 0.141 |
| Margaric acid (17:0) | 0.30 ± 0.01 | 0.31 ± 0.01 | 0.428 |
| Stearic acid (18:0) | 20.55 ± 0.29 | 19.99 ± 0.30 | 0.201 |
| Elaidic acid (18:1 trans) | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.911 |
| Oleic acid (18:1 n-9) | 17.58 ± 0.68 | 18.44 ± 1.08 | 0.496 |
| Cis-vaccenic acid (18:1 n-7) | 4.15 ± 0.21 | 4.37 ± 0.21 | 0.462 |
| Linoelaidic acid (18:2 all trans-9,12) | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.374 |
| Linoelaidic acid (18:2 trans-12) | 0.03 ± 0.03 | 0.00 ± 0.00 | 0.374 |
| Linoleic acid (18:2 n-6) | 0.86 ± 0.09 | 0.76 ± 0.12 | 0.478 |
| α-linolenic acid (18:3 n-3) | 0.34 ± 0.04 | 0.34 ± 0.06 | 0.960 |
| Arachidic acid (20:0) | 0.27 ± 0.01 | 0.27 ± 0.02 | 0.923 |
| Gondoic acid (20:1 n-9) | 0.79 ± 0.10 | 0.89 ± 0.16 | 0.568 |
| Eicosadienoic acid (20:2 n-6) | 0.23 ± 0.02 | 0.29 ± 0.02 | 0.075 |
| Dihomo-γ-linolenic acid (20:3 n-6) | 1.08 ± 0.07 | 1.03 ± 0.08 | 0.623 |
| Eicosatrienoic acid (20:3 n-3) | 0.02 ± 0.02 | 0.00 ± 0.00 | 0.374 |
| Arachidonic acid (20:4 n-6, AA) | 8.83 ± 0.35 | 8.60 ± 0.48 | 0.697 |
| Eicosapentaenoic acid (20:5 n-3, EPA) | 0.05 ± 0.04 | 0.00 ± 0.00 | 0.174 |
| Behenic acid (22:0) | 0.48 ± 0.08 | 0.65 ± 0.08 | 0.159 |
| Erucic acid (22:1 n-9) | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.073 |
| Adrenic acid (22:4 n-6) | 4.78 ± 0.14 | 4.32 ± 0.17 | 0.051 |
| Docosapentaenoic acid (22:5 n-6, DPA) | 1.23 ± 0.10 | 1.89 ± 0.23* | 0.022 |
| Docosapentaenoic acid (22:5 n-3) | 0.41 ± 0.07 | 0.26 ± 0.03 | 0.079 |
| Docosahexaenoic acid (22:6 n-3, DHA) | 11.83 ± 0.80 | 10.77 ± 1.20 | 0.458 |
| Tricosylic acid (23:0) | 0.14 ± 0.07 | 0.10 ± 0.06 | 0.679 |
| Lignoceric acid (24:0) | 1.05 ± 0.16 | 1.20 ± 0.29 | 0.653 |
| Nervonic acid (24:1 n-9) | 3.63 ± 0.53 | 4.00 ± 0.98 | 0.734 |
| SFA | 42.88 ± 0.47 | 42.77 ± 0.79 | 0.903 |
| MUFA | 27.37 ± 1.32 | 28.93 ± 2.12 | 0.525 |
| PUFA | 29.67 ± 0.94 | 28.26 ± 1.41 | 0.402 |
| TRANS | 0.09 ± 0.04 | 0.05 ± 0.01 | 0.349 |
| PUFA n-6 | 17.02 ± 0.37 | 16.89 ± 0.51 | 0.832 |
| PUFA n-3 | 12.65 ± 0.78 | 11.37 ± 1.14 | 0.355 |
| n-6/n-3 | 1.44 ± 0.15 | 1.69 ± 0.27 | 0.410 |
| LC-PUFA n-6 | 16.16 ± 0.33 | 16.13 ± 0.53 | 0.966 |
| LC-PUFA n-3 | 12.31 ± 0.82 | 11.03 ± 1.19 | 0.374 |
| Total Fatty Acid concentration (mg/g) | 23.82 ± 1.09 | 22.37 ± 1.62 | 0.454 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Campillo, M.; Ruiz-Pastor, M.J.; Gázquez, A.; Marín-Muñoz, J.; Noguera-Perea, F.; Ruiz-Alcaraz, A.J.; Manzanares-Sánchez, S.; Antúnez, C.; Larqué, E. Decreased Blood Level of MFSD2a as a Potential Biomarker of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 70. https://doi.org/10.3390/ijms21010070
Sánchez-Campillo M, Ruiz-Pastor MJ, Gázquez A, Marín-Muñoz J, Noguera-Perea F, Ruiz-Alcaraz AJ, Manzanares-Sánchez S, Antúnez C, Larqué E. Decreased Blood Level of MFSD2a as a Potential Biomarker of Alzheimer’s Disease. International Journal of Molecular Sciences. 2020; 21(1):70. https://doi.org/10.3390/ijms21010070
Chicago/Turabian StyleSánchez-Campillo, María, María José Ruiz-Pastor, Antonio Gázquez, Juan Marín-Muñoz, Fuensanta Noguera-Perea, Antonio J. Ruiz-Alcaraz, Salvadora Manzanares-Sánchez, Carmen Antúnez, and Elvira Larqué. 2020. "Decreased Blood Level of MFSD2a as a Potential Biomarker of Alzheimer’s Disease" International Journal of Molecular Sciences 21, no. 1: 70. https://doi.org/10.3390/ijms21010070
APA StyleSánchez-Campillo, M., Ruiz-Pastor, M. J., Gázquez, A., Marín-Muñoz, J., Noguera-Perea, F., Ruiz-Alcaraz, A. J., Manzanares-Sánchez, S., Antúnez, C., & Larqué, E. (2020). Decreased Blood Level of MFSD2a as a Potential Biomarker of Alzheimer’s Disease. International Journal of Molecular Sciences, 21(1), 70. https://doi.org/10.3390/ijms21010070





