Improving Phenolic Total Content and Monoterpene in Mentha x piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation
Abstract
1. Introduction
2. Results
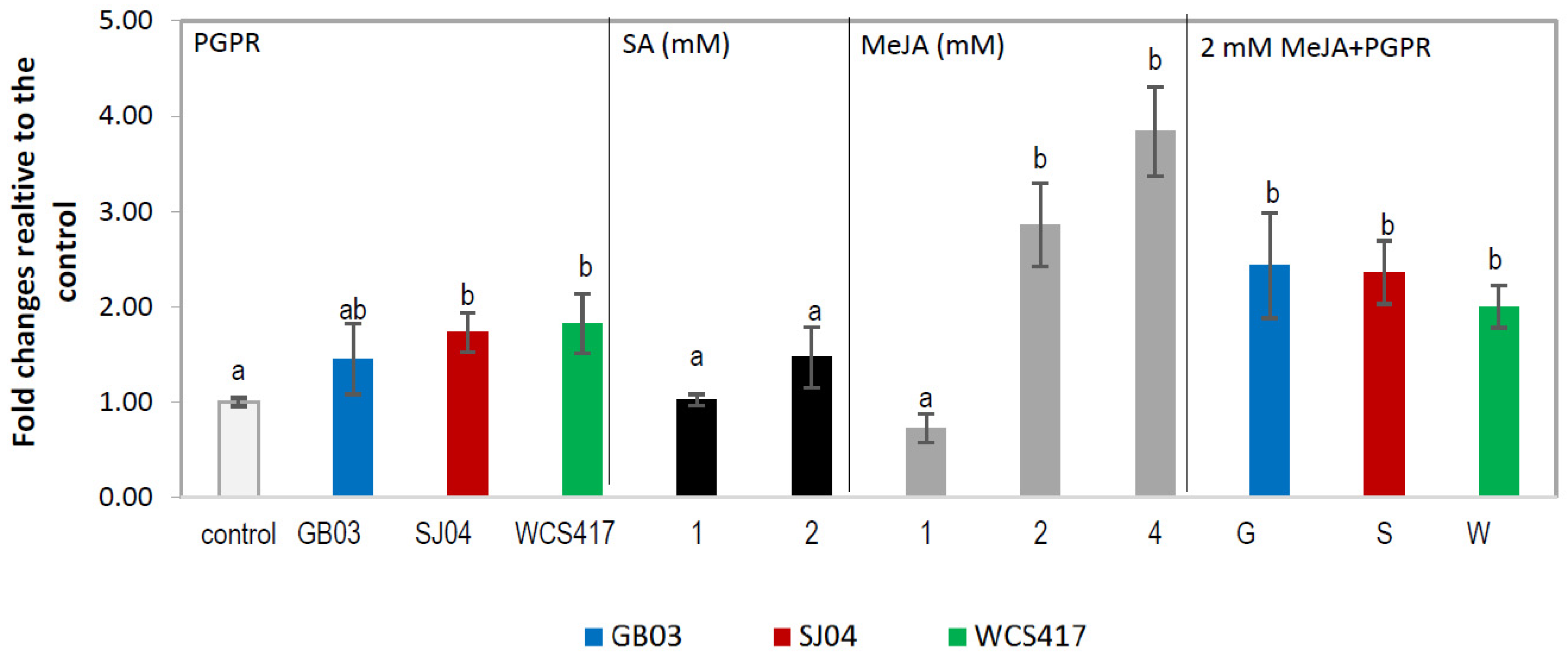
2.1. Total Phenolic Content (TPC)
2.2. Phenylalanine Ammonia Lyase Activity (PAL)
2.3. Quantification of Main EO Components
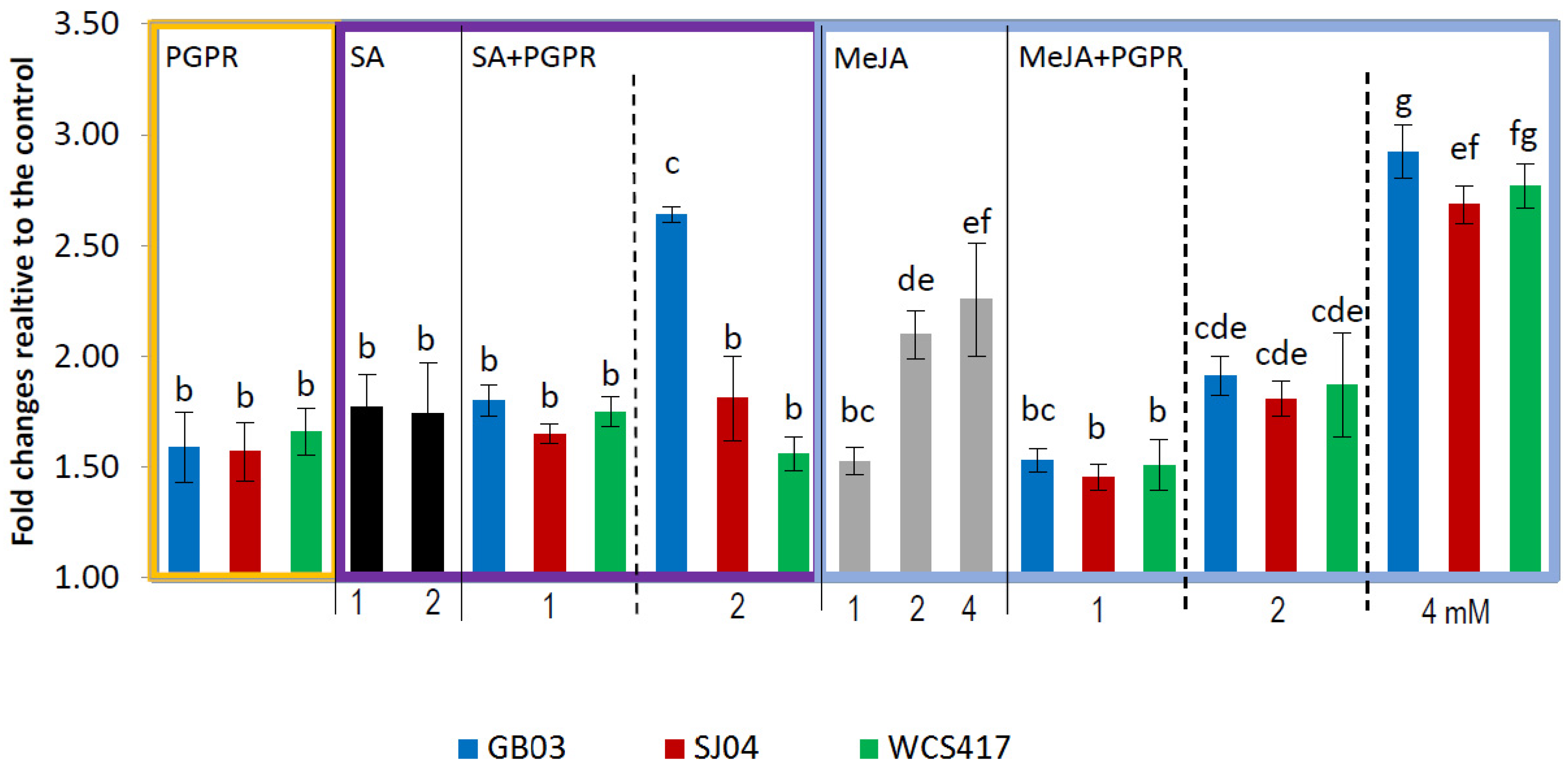
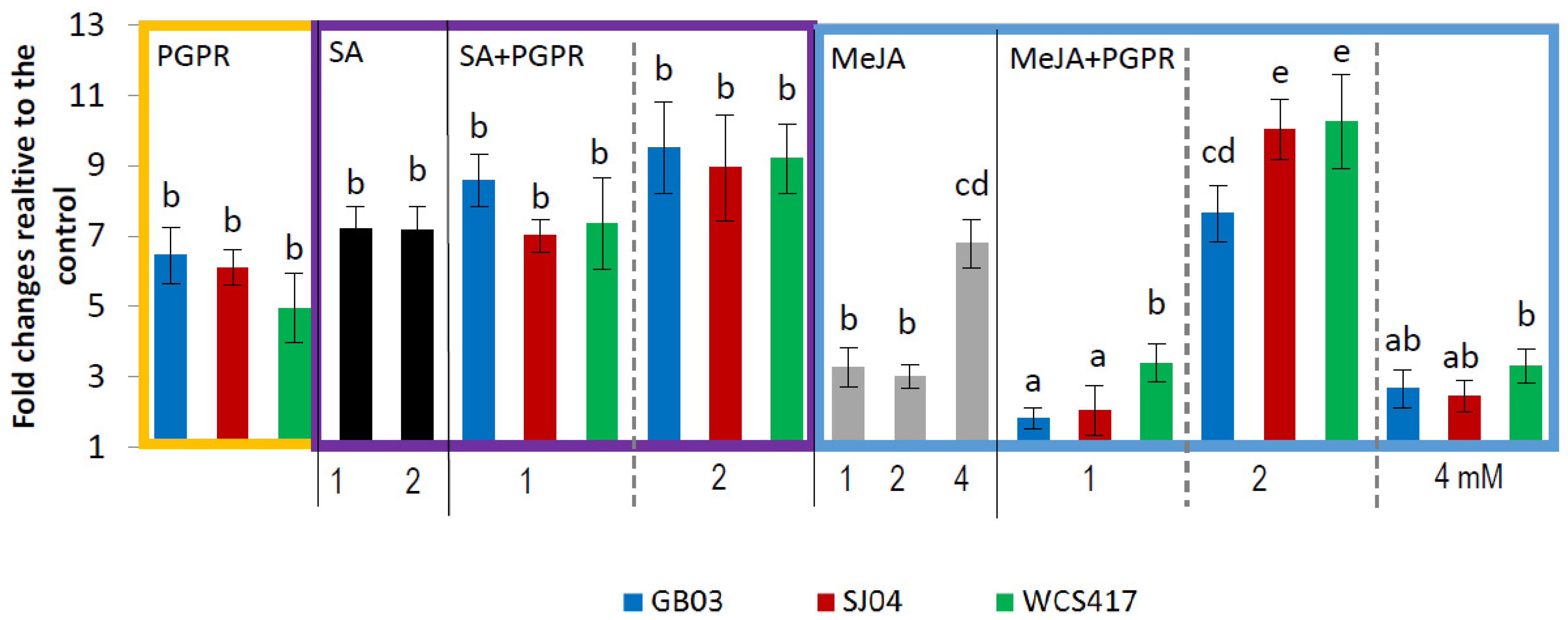
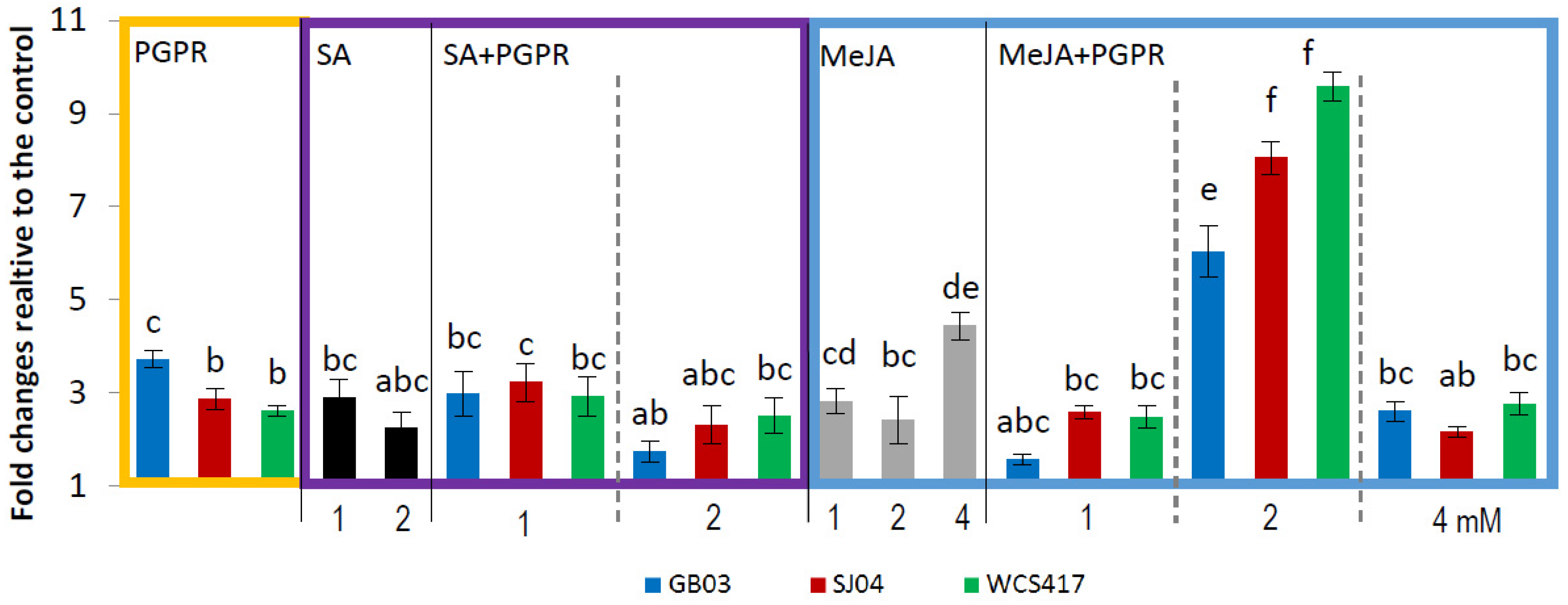
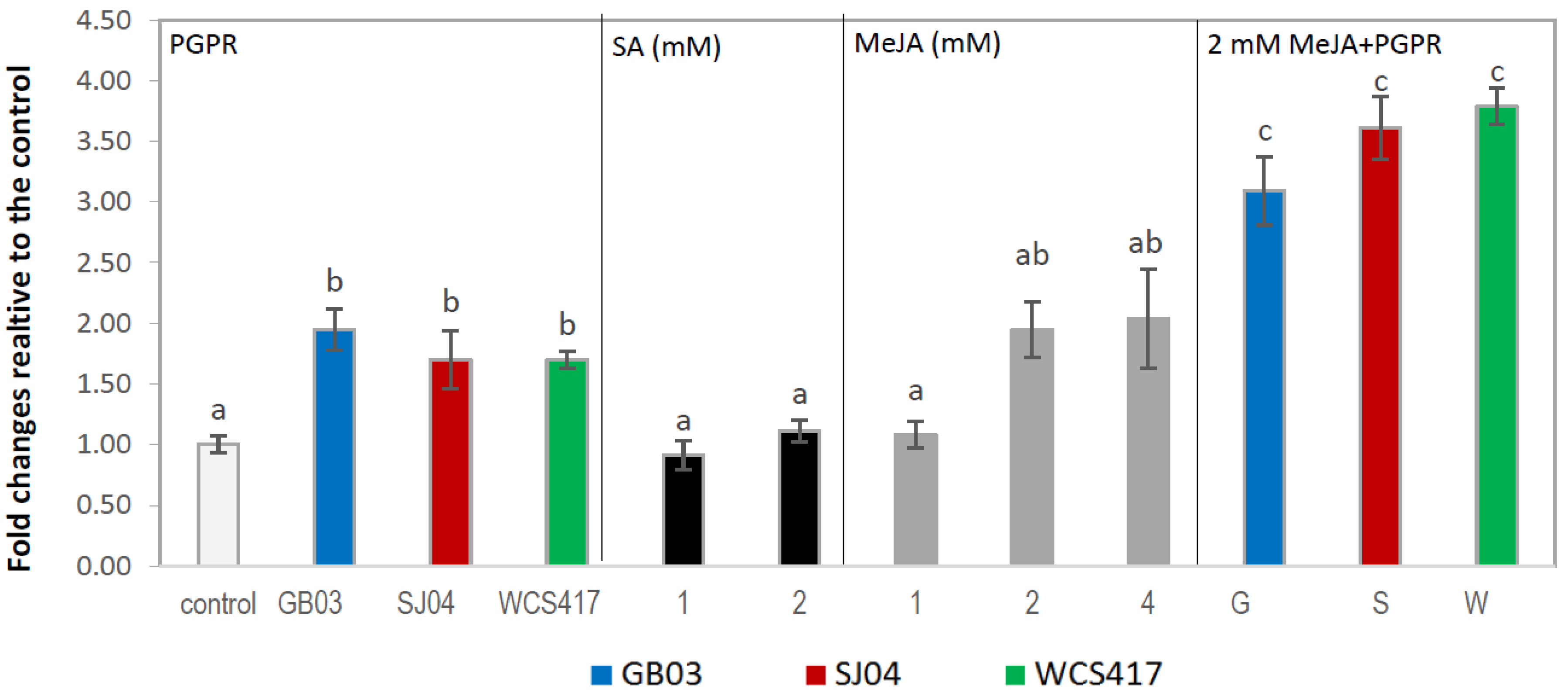
2.4. PGPR Inoculation and External Phytohormones Application Induces Terpenoid Gene Expression in M. piperita
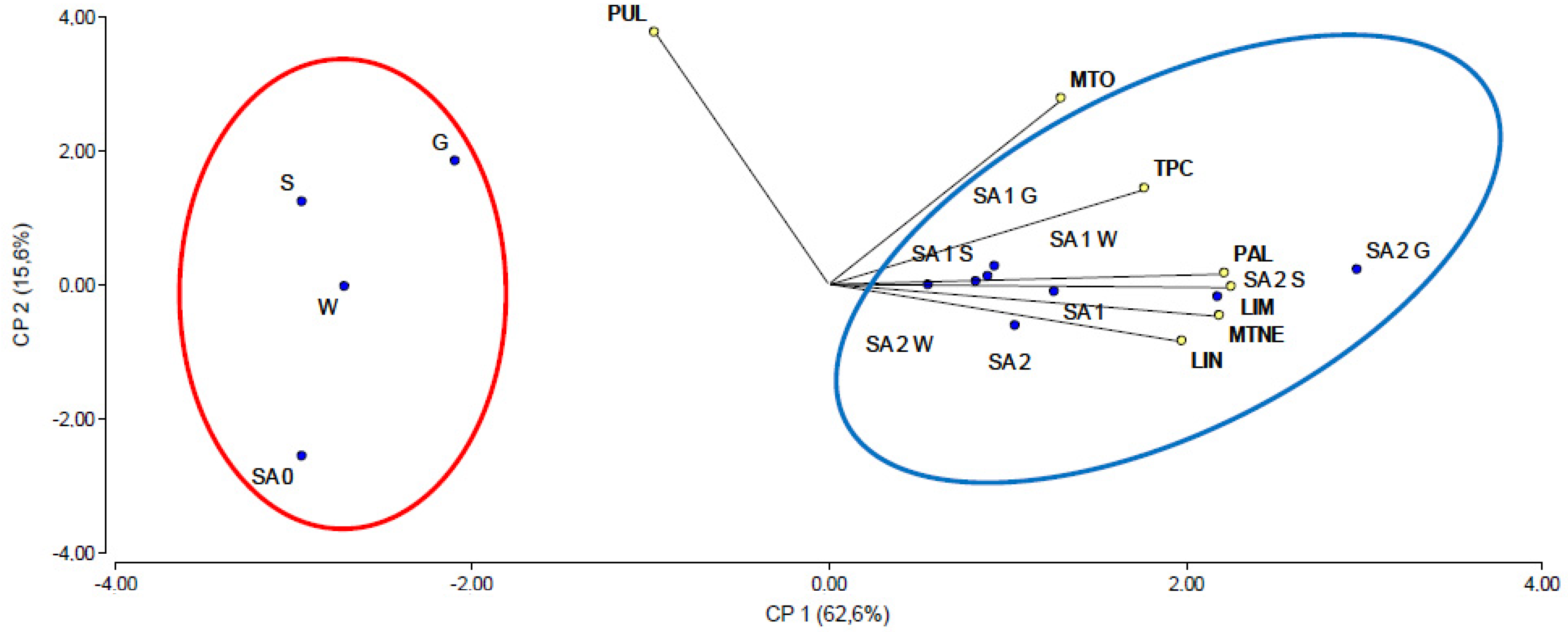
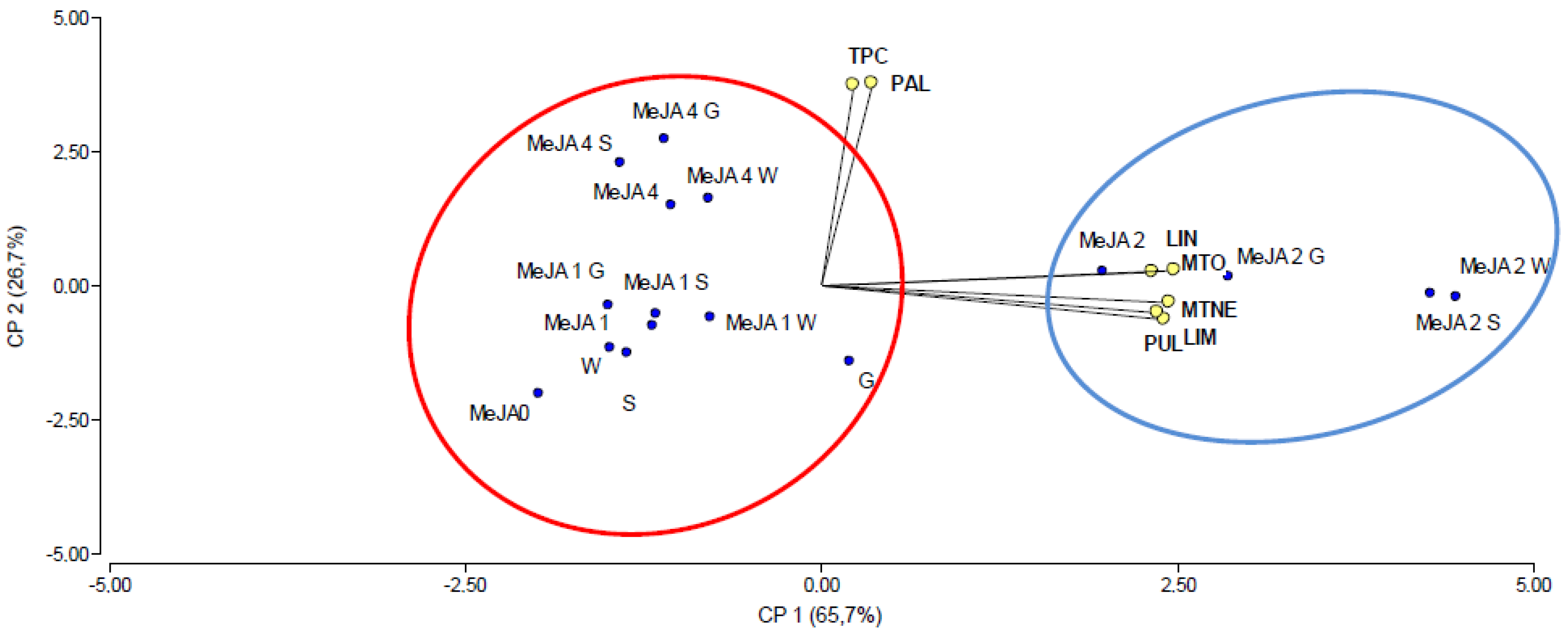
2.5. Principal Component Analysis
3. Discussion
4. Material and Methods
4.1. Plant Material, Bacterial Inoculation, and Treatments
4.2. Greenhouse Experiments
4.3. Determination of Total Phenolic Content
4.4. Determination of PAL Enzyme Activity
4.5. Extraction and Quantification of Main Monoterpene EO Components
4.6. Total RNA Extraction and Quantitative Real-Time PCR
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | essential oil |
| PGPR | plant growth-promoting rhizobacteria |
| JA | jasmonic acid |
| MeJA | methyl jasmonate |
| SA | salicylic acid |
| TPC | total phenolic content |
| PAL | phenylalanine ammonia lyase |
References
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Romano, A. Production of Plant Secondary Metabolites by Using Biotechnological Tools. In Secondary Metabolites—Sources and Applications; Vijayakumar, R., Raja, S.S.S., Eds.; IntechOpen: London, UK, 2018; pp. 81–99. [Google Scholar] [CrossRef]
- Sing, P.; Pandey, A.K. Prospective of Essential Oils of the Genus Mentha as Biopesticides: A Review. Front. Plant Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.; Koşar, M.; Başer, K.H.; Hiltunen, R. Phenolic profile and antioxidant evaluation of Mentha × piperita L. (peppermint) extracts. Nat. Prod. Comun. 2009, 4, 535–542. [Google Scholar] [CrossRef]
- Farnad, N.; Heidari, R.; Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint (Mentha piperita). J. Food Meas. Charact. 2014, 8, 113–121. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Figueroa Pérez, M.G.; Rocha-Guzmán, N.E.; Mercado-Silva, E.; Loarca-Piña, G.; Reynoso Camacho, R. Effect of chemical elicitors on peppermint (Mentha piperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem. 2014, 156, 273–278. [Google Scholar] [CrossRef]
- Figueroa-Pérez, M.G.; Rocha-Guzmán, N.E.; Pérez-Ramírez, I.F.; Mercado-Silva, E.; Reynoso-Camacho, R. Metabolite profile, antioxidant capacity, and inhibition of digestive enzymes in infusions of peppermint (Mentha piperita) grown under drought stress. J. Agric. Food Chem. 2014, 62, 12027–12033. [Google Scholar] [CrossRef]
- Riachi, L.G.; De Maria, C.A.B. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Yang, L.; Yang, C.; Li, C. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. 2016, 61, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Wheatley, R.E. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 2002, 81, 357–364. [Google Scholar] [CrossRef]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Rao, S.M.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolities. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Vanisree, M.; Lee, C.-Y.; Lo, S.-F.; Nalawade, S.M.; Lin, C.Y.; Tsay, H.-S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2004, 45, 1–22. [Google Scholar]
- Thiruvengadam, M.; Rekha, K.; Chung, I.M. Induction of hairy roots by Agrobacterium rhizogenes-mediated transformation of spine gourd (Momordica dioica Roxb. ex. willd) for the assessment of phenolic compounds and biological activities. Sci. Hort. 2016, 198, 132–141. [Google Scholar] [CrossRef]
- Farmer, E.E.; Alméras, E.; Krishnamurthy, V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 2003, 6, 372–378. [Google Scholar] [CrossRef]
- Koo, A.J.K.; Howe, G.A. The wound hormone jasmonate. Phytochemistry 2009, 70, 1571–1580. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates are signals in the biosynthesis of secondary metabolites—Pathways, transcription factors and applied aspects—A brief review. N. Biotechnol. 2019, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Optimization of Methyl Jasmonate Application to Broccoli Florets to Enhance Health-promoting Phytochemical Content. J. Sci. Food Agric. 2014, 94, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on methyl jasmonate. Food Chem. Toxicol. 2012, 50, S572–S576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Taguchi, G.; Yazawa, T.; Hayashida, N.; Okazaki, M. Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrate specificity and are induced by salicylic acid and auxin. Eur. J. Biochem. 2001, 268, 4086–4094. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 14, 2097. [Google Scholar] [CrossRef]
- Kloepper, J.W. Plant-Growth-Promoting Rhizobacteria as Biological Control Agents. In Soil Microbial Ecology: Applications in Agricultural and Environmental Management; Metting, F.B., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1993; pp. 255–273. [Google Scholar]
- Niranjan, R.S.; Shetty, H.S.; Reddy, M.S. Plant Growth Promoting Rhizobacteria: Potential Green Alternative for Plant Productivity. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 197–216. [Google Scholar]
- van Loon, L.C. Plant response to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; van Wees, S.C.M.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 1013–1020. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Banchio, E.; Xie, X.; Zhang, H.; Paré, P.W. Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J. Agric. Food Chem. 2009, 5, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Banchio, E.; Bogino, P.; Santoro, M.V.; Torres, L.; Zygadlo, J.; Giordano, W. Systemic induction of monoterpene biosynthesis in Origanum x majoricum by soil bacteria. J. Agric. Food Chem. 2010, 58, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.; Santoro, M.V.; Nievas, F.; Giordano, W.; Banchio, E. Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2013, 70, 16–22. [Google Scholar] [CrossRef]
- Santoro, M.V.; Zygadlo, J.; Giordano, W.; Banchio, E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol. Biochem. 2011, 49, 1177–1182. [Google Scholar] [CrossRef]
- Cappellari, L.; Santoro, V.M.; Schmidt, A.; Gershenzon, J.; Banchio, E. Induction of essential oil production in Mentha x piperita by plant growth promoting bacteria was correlated with an increase in jasmonate and salicylate levels and a higher density of glandular trichomes. Plant Physiol. Biochem. 2019, 141, 142–153. [Google Scholar] [CrossRef]
- McConkey, M.E.; Gershenzon, J.; Croteau, R.B. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol. 2000, 122, 215–224. [Google Scholar] [CrossRef]
- Turner, G.W.; Gershenzon, J.; Nielson, E.E.; Froehlich, J.E.; Croteau, R.B. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999, 120, 879–886. [Google Scholar] [CrossRef]
- Cappellari, L.R.; Chiappero, J.; Santoro, M.; Giordano, W.; Banchio, E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci. Hort. 2017, 220, 193–198. [Google Scholar] [CrossRef]
- Sudha, G.; Ravishankar, G.A. Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Organ. Cult. 2002, 71, 181–212. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha × piperita cell suspension cultures. Plant Cell Tiss. Organ Cult. 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 2007, 12, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 3, e00273. [Google Scholar] [CrossRef] [PubMed]
- Nafie, E.; Hathout, T.; Mokadem, A.S.A. Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. cells. Braz. J. Plant Physiol. 2011, 23, 161–174. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C. Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2007, 12, 5510366. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Chun, S.W.; Chung, Y.S.; Lee, S.Y.; Park, S.U. Influence of chitosan, salicylic acid and jasmonic acid on phenylpropanoid accumulation in germinated buckwheat (Fagopyrum esculentum Moench). Foods 2019, 6, 153. [Google Scholar] [CrossRef]
- Chen, H.; Seguin, P.; Archambault, A.; Constan, L.; Jabaji, S. Gene expression and isoflavone concentrations in soybean sprouts treated with chitosan. Crop Sci. 2009, 49, 224–236. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.K.; Uddin, M.R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.; Lee, J.-H.; Park, S.U. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef]
- Khalil, N.; Fekry, M.; Bishr, M.; El-Zalabani, S.; Salama, O. Foliar spraying of salicylic acid induced accumulation of phenolics, increased radical scavenging activity and modified the composition of the essential oil of water stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Gorni, P.H.; Pacheco, A.C. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Afr. J. Biotechnol. 2016, 15, 657–665. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Q.-H.; Yang, H.-R.; Liu, Y.-Y.; Huang, W.-D. Relationship between H2O2 and jasmonic acid in pea leaf wounding response. Russ. J. Plant Physl. 2008, 55, 765. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A. Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tissue Organ. Cult. 2007, 89, 1–13. [Google Scholar] [CrossRef]
- Lavania, M.; Chauhan, P.S.; Chauhan, S.V.S.; Singh, H.B.; Nautiyal, C.H. Induction of plant defense enzymes and phenolics by treatment with plant growth promoting rhizobacteria Serratia marcescens NBRI1213. Curr. Microbiol. 2006, 52, 363–368. [Google Scholar] [CrossRef]
- Singh, U.P.; Sarma, B.K.; Singh, D.P. Effect of plant growth-promoting rhizobacteria and culture filtrate of Sclerotium rolfsii on phenolic and salicylic acid contents in Chickpea (Cicer arietinum). Curr. Microbiol. 2003, 46, 131–140. [Google Scholar] [CrossRef]
- Salla, T.D.; Ramos, T.; Astarita, L.V.; Santarém, E.R. Streptomyces rhizobacteria modulate the secondary metabolism of Eucalyptus plants. Plant Physiol. Biochem. 2014, 85, 14–20. [Google Scholar] [CrossRef]
- Panka, D.; Piesik, D.; Jeske, M.; Baturo-Ciesniewska, A. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J. Plant Physiol. 2013, 170, 1010–1019. [Google Scholar] [CrossRef]
- Vanitha, S.C.; Umesha, S. Pseudomonas fluorescens mediated systemic resistance in tomato is driven through an elevated synthesis of defense enzymes. Biol. Plant 2011, 55, 317. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Samiyappan, R. Induction of defenserelatedgenes in Pseudomonas fluorescens treated chilli plants in response to infection by Colletotrichum capsici. J. Mycol. Plant Pathol. 2001, 31, 146–155. [Google Scholar]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 1, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Gershenzon, J.; Lardau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.; Santoro, M.; Reinoso, H.; Travaglia, C.; Giordano, W.; Banchio, E. Anatomical, morphological, and phytochemical effects of inoculation with plant growth promoting rhizobacteria on peppermint (Mentha piperita). J. Chem. Ecol. 2015, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Banchio, E.; Bogino, P.; Zygadlo, J.; Giordano, W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008, 36, 766–771. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Wang, J. Molecular characterization and expression of 1-deoxy- d-xylulose 5-phosphate reductoisomerase (DXR) gene from Salvia miltiorrhiza. Acta Physiol. Plant 2009, 31, 1015. [Google Scholar] [CrossRef]
- Cao, X.Y.; Li, C.G.; Miao, Q.; Zheng, Z.J.; Jiang, J.H. Molecular cloning and expression analysis of a leaf-specific expressing 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase gene from Michelia chapensis Dandy. J. Med. Plants Res. 2011, 5, 3868–3875. [Google Scholar] [CrossRef]
- Cao, X.Y.; Yin, T.; Miao, Q.; Li, C.G.; Ju, X.Y.; Sun, Y. Molecular characterization and expression analysis of a gene encoding for farnesyl diphosphate synthase from Euphorbia pekinensis Rupr. Mol. Biol. Rep. 2012, 39, 1487–1492. [Google Scholar] [CrossRef]
- Shabani, L.; Ehsanpour, A.A.; Asghari, G.; Emami, J. Glycyrrhizin production by in vitro cultured glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ. J. Plant Physiol. 2009, 56, 621–626. [Google Scholar] [CrossRef]
- Kai, M.; Crespo, E.; Cristescu, S.M.; Harren, F.J.M.; Francke, W.; Piechulla, B. Serratia odorifera: Analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 2010, 88, 965–976. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Peñuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2003, 217, 758–766. [Google Scholar] [CrossRef]
- Eraslan, F.; Inal, A.; Gunes, A.; Alpaslan, M. Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci. Hort. 2007, 113, 120–128. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J.; Filella, I. Methyl salicylate fumigation increases monoterpene emission rates. Biol. Plant. 2007, 51, 372–376. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Li, K.; Yu, D. Identification and characterization of a novel monoterpene synthase from soybean restricted to neryldiphosphate precursor. PLoS ONE 2013, 4, e75972. [Google Scholar] [CrossRef]
- Pateraki, I.; Kanellis, A.K. Stress and developmental responses of terpenoid biosynthetic genes in Cistus creticus subsp. Creticus. Plant Cell Rep. 2010, 29, 629–641. [Google Scholar] [CrossRef]
- Xu, Y.W.; Lv, S.S.; Zhao, D.; Chen, J.W.; Yang, W.T.; Wu, W. Effects of salicylic acid on monoterpene production and antioxidant systems in Houttuynia cordata. Afr. J. Biotechnol. 2012, 11, 1364–1372. [Google Scholar]
- Anand, A.; Uppalapati, S.R.; Ryu, C.M.; Allen, S.N.; Kang, L.; Tang, Y.; Mysore, K.S. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 2008, 146, 703–715. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005, 46, 923–930. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University Chicago Press: Chicago, IL, USA, 1997; pp. 33–38. [Google Scholar]
- Hu, P.; Zhou, W.; Cheng, Z.; Fan, M.; Wang, L.; Xie, D. JAV1 controls jasmonate-regulated plant defense. Mol. Cell 2013, 50, 504–515. [Google Scholar] [CrossRef]
- Kautz, S.; Trisel, J.A.; Ballhorn, D.J. Jasmonic acid enhances plant cyanogenesis and resistance to herbivory in lima bean. J. Chem. Ecol. 2014, 40, 1186–1996. [Google Scholar] [CrossRef]
- Tian, D.; Peiffer, M.; De Moraes, C.M.; Felton, G.W. Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta 2014, 239, 577–589. [Google Scholar] [CrossRef]
- Yan, C.; Xie, D. Jasmonate in plant defence: Sentinel or double agent? Plant Biotech. J. 2015, 13, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A. Cyclopentenone signals for plant defense: Remodeling the jasmonic acid response. Proc. Natl. Acad. Sci. USA 2001, 98, 12317–12319. [Google Scholar] [CrossRef] [PubMed]
- Maes, L.; Van Nieuwerburgh, F.C.; Zhang, Y.; Reed, D.W.; Pollier, J.; Vande Casteele, S.R.; Inzé, D.; Covello, P.S.; Deforce, D.L.; Goossens, A. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2011, 189, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transciption. J. Exp. Bot. 2016, 68, 1303–1321. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Nagel, R.; Krekling, T.; Christiansen, E.; Gershenzon, J.; Krokene, P. Induction of isoprenyl diphosphate synthases, plant hormones and defense signalling genes correlates with traumatic resin duct formation in Norway spruce (Picea abies). Plant Mol. Biol. 2011, 77, 577–590. [Google Scholar] [CrossRef]
- Henery, M.L.; Wallis, I.R.; Stone, C.; Foley, W.J. Methyl jasmonate does not induce changes in Eucalyptus grandis leaves that alter the effect of constitutive defense on larvae of a specialist herbivore. Oecologia 2008, 56, 847–859. [Google Scholar] [CrossRef]
- Rodríguez-Saona, C.; Crafts-Brandner, S.J.; Paré, P.W.; Henneberry, T.J. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J. Chem. Ecol. 2001, 27, 679–695. [Google Scholar] [CrossRef]
- Złotek, U.; Michalak-Majewska, M.; Szymanowska, U. Effect of jasmonic acid elicitation on the yield, chemical composition, and antioxidant and anti-inflammatory properties of essential oil of lettuce leaf basil (Ocimum basilicum L.). Food Chem. 2016, 213, 1–7. [Google Scholar] [CrossRef]
- Lange, B.M.; Mahmoud, S.S.; Wildung, M.R.; Turner, G.W.; Davis, E.M.; Lange, I.; Baker, R.C.; Boydston, R.A.; Croteau, R.B. Improving peppermint essential oil yield and composition by metabolic engineering. Proc. Natl. Acad. Sci. USA 2011, 108, 16944–16949. [Google Scholar] [CrossRef]
- Lange, B.M.; Turner, G.W. Terpenoid biosynthesis in trichomes-current status and future opportunities. Plant Biotechnol. J. 2013, 11, 2–22. [Google Scholar] [CrossRef]
- Gershenzon, J.; McCaskill, D.; Rajaonarivony, J.I.M.; Mihaliak, C.; Karp, F.; Croteau, R. Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Anal. Biochem. 1992, 200, 130–138. [Google Scholar] [CrossRef]
- McCaskill, D.; Gershenzon, J.; Croteau, R. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.). Planta 1992, 187, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Methyl jasmonate application induces increased densities of glandular trichomes on tomate, Lycopersicon esculentum. J. Chem. Ecol. 2005, 31, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.; Colangelo, C.; Williams, K. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef]
- van der Fits, L.; Memelink, J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 2000, 289, 295–297. [Google Scholar] [CrossRef]
- Schenk, P.M.; Kazan, K.; Wilson, I.; Anderson, J.P.; Richmond, T.; Somerville, S.C.; Manners, J.M. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 2000, 97, 11655–11660. [Google Scholar] [CrossRef]
- Reymond, P.; Bodenhausen, N.; Van Poecke, R.M.P.; Krishnamurthy, V.; Dicke, M.; Farmer, E.E. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 2000, 16, 3132–3147. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Guo, X.; Stotz, H.U. Defense against Sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol. Plant Microbe Interact. 2007, 20, 1384–1395. [Google Scholar] [CrossRef]
- Memelink, J.; Verpoorte, R.; Kijne, J.W. ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci. 2001, 6, 212–219. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant 2014, 36, 2537–2546. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K.G., Me’rillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Huxley, R.R.; Neil, H.A. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- Basbagci, G.; Erler, F. Evaluation of some essential oils and their major components against mushroom scatopsid flies as fumigants. Fresenius Environ. Bull. 2013, 22, 3173–3181. [Google Scholar]
- Dorman, H.J.D.; Deans, S.G. Antibacterial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Rhouma, A.; Daoud, H.B.; Ghanmi, S.; Salah, H.B.; Romdhane, M.; Demak, M. Antimicrobial activities of leaf extracts of Pistacia and Schinus species against some plant pathogenic fungi and bacteria. J. Plant Pathol. 2009, 91, 339–345. [Google Scholar]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Berendsen, R.L.; van Verk, M.C.; Stringlis, I.A. Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genom. 2015, 16, 539. [Google Scholar] [CrossRef] [PubMed]
- Santoro, V.M.; Bogino, P.C.; Nocelli, N.; Cappellari, L.; Giordano, W.F.; Banchio, E. Analysis of plant growth-promoting effects of fluorescent Pseudomonas strains isolated from Mentha piperita rhizosphere and effects of their volatile organic compounds on essential oil composition. Front. Microb. 2016, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Jeong, H.; Kloepper, J.W.; Ryu, C.M. Genome sequence of Bacillus amyloliquefaciens GB03, an active ingredient of the first commercial biological control product. Genome Announc. 2014, 2, e01092-14. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bradford, M.A. Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beaudoin-Eagan, L.D.; Thorpe, T.A. Tyrosine and phenylalanine ammonia-lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol. 1985, 78, 438–441. [Google Scholar] [CrossRef]

| Treatments | Linalool (µg/g fw) | (−)-Limonene (µg/g fw) | (−)-Menthone (µg/g fw) |
|---|---|---|---|
| Control | 0.15 ± 0.01 a | 0.20 ± 0.02 a | 0.50 ± 0.03 a |
| G | 0.61 ± 0.07 b | 0.76 ± 0.06 b | 1.15± 0.05 bc |
| S | 0.48 ± 0.08 b | 0.73 ± 0.08 b | 1.34 ± 0.15 c |
| W | 0.52 ± 0.05 b | 0.65 ± 0.07 b | 0.99 ± 0.09 b |
| Control for SA | 0.14 ± 0.02 a | 0.19 ± 0.04 a | 0.43 ± 0.09 a |
| 1 mM SA | 0.54 ± 0.07 b | 1.09 ± 0.18 b | 1.30 ± 0.09 b |
| 2 mM SA | 0.40 ± 0.07 ab | 1.01 ± 0.20 b | 1.40 ± 0.09 b |
| 1 mM SA + G | 0.41 ± 0.03 cd | 0.91 ± 0.06 b | 1.34 ± 0.07 b |
| 1 mM SA + S | 0.32 ± 0.03 ab | 0.99 ± 0.18 b | 1.71 ± 0.35 c |
| 1 mM SA + W | 0.40 ± 0.05 ab | 0.66 ± 0.17 ab | 1.45 ± 0.05 b |
| 2 mM SA + G | 0.34 ± 0.09 ab | 1.14 ± 0.03 b | 1.50 ± 0.22 b |
| 2 mM SA + S | 0.41 ± 0.05 ab | 1.18 ± 0.08 b | 1.53 ± 0.06 b |
| 2 mM SA + W | 0.22 ± 0.06 a | 1.06 ± 0.17 b | 1.31 ± 0.05 b |
| Treatments | Linalool (µg/g fw) | (−)-Limonene (µg/g fw) | (−)-Menthone (µg/g fw) |
|---|---|---|---|
| Control MeJA | 0.15 ± 0.02 a | 0.25 ± 0.02 a | 0.35 ± 0.03 a |
| 1 mM + MeJA | 0.16 ± 0.02 a | 0.25 ± 0.05 a | 0.43 ± 0.08 a |
| 2 mM + MeJA | 0.22 ± 0.04 a | 0.21 ± 0.04 a | 0.47 ± 0.13 a |
| 4 mM + MeJA | 0.97 ± 0.12 c | 0.83 ± 0.07 b | 1.42 ± 0.14 b |
| 1 mM MeJA + G | 0.23 ± 0.04 a | 0.40 ± 0.05 a | 0.23 ± 0.03 a |
| 1 mM MeJA + S | 0.32 ± 0.07 a | 0.25 ± 0.03 a | 0.51 ± 0.13 a |
| 1 mM MeJA + W | 0.35 ± 0.07 ab | 0.37 ± 0.04 a | 0.44 ± 0.13 a |
| 2 mM MeJA + G | 0.79 ± 0.15 bc | 1.09 ± 0.07 bc | 1.75 ± 0.17 bc |
| 2 mM MeJA + S | 1.12 ± 0.17 c | 1.23 ± 0.07 c | 1.95 ± 0.17 bc |
| 2 mM MeJA + W | 0.84 ± 0.21 c | 1.11 ± 0.10 bc | 2.54 ± 0.45 c |
| 4 mM MeJA + G | 0.21 ± 0.08 a | 0.22 ± 0.04 a | 0.27 ± 0.07 a |
| 4 mM MeJA + S | 0.20 ± 0.02 a | 0.23 ± 0.01a | 0.20 ± 0.04 a |
| 4 mM MeJA + W | 0.23 ± 0.02 a | 0.27 ± 0.09 a | 0.42 ± 0.12 a |
| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Act | GCTCCAAGGGCTGTGTTCC | TCTTTCTGTCCCATGCCAAC |
| Ls | TTGTGGCGAATTCTCTCGCT | GGCTTCTGAGCTGGTCACTT |
| Pr | GCATGGAGATCCCAGATGGC | AGTAGAGCCAGGAAGGATGGA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappellari, L.d.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Improving Phenolic Total Content and Monoterpene in Mentha x piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation. Int. J. Mol. Sci. 2020, 21, 50. https://doi.org/10.3390/ijms21010050
Cappellari LdR, Santoro MV, Schmidt A, Gershenzon J, Banchio E. Improving Phenolic Total Content and Monoterpene in Mentha x piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation. International Journal of Molecular Sciences. 2020; 21(1):50. https://doi.org/10.3390/ijms21010050
Chicago/Turabian StyleCappellari, Lorena del Rosario, Maricel Valeria Santoro, Axel Schmidt, Jonathan Gershenzon, and Erika Banchio. 2020. "Improving Phenolic Total Content and Monoterpene in Mentha x piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation" International Journal of Molecular Sciences 21, no. 1: 50. https://doi.org/10.3390/ijms21010050
APA StyleCappellari, L. d. R., Santoro, M. V., Schmidt, A., Gershenzon, J., & Banchio, E. (2020). Improving Phenolic Total Content and Monoterpene in Mentha x piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation. International Journal of Molecular Sciences, 21(1), 50. https://doi.org/10.3390/ijms21010050





