The Use of Proton Pump Inhibitors May Increase Symptoms of Muscle Function Loss in Patients with Chronic Illnesses
Abstract
1. Introduction
1.1. Cachexia in Chronic Illness
1.2. Pathophysiology of Cachexia
1.3. Sarcopenic Obesity in Chronic Illness
1.4. Adverse Effects of Long Term Use of PPIs
1.5. Aim and Scope
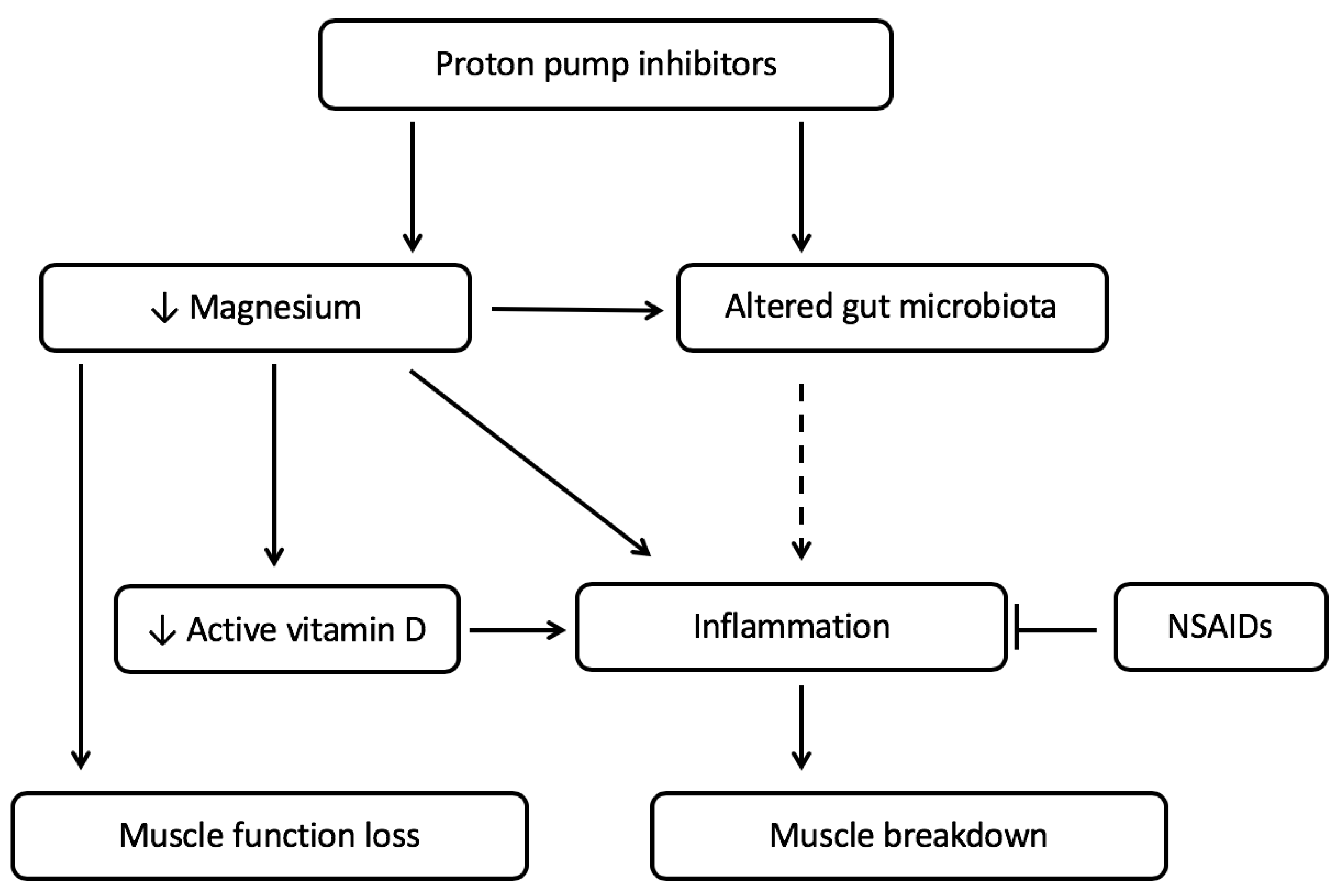
2. Relation between PPI Use, Micronutrient Status and Muscle Function
2.1. PPI Use Is Associated with Low Magnesium Status
2.2. Low Magnesium Levels Are Related to Decreased Muscle Function
2.3. Magnesium and Vitamin D Deficiency, Together, Can Lead to Increased Inflammation Affecting the Muscle
3. PPI Use and the Gut Microbiota in Relation with Cachexia
3.1. The Gut Microbiota Is Altered by PPI Use
3.2. Alterations in Gut Microbiota Can Contribute to Both Muscle Wasting and Obesity
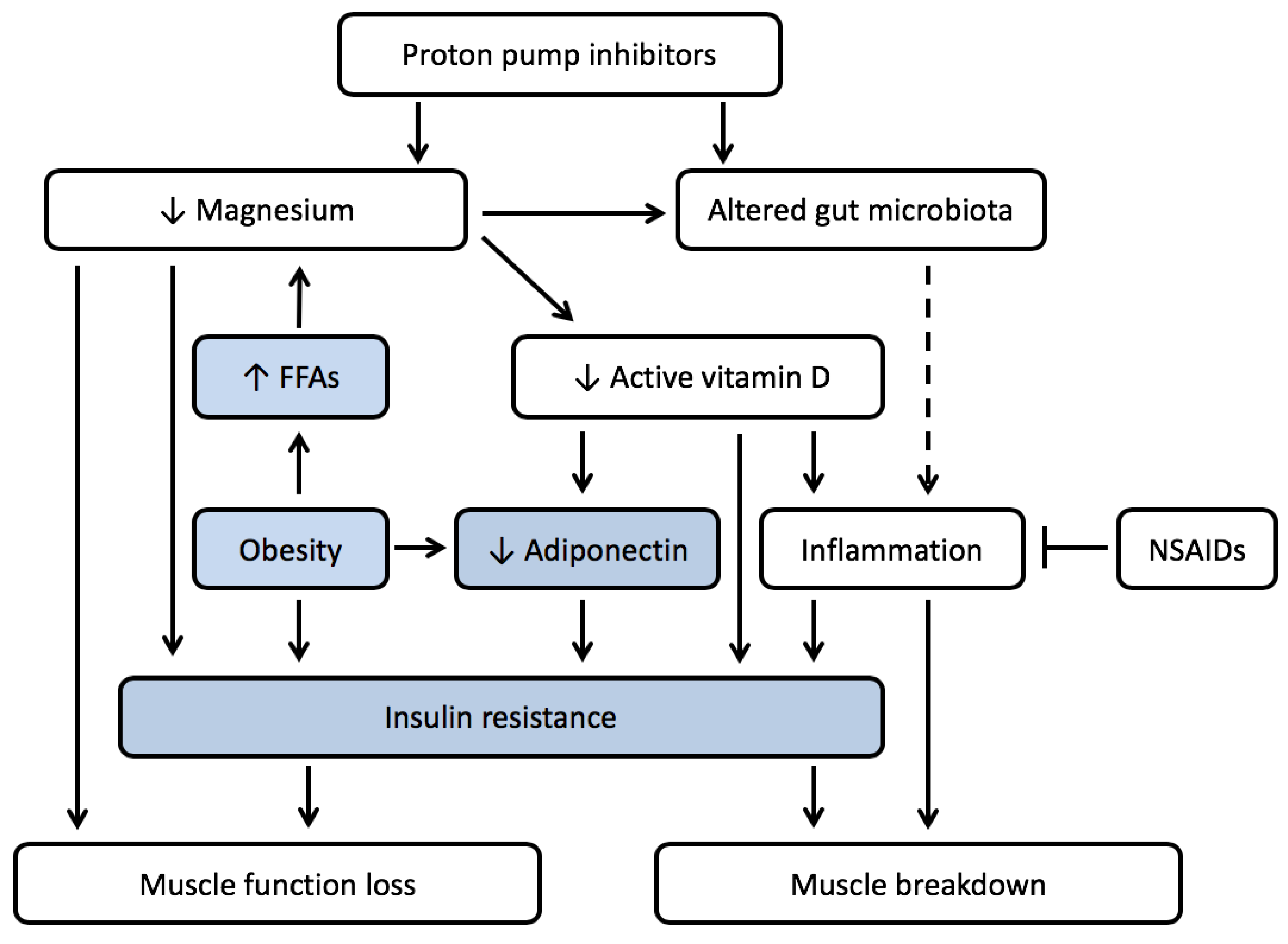
4. PPI Use and Muscle Wasting in the Presence of Obesity
4.1. Low Magnesium Levels Are Often Seen in Obesity
4.2. Low Magnesium Levels Lead to Insulin Resistance
4.3. Vitamin D Deficiency Is Prevalent in Obesity
4.4. PPI Use Might Increase Prevalence of Micronutrient Deficiences and Enhance Development of Sarcopenic Obesity
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CCK | Cholecystokinin |
| COPD | chronic obstructive pulmonary disease |
| FFAs | free fatty acids |
| GLP-1 | Glucagon-like peptide 1 |
| HF | heart failure |
| IFN-γ | interferon-γ |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| IR | insulin resistance |
| MHOB | Metabolically Healthy Obesity |
| MONW | Metabolically Obese Normal Weight |
| MUO | Metabolically Unhealthy Obesity |
| NFκB | Nuclear Factor kappa B |
| NMDA | N-methyl-d-aspartate |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| PGE2 | prostaglandin E2 |
| PPIs | proton pump inhibitors |
| PYY | Peptide YY |
| T2DM | type II diabetes mellitus |
| TNF-α | tumor necrosis factor- α |
| TRPM6 | transient receptor potential melastatin 6 |
| TRPM7 | transient receptor potential melastatin 7 |
| VDBP | vitamin D binding protein |
References
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S. Cancer Cachexia Study Group Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.A.; Reid, J.; Hill, L.; Fitzsimons, D. The need for a specific definition of cardiac cachexia. Eur. J. Cardiovasc. Nurs. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Anker, M.S.; Anker, S.D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: Facts and numbers update 2016. J. Cachexia Sarcopenia Muscle 2016, 7, 507–509. [Google Scholar] [CrossRef]
- Society on Sarcopenia Cachexia and Wasting Disorders Definition of Cachexia and Sarcopenia. Available online: http://society-scwd.org/cachexia-definition/ (accessed on 15 May 2018).
- Scherbakov, N.; Doehner, W. Cachexia as a common characteristic in multiple chronic disease. J. Cachexia Sarcopenia Muscle 2019, 97, 1189–1191. [Google Scholar] [CrossRef]
- Garcia, J.M. What is next after anamorelin? Curr. Opin. Support. Palliat. Care 2017, 11, 266–271. [Google Scholar] [CrossRef]
- Tessier, A.J.; Wing, S.S.; Rahme, E.; Morais, J.A.; Chevalier, S. Physical function-derived cut-points for the diagnosis of sarcopenia and dynapenia from the Canadian longitudinal study on aging. J. Cachexia Sarcopenia Muscle 2019, 10, 985–999. [Google Scholar] [CrossRef]
- Laviano, A.; Koverech, A.; Seelaender, M. Assessing pathophysiology of cancer anorexia. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 340–345. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Yoshikawa, T.; Matsumoto, A.; Svaninger, G.; Gelin, J. Are cytokines possible mediators of cancer cachexia? Surg. Today 1996, 26, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Ebner, N.; dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Dwarkasing, J.T.; Marks, D.L.; Witkamp, R.F.; Van Norren, K. Hypothalamic inflammation and food intake regulation during chronic illness. Peptides 2016, 77, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Dwarkasing, J.T.; van Dijk, M.; Dijk, F.J.; Boekschoten, M.V.; Faber, J.; Argilès, J.M.; Laviano, A.; Müller, M.; Witkamp, R.F.; van Norren, K. Hypothalamic food intake regulation in a cancer-cachectic mouse model. J. Cachexia Sarcopenia Muscle 2014, 5, 159–169. [Google Scholar] [CrossRef]
- Van der Ende, M.; Grefte, S.; Plas, R.; Meijerink, J.; Witkamp, R.F.; Keijer, J.; van Norren, K. Mitochondrial dynamics in cancer-induced cachexia. Biochim. Biophys. Acta 2018, 1870, 137–150. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Muscle wasting in cancer: The role of mitochondria. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 221–225. [Google Scholar] [CrossRef]
- Argilés, J.M.; Fontes-Oliveira, C.C.; Toledo, M.; López-Soriano, F.J.; Busquets, S. Cachexia: A problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 2014, 5, 279–286. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef]
- Martin, L.; Hopkins, J.; Malietzis, G.; Jenkins, J.T.; Sawyer, M.B.; Brisebois, R.; MacLean, A.; Nelson, G.; Gramlich, L.; Baracos, V.E. Assessment of Computed Tomography (CT)-Defined Muscle and Adipose Tissue Features in Relation to Short-Term Outcomes After Elective Surgery for Colorectal Cancer: A Multicenter Approach. Ann. Surg. Oncol. 2018, 25, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.L.; Birdsell, L.A.; Martin, L.; Baracos, V.E.; Fearon, K.C.H. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin. Cancer Res. 2009, 15, 6973–6979. [Google Scholar] [CrossRef] [PubMed]
- Vecchié, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Frühbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Boucherie, Q.; Rouby, F.; Frankel, D.; Roll, P.; Micallef, J. Proton pump inhibitors prescriptions in France: Main trends from 2006 to 2016 on French health insurance database. Therapie 2018, 73, 385–388. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, S.; Pan, K.; Li, X.; Zhao, X.; Zhou, Y.; Cui, Y.; Liu, X.M. Potentially inappropriate medications in hospitalized older patients: A cross-sectional study using the Beers 2015 criteria versus the 2012 criteria. Clin. Interv. Aging 2017, 12, 1697–1703. [Google Scholar] [CrossRef]
- Parsons, C.; Johnston, S.; Mathie, E.; Baron, N.; MacHen, I.; Amador, S.; Goodman, C. Potentially inappropriate prescribing in older people with dementia in care homes: A retrospective analysis. Drugs Aging 2012, 29, 143–155. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Ishimura, N.; Ishihara, S. Advantages and disadvantages of long-term proton pump inhibitor use. J. Neurogastroenterol. Motil. 2018, 24, 182–196. [Google Scholar] [CrossRef]
- Scarpignato, C.; Gatta, L.; Zullo, A.; Blandizzi, C. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016, 14, 179. [Google Scholar] [CrossRef]
- Benmassaoud, A.; McDonald, E.G.; Lee, T.C. Potential harms of proton pump inhibitor therapy: Rare adverse effects of commonly used drugs. CMAJ 2016, 188, 657–662. [Google Scholar] [CrossRef]
- Shin, J.M.; Sachs, G. Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Asahara, T.; Nagahara, A.; Takeda, T.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Asaoka, D.; Takahashi, T.; Nomoto, K.; et al. Gut Microbiota Composition Before and After Use of Proton Pump Inhibitors. Dig. Dis. Sci. 2018, 63, 2940–2949. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Important drug-micronutrient interactions: A selection for clinical practice. Crit. Rev. Food Sci. Nutr. 2018, 1–19. [Google Scholar] [CrossRef]
- Mohn, E.; Kern, H.; Saltzman, E.; Mitmesser, S.; McKay, D. Evidence of Drug–Nutrient Interactions with Chronic Use of Commonly Prescribed Medications: An Update. Pharmaceutics 2018, 10, 36. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef]
- Sheen, E.; Triadafilopoulos, G. Adverse effects of long-term proton pump inhibitor therapy. Dig. Dis. Sci. 2011, 56, 931–950. [Google Scholar] [CrossRef]
- Cernea, A.; Fernández-Martínez, J.L.; de Andrés-Galiana, E.J.; Fernández-Muñiz, Z.; Bermejo-Millo, J.C.; González-Blanco, L.; Solano, J.J.; Abizanda, P.; Coto-Montes, A.; Caballero, B. Prognostic networks for unraveling the biological mechanisms of Sarcopenia. Mech. Ageing Dev. 2019, 182, 111129. [Google Scholar] [CrossRef]
- Clark, D.W.J.; Strandell, J. Myopathy including polymyositis: A likely class adverse effect of proton pump inhibitors? Eur. J. Clin. Pharmacol. 2006, 62, 473–479. [Google Scholar] [CrossRef]
- Capogrosso Sansone, A.; Convertino, I.; Galiulo, M.T.; Salvadori, S.; Pieroni, S.; Knezevic, T.; Mantarro, S.; Marino, A.; Hauben, M.; Blandizzi, C.; et al. Muscular Adverse Drug Reactions Associated with Proton Pump Inhibitors: A Disproportionality Analysis Using the Italian National Network of Pharmacovigilance Database. Drug Saf. 2017, 40, 895–909. [Google Scholar] [CrossRef]
- Vaezi, M.F.; Yang, Y.X.; Howden, C.W. Complications of Proton Pump Inhibitor Therapy. Gastroenterology 2017, 153, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Iván, I.; Gábor, S.; Sándor, B.; Miklós, S. Adverse effects of long-term proton-pump inhibitor therapy on adults. Orv. Hetil. 2018, 159, 735–740. [Google Scholar]
- Van Dijk, M.; Dijk, F.J.; Hartog, A.; van Norren, K.; Verlaan, S.; van Helvoort, A.; Jaspers, R.T.; Luiking, Y. Reduced dietary intake of micronutrients with antioxidant properties negatively impacts muscle health in aged mice. J. Cachexia Sarcopenia Muscle 2018, 9, 146–159. [Google Scholar] [CrossRef]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef] [PubMed]
- Vinke, P.; Jansen, S.M.; Witkamp, R.F.; van Norren, K. Increasing quality of life in pulmonary arterial hypertension: Is there a role for nutrition? Heart Fail. Rev. 2018, 23, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Aslani, S.; Ataollahi, M.R.; Mahmoudi, M. The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019, 27, 649–661. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- William, J.H.; Danziger, J. Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms. World J. Nephrol. 2016, 5, 152. [Google Scholar] [CrossRef]
- Thongon, N.; Krishnamra, N. Apical acidity decreases inhibitory effect of omeprazole on Mg2+ absorption and claudin-7 and -12 expression in Caco-2 monolayers. Exp. Mol. Med. 2012, 44, 684–693. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Ong, E.; Wilson, R. Hypomagnesaemia associated with long-term use of proton pump inhibitors. Gastroenterol. Rep. 2015, 3, 243–253. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, E.H.; Roh, Y.H.; Kim, H.Y.; Lee, S.K. The Association between the Use of Proton Pump Inhibitors and the Risk of Hypomagnesemia: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e112558. [Google Scholar] [CrossRef] [PubMed]
- Van Orten-Luiten, A.C.B.; Janse, A.; Verspoor, E.; Brouwer-Brolsma, E.M.; Witkamp, R.F. Drug use is associated with lower plasma magnesium levels in geriatric outpatients; possible clinical relevance. Clin. Nutr. 2018, 38, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Kittanamongkolchai, W.; Srivali, N.; Edmonds, P.J.; Ungprasert, P.; O’Corragain, O.A.; Korpaisarn, S.; Erickson, S.B. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren. Fail. 2015, 37, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Lauretani, F.; Bandinelli, S.; Bos, A.; Corsi, A.M.; Simonsick, E.M.; Ferrucci, L. Magnesium and muscle performance in older persons: The InCHIANTI study. Am. J. Clin. Nutr. 2006, 84, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary Magnesium Is Positively Associated with Skeletal Muscle Power and Indices of Muscle Mass and May Attenuate the Association between Circulating C-Reactive Protein and Muscle Mass in Women. J. Bone Miner. Res. 2016, 31, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; de Groot, L.C.P.G.M.; Mijnarends, D.M.; de Vries, J.H.M.; Verlaan, S.; Meijboom, S.; Luiking, Y.C.; Schols, J.M.G.A. Differences in Nutrient Intake and Biochemical Nutrient Status Between Sarcopenic and Nonsarcopenic Older Adults-Results From the Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef]
- Veronese, N.; Berton, L.; Carraro, S.; Bolzetta, F.; De Rui, M.; Perissinotto, E.; Toffanello, E.D.; Bano, G.; Pizzato, S.; Miotto, F.; et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 974–981. [Google Scholar] [CrossRef]
- Van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11. [Google Scholar] [CrossRef]
- Garrison, S.R.; Allan, G.M.; Sekhon, R.K.; Musini, V.M.; Khan, K.M. Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev. 2012, 9, CD009402. [Google Scholar] [CrossRef]
- Young, G.; Jewell, D. Interventions for leg cramps in pregnancy. Cochrane Database Syst. Rev. 2002, 1, CD000121. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Mediators of cachexia in cancer patients. Nutrition 2019, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rayssiguier, Y.; Libako, P.; Nowacki, W.; Rock, E. Magnesium deficiency and metabolic syndrome: Stress and inflammation may reflect calcium activation. Magnes. Res. 2010, 23, 73–80. [Google Scholar] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Risco, F.; Traba, M.L. Influence of magnesium on the in vitro synthesis of 24,25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3. Magnes. Res. 1992, 5, 5–14. [Google Scholar]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Gonçalves De Carvalho, C.M.R.; Ribeiro, S.M.L. Aging, low-grade systemic inflammation and Vitamin D: A mini-review. Eur. J. Clin. Nutr. 2017, 71, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Feldman, D. Mechanisms of the Anti-Cancer and Anti-Inflammatory Actions of Vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, S.; Ditah, I.; Adler, D.G.; Ehrinpreis, M.N. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am. J. Gastroenterol. 2012, 107, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Bouwknegt, M.; van Pelt, W.; Kubbinga, M.E.; Weda, M.; Havelaar, A.H. Potential association between the recent increase in campylobacteriosis incidence in the Netherlands and proton-pump inhibitor use—An ecological study. Eurosurveillance 2014, 19, 21–26. [Google Scholar] [CrossRef]
- Leonard, J.; Marshall, J.K.; Moayyedi, P. Systematic Review of the Risk of Enteric Infection in Patients Taking Acid Suppression. Am. J. Gastroenterol. 2007, 102, 2047–2056. [Google Scholar] [CrossRef]
- McDonald, E.G.; Milligan, J.; Frenette, C.; Lee, T.C. Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA Intern. Med. 2015, 175, 784. [Google Scholar] [CrossRef]
- Pachikian, B.D.; Neyrinck, A.M.; Deldicque, L.; De Backer, F.C.; Catry, E.; Dewulf, E.M.; Sohet, F.M.; Bindels, L.B.; Everard, A.; Francaux, M.; et al. Changes in Intestinal Bifidobacteria Levels Are Associated with the Inflammatory Response in Magnesium-Deficient Mice. J. Nutr. 2010, 140, 509–514. [Google Scholar] [CrossRef]
- Hess, M.W.; De Baaij, J.H.F.; Broekman, M.; Bisseling, T.M.; Haarhuis, B.; Tan, A.; Te Morsche, R.; Hoenderop, J.G.J.; Bindels, R.J.M.; Drenth, J.P.H. Inulin significantly improves serum magnesium levels in proton pump inhibitor-induced hypomagnesaemia. Aliment. Pharmacol. Ther. 2016, 43, 1178–1185. [Google Scholar] [CrossRef]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sorensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef]
- Gommers, L.M.M.; Ederveen, T.H.A.; van der Wijst, J.; Overmars-Bos, C.; Kortman, G.A.M.; Boekhorst, J.; Bindels, R.J.M.; de Baaij, J.H.F.; Hoenderop, J.G.J. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI-induced hypomagnesemia. FASEB J. 2019, 33, 11235–11246. [Google Scholar] [CrossRef] [PubMed]
- Van Norren, K.; Dwarkasing, J.T.; Witkamp, R.F. The role of hypothalamic inflammation, the hypothalamic-pituitary-Adrenal axis and serotonin in the cancer anorexia-cachexia syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Witkamp, R.F.; van Norren, K. Let thy food be thy medicine….when possible. Eur. J. Pharmacol. 2018, 836, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Luttikhold, J.; De Ruijter, F.M.; Van Norren, K.; Diamant, M.; Witkamp, R.F.; Van Leeuwen, P.A.M.; Vermeulen, M.A.R. Review article: The role of gastrointestinal hormones in the treatment of delayed gastric emptying in critically ill patients. Aliment. Pharmacol. Ther. 2013, 38, 573–583. [Google Scholar] [CrossRef]
- Sanders, K.J.C.; Kneppers, A.E.M.; van de Bool, C.; Langen, R.C.J.; Schols, A.M.W.J. Cachexia in chronic obstructive pulmonary disease: New insights and therapeutic perspective. J. Cachexia Sarcopenia Muscle 2016, 7, 5–22. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Flores-García, A.; Saldaña-Guerrero, S.; Simental-Mendía, L.E.; Rodríguez-Morán, M. Obesity and hypomagnesemia. Eur. J. Intern. Med. 2016, 34, 29–33. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium, inflammation, and obesity in chronic disease. Nutr. Rev. 2010, 68, 333–340. [Google Scholar] [CrossRef]
- Johansson, H.E.; Zethelius, B.; Öhrvall, M.; Sundbom, M.; Haenni, A. Serum magnesium status after gastric bypass surgery in obesity. Obes. Surg. 2009, 19, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Günther, T. Hypomagnesemia, obesity and inflammatory cytokines. Magnes. Res. 2011, 24, 19–20. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, I.; Vansant, G.; Van Gaal, L. Magnesium and obesity: Influence of gender, glucose tolerance, and body fat distribution on circulating magnesium concentrations. Magnes. Res. 1992, 5, 183–187. [Google Scholar] [PubMed]
- Farhanghi, M.A.; Mahboob, S.; Ostadrahimi, A. Obesity induced Magnesium deficiency can be treated by vitamin D supplementation. J. Pak. Med. Assoc. 2009, 59, 258–261. [Google Scholar] [PubMed]
- Resnick, L.M. Cellular ions in hypertension, insulin resistance, obesity, and diabetes: A unifying theme. J. Am. Soc. Nephrol. 1992, 3, S78–S85. [Google Scholar]
- De Oliveira, A.R.S.; Cruz, K.J.C.; Severo, J.S.; Morais, J.B.S.; De Freitas, T.E.C.; Araújo, R.S.; Do Nascimento Marreiro, D. Hypomagnesemia and its relation with chronic low-grade inflammation in obesity. Rev. Assoc. Med. Bras. 2017, 63, 156–163. [Google Scholar] [CrossRef]
- Mostafavi, E.; Nargesi, A.A.; Asbagh, F.A.; Ghazizadeh, Z.; Heidari, B.; Mirmiranpoor, H.; Esteghamati, A.; Vigneron, C.; Nakhjavani, M. Abdominal obesity and gestational diabetes: The interactive role of magnesium. Magnes. Res. 2015, 28, 116–125. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; Dos Santos, L.R.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Kurpad, A.V.; Aeberli, I. Low serum magnesium and obesity—Causal role or diet biomarker? Indian Pediatr. 2012, 49, 100–101. [Google Scholar]
- Morrell, J.S.; Lofgren, I.E.; Burke, J.D.; Reilly, R.A. Metabolic syndrome, obesity, and related risk factors among college men and women. J. Am. Coll. Heal. 2012, 60, 82–89. [Google Scholar] [CrossRef]
- Liu, D.; Yu, L.; Li, S.; Zhang, Q.; Zhu, L.; Liu, Q.; Lin, H.; Zhang, J. Association between serum magnesium and blood count: Influence of type 2 diabetes and central obesity. Br. J. Nutr. 2019, 121, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Grantham, N.; Ebeling, P.R.; Daly, R.M. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: Results from a national, population-based prospective study (the Australian diabetes, obesity and lifestyle study). Diabetes Care 2011, 34, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Bügel, S. Overfed but undernourished: Recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int. J. Obes. 2019, 43, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card. Fail. 2019, 25, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Kurstjens, S.; de Baaij, J.H.F.; Overmars-Bos, C.; van den Munckhof, I.C.L.; Garzero, V.; de Vries, M.A.; Burggraaf, B.; van Diepen, J.A.; Riksen, N.P.; Rutten, J.H.W.; et al. Increased NEFA levels reduce blood Mg2+ in hypertriacylglycerolaemic states via direct binding of NEFA to Mg2+. Diabetologia 2019, 62, 311–321. [Google Scholar] [CrossRef] [PubMed]
- McGill, A.T.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of low serum vitamin D 3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Slusher, A.L.; McAllister, M.J.; Huang, C.J. A therapeutic role for vitamin D on obesity-associated inflammation and weight-loss intervention. Inflamm. Res. 2015, 64, 565–575. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Stokić, E.; Kupusinac, A.; Tomic-Naglic, D.; Smiljenic, D.; Kovacev-Zavisic, B.; Srdic-Galic, B.; Soskic, S.; Isenovic, E.R. Vitamin D and Dysfunctional Adipose Tissue in Obesity. Angiology 2015, 66, 613–618. [Google Scholar] [CrossRef]
- Gao, D.; Trayhurn, P.; Bing, C. 1,25-Dihydroxyvitamin D 3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int. J. Obes. 2013, 37, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Lanas-Gimeno, A.; Hijos, G.; Lanas, Á. Proton pump inhibitors, adverse events and increased risk of mortality. Expert Opin. Drug Saf. 2019, 18, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.F.; Liao, H.F.; Perng, D.W.; Chou, Y.C.; Hsu, C.C.; Chou, C.L.; Chang, Y.L.; Yen, J.C.; Chen, T.J.; Chou, T.C. Proton pump inhibitors use is associated with a lower risk of acute exacerbation and mortality in patients with coexistent COPD and GERD. Int. J. COPD 2018, 13, 2907–2915. [Google Scholar] [CrossRef]
- Cabras, P.; Anedda, M.; Caddeo, L.; Francesco, M.; Antonella, M. Hypomagnesemia and Hypocalcemia Caused by Proton-Pump Inhibitors Long-Term Therapy. Am. J. Ther. 2019. [Google Scholar] [CrossRef]
- Liao, S.; Gan, L.; Mei, Z. Does the use of proton pump inhibitors increase the risk of hypomagnesemia: An updated systematic review and meta-analysis. Medicine 2019, 98, e15011. [Google Scholar] [CrossRef]
- Klein, G.L.; Petschow, B.W.; Shaw, A.L.; Weaver, E. Gut barrier dysfunction and microbial translocation in cancer cachexia: A new therapeutic target. Curr. Opin. Support. Palliat. Care 2013, 7, 361–367. [Google Scholar] [CrossRef]
- Cascino, T.M.; Hummel, S.L. Nutrient deficiencies in heart failure: A micro problem with macro effects? J. Am. Heart Assoc. 2018, 7, 1–3. [Google Scholar] [CrossRef]
- Soukoulis, V.; Dihu, J.B.; Sole, M.; Anker, S.D.; Cleland, J.; Fonarow, G.C.; Metra, M.; Pasini, E.; Strzelczyk, T.; Taegtmeyer, H.; et al. Micronutrient Deficiencies. An Unmet Need in Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 1660–1673. [Google Scholar] [CrossRef]
- Sundaram, V.; Fang, J.C. Gastrointestinal and Liver Issues in Heart Failure. Circulation 2016, 133, 1696–1703. [Google Scholar] [CrossRef]
- Schols, A.M.W.J. Nutrition as a metabolic modulator in COPD. Chest 2013, 144, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Horadagoda, C.; Dinihan, T.; Roberts, M.; Kairaitis, K. Body composition and micronutrient deficiencies in patients with an acute exacerbation of chronic obstructive pulmonary disease. Intern. Med. J. 2017, 47, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Rutten-Van Mölken, M.; Slinde, F.; Steiner, M.C.; et al. Nutritional assessment and therapy in COPD: A European respiratory society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Witkowski, M.; Hubert, J.; Mazur, A. Methods of assessment of magnesium status in humans: A systematic review. Magnes. Res. 2011, 24, 163–180. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Rjater, R.G.; Kale-Pradhan, P.B. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev. Clin. Pharmacol. 2013, 6, 443–451. [Google Scholar] [CrossRef]
- Yu, L.Y.; Sun, L.N.; Zhang, X.H.; Li, Y.Q.; Yu, L.; Meng, L.; Zhang, H.W.; Wang, Y.Q. A Review of the Novel Application and Potential Adverse Effects of Proton Pump Inhibitors. Adv. Ther. 2017, 34, 1070–1086. [Google Scholar] [CrossRef]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinke, P.; Wesselink, E.; van Orten-Luiten, W.; van Norren, K. The Use of Proton Pump Inhibitors May Increase Symptoms of Muscle Function Loss in Patients with Chronic Illnesses. Int. J. Mol. Sci. 2020, 21, 323. https://doi.org/10.3390/ijms21010323
Vinke P, Wesselink E, van Orten-Luiten W, van Norren K. The Use of Proton Pump Inhibitors May Increase Symptoms of Muscle Function Loss in Patients with Chronic Illnesses. International Journal of Molecular Sciences. 2020; 21(1):323. https://doi.org/10.3390/ijms21010323
Chicago/Turabian StyleVinke, Paulien, Evertine Wesselink, Wout van Orten-Luiten, and Klaske van Norren. 2020. "The Use of Proton Pump Inhibitors May Increase Symptoms of Muscle Function Loss in Patients with Chronic Illnesses" International Journal of Molecular Sciences 21, no. 1: 323. https://doi.org/10.3390/ijms21010323
APA StyleVinke, P., Wesselink, E., van Orten-Luiten, W., & van Norren, K. (2020). The Use of Proton Pump Inhibitors May Increase Symptoms of Muscle Function Loss in Patients with Chronic Illnesses. International Journal of Molecular Sciences, 21(1), 323. https://doi.org/10.3390/ijms21010323




