CT2A Cell Viability Modulated by Electromagnetic Fields at Extremely Low Frequency under No Thermal Effects
Abstract
:1. Introduction
2. Results
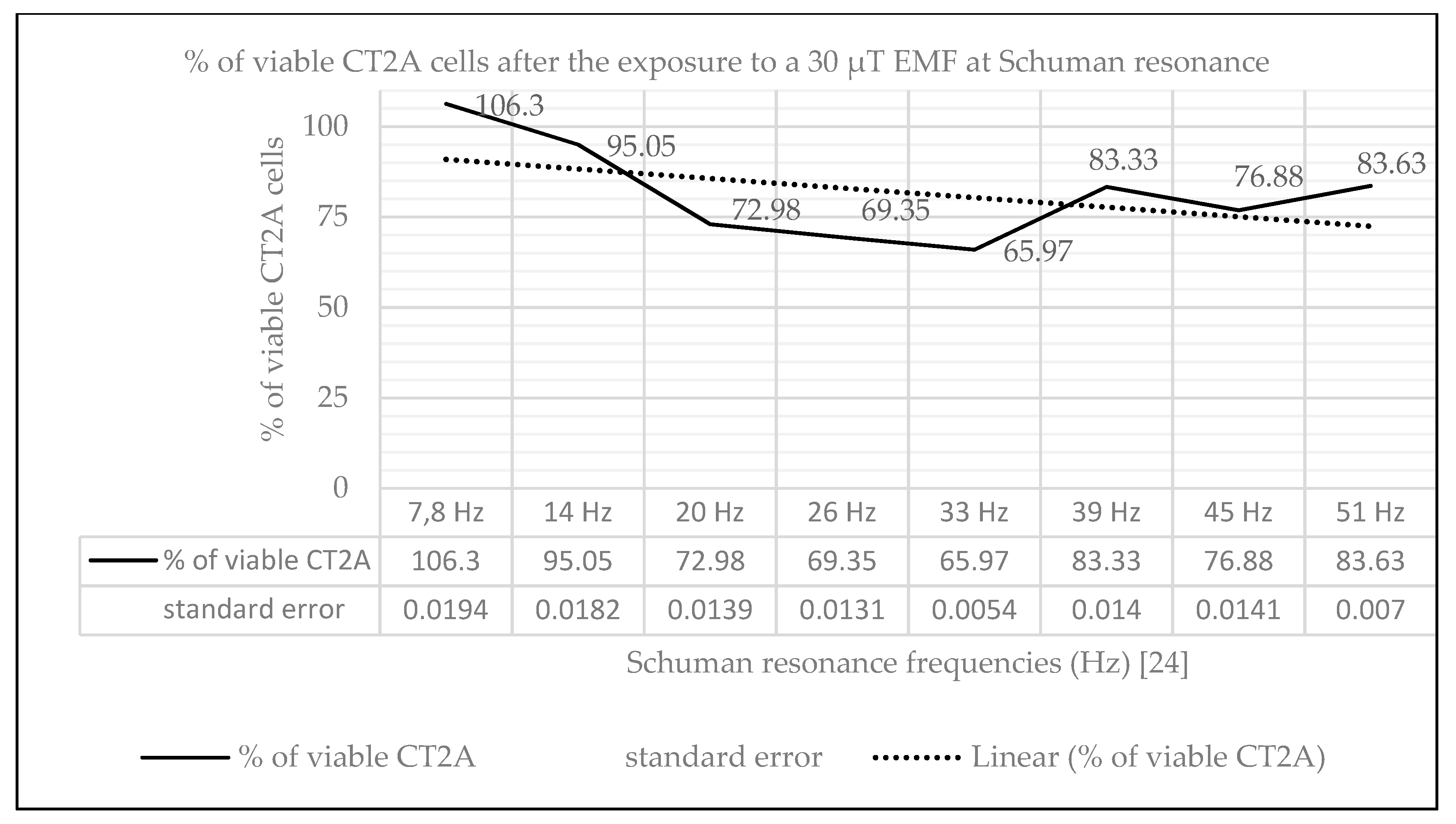
2.1. Effect of Natural EMF on Cell Viability
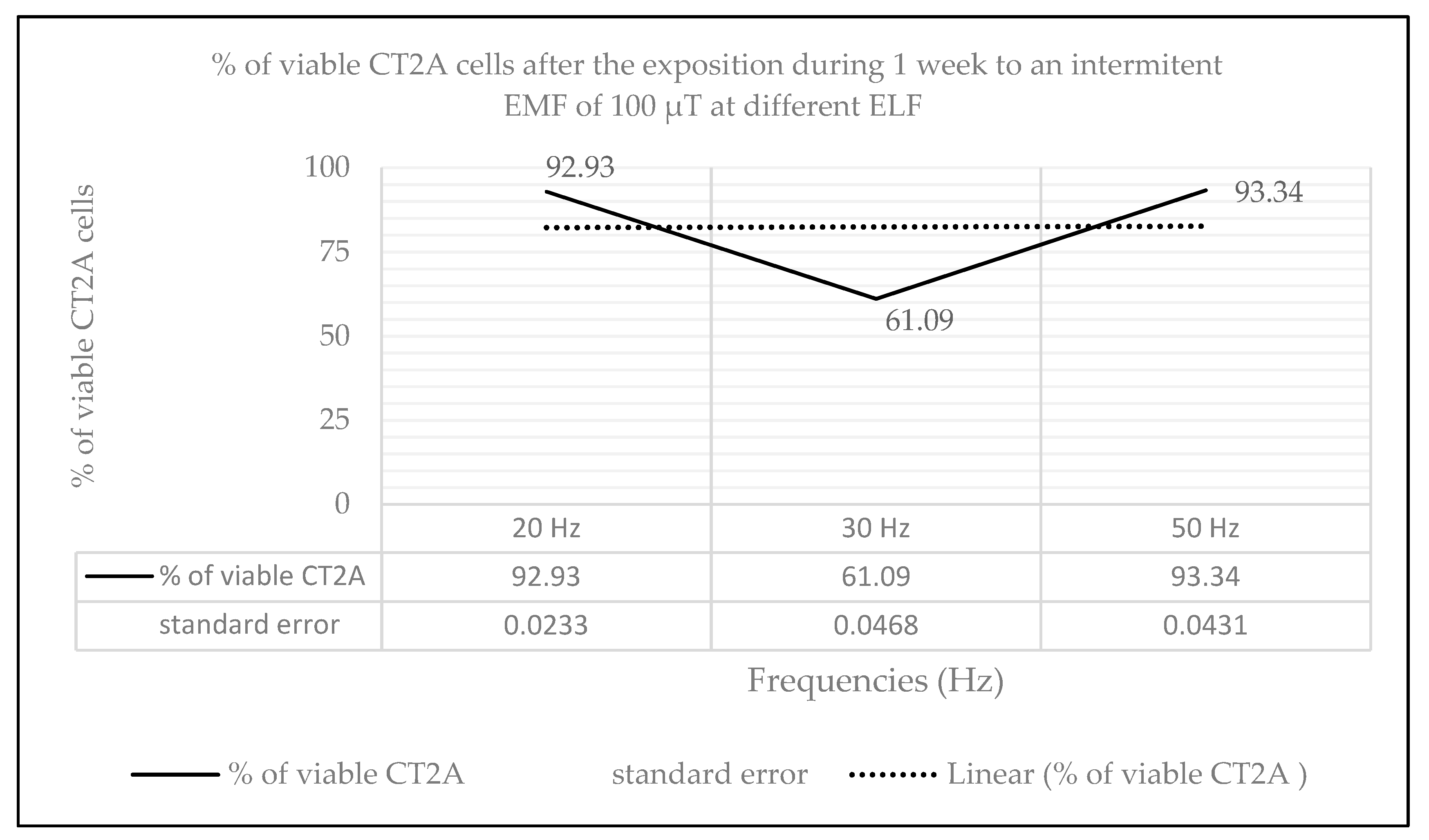
2.2. Effects of Long-Term and Intermittent Exposure to a 100 µT EMF at Different ELF
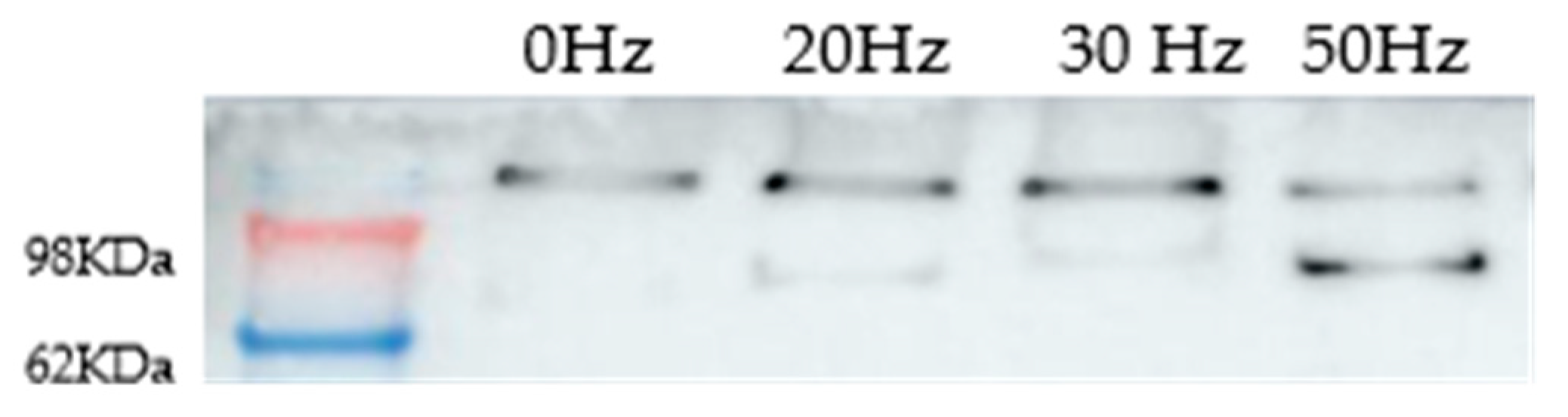
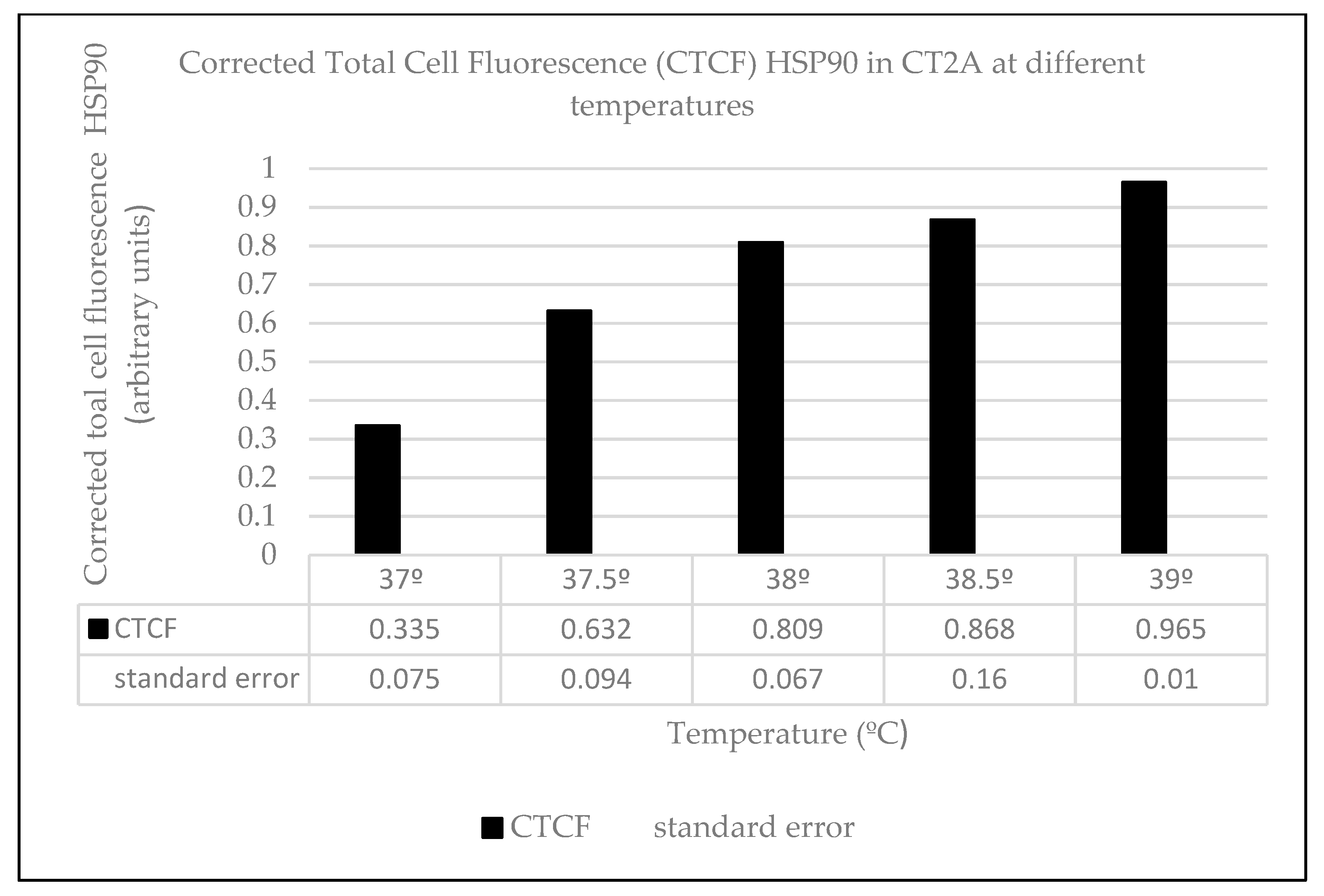
2.3. Heat Effects Produced of CT2A Exposed to ELF-EMF of 100 µT
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Exposure System
4.3. Methodology
4.4. Cell Viability
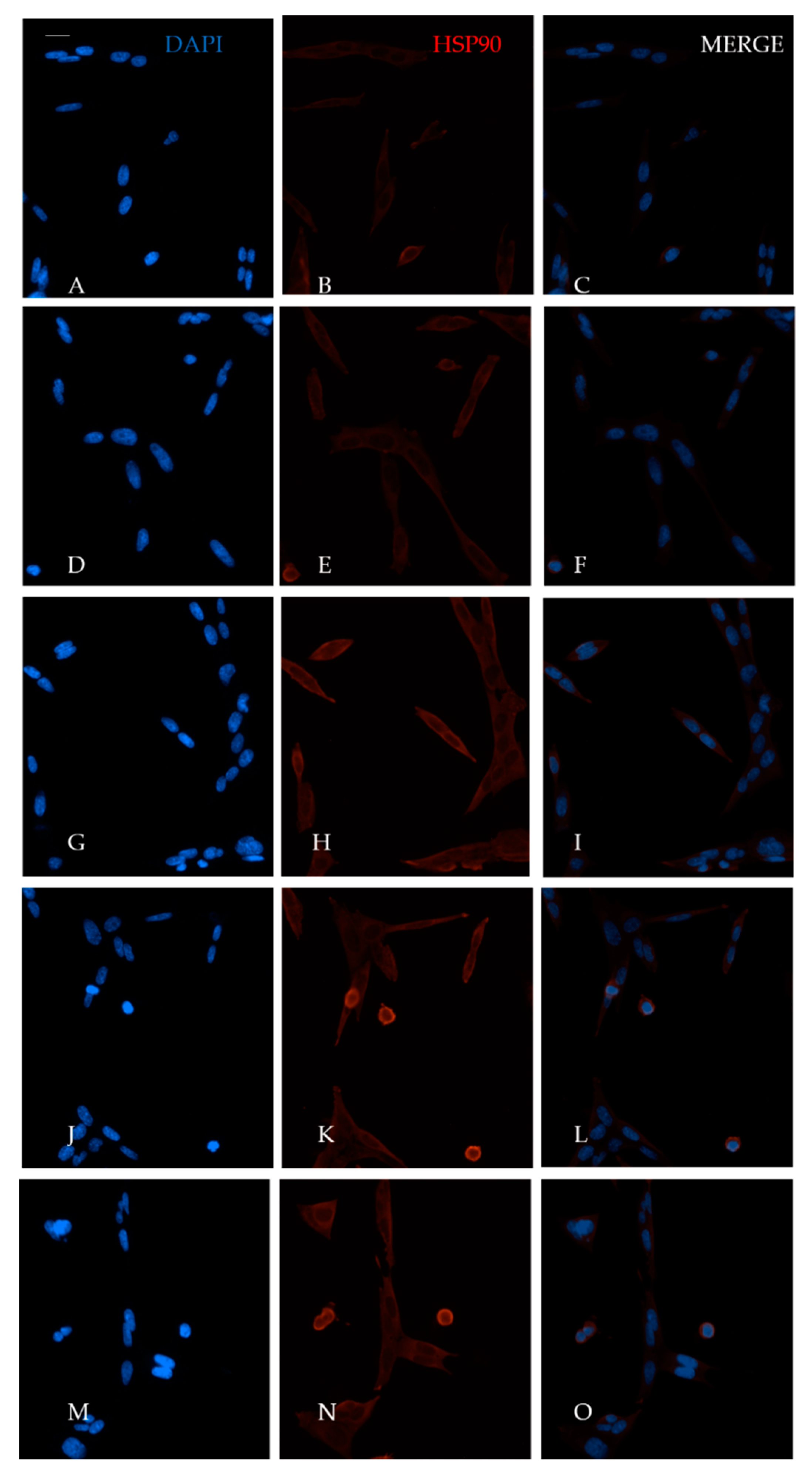
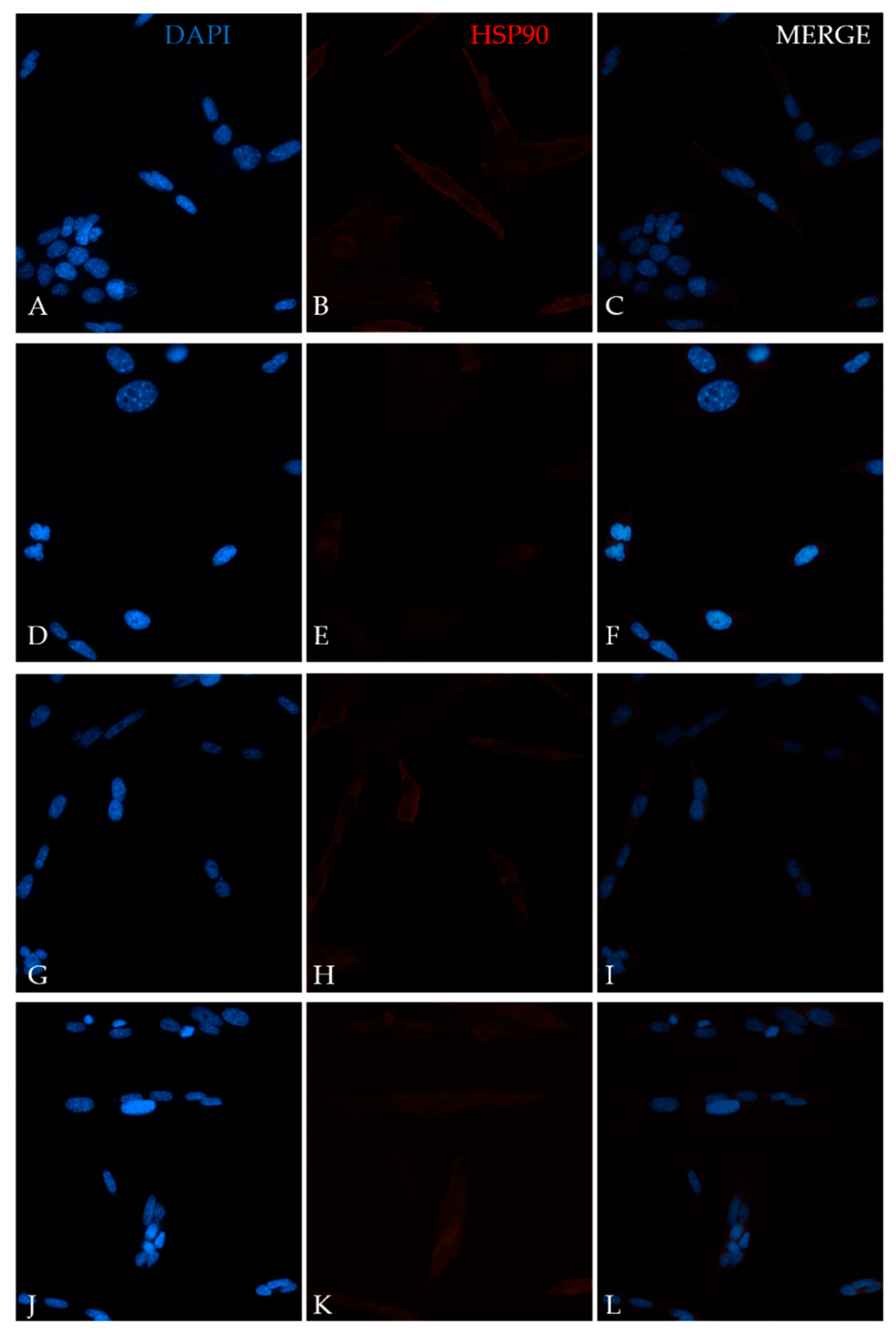
4.5. Immunofluorescence Assay
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Korada, U. Introduction to Engineering Electromagnetic Fields. In Advanced Series in Electrical and Computer Engineering; World Scientific Pub Co Inc.: Singapore, 1989. [Google Scholar]
- WHO. What Are Electromagnetic Fields? Available online: https://www.who.int/peh-emf/about/WhatisEMF/en/ (accessed on 28 October 2019).
- Council Recommendation 1999/519/EC. Council Recommendation of 12 July 1999 on the Limitation of Exposure of the General Public to Electromagnetic Fields (0 Hz to 300 GHz). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1999:199:0059:0070:EN:PDF (accessed on 20 October 2019).
- Adlkofer, F.; Tauber, R.W.; Rüdiger, H.G.; Wobus, A.M.; Trillo, A.; Leszczynski, D.; Kolb, H.A.; Bersani, F.; Lagroye, I.; Kuster, N.; et al. Risk Evaluation of Potential Environmental Hazards from Low Energy Electromagnetic Field Exposure Using Sensitive in vitro Methods. In Final Report of a Project Funded by the European Union under the Programme Quality of Life and Management of Living Resources; Springer: Dordrecht, The Netherlands, 2004; Available online: https://itis.swiss/assets/Downloads/Papers-Reports/Reports/REFLEXFinal-Report171104.pdf (accessed on 12 May 2019).
- García-Minguillán López, O.; Jiménez Valbuena, A.; Maestú Unturbe, C. Significant Cellular Viability Dependence on Time Exposition at ELF-EMF and RF-EMF In Vitro Studies. Int. J. Environ. Res. Public Health 2019, 16, 2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walleczek, J. Electromagnetic field effects on cells of the immune system: The role of calcium signaling. FASEB J. 1992, 6, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Grassi, C.; D’Ascenzo, M.; Torsello, A.; Martinotti, G.; Wolf, F.; Cittadini, A.; Azzena, G.B. Effects of 50 Hz electromagnetic fields on voltage-gated Ca2+ channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calc. 2004, 35, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kum, C.; Choi, H.J.; Park, E.S.; Sohn, U.D. Extremely low frequency magnetic field induces hyperalgesia in mice modulated by nitric oxide synthesis. Life Sci. 2006, 78, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Lorich, D.G.; Brighton, C.T.; Gupta, R.; Corsetti, J.R.; Levine, S.E.; Gelb, I.D.; Seldes, R.; Pollack, S.R. Biochemical pathway mediating the response of bone cells to capacitive coupling. Clin. Orthop. Relat. Res. 1998, 350, 246–256. [Google Scholar] [CrossRef]
- Piacentini, R.; Ripoli, C.; Mezzogori, D.; Azzena, G.B.; Grassi, C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J. Cell. Physiol. 2008, 215, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Intracellular calcium homeostasis and signaling. Met. Ions Life Sci. 2013, 12, 119–168. [Google Scholar] [CrossRef]
- Brini, M.; Ottolini, D.; Calì, T.; Carafoli, E. Calcium in health and disease. Met. Ions Life Sci. 2013, 13, 81–137. [Google Scholar] [CrossRef]
- Martin, L.P. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef]
- Luukkonen, J.; Höytö, A.; Sokka, M.; Liimatainen, A.; Syväoja, J.; Juutilainen, J.; Naarala, J. Modification of p21 level and cell cycle distribution by 50 Hz magnetic fields in human SH-SY5Y neuroblastoma cells. Int. J. Radiat. Biol. 2017, 93, 240–248. [Google Scholar] [CrossRef]
- Qinlong, M.; Ping, D.; Gang, Z.; Chuan, L.; Lei, Z.; Zhou, Z.; Xue, L.; Min, L.; Min, Z.; Zhengping, Y.; et al. Extremely Low-Frequency Electromagnetic Fields Affect Transcript Levels of Neuronal Differentiation-Related Genes in Embryonic Neural Stem Cells. PLoS ONE 2014, 9, e90041. [Google Scholar] [CrossRef]
- Solek, P.; Majchrowicz, L.; Bloniarz, D.; Krotoszynska, E.; Koziorowski, M. Pulsed or continuous electromagnetic field induce p53/p21-mediated apoptotic signaling pathway in mouse spermatogenic cells in vitro and thus may affect male fertility. Toxicology 2017, 382, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Akdag, M.Z.; Dasdag, S.; Uzunlar, A.K.; Ulukaya, E.; Oral, A.Y.; Çelik, N.; Akşen, F. Can safe and long-term exposure to extremely low frequency (50 Hz) magnetic fields affect apoptosis, reproduction, and oxidative stress? Int. J. Radiat. Biol. 2013, 89, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Tai, J.L.; Li, G.Q.; Zhang, Z.W.; Xue, J.H.; Liu, H.S.; Zhu, H.; Cheng, J.D.; Liu, Y.L.; Li, A.M.; et al. Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS ONE 2012, 7, e42332. [Google Scholar] [CrossRef] [Green Version]
- Lantow, M.; Lupke, M.; Frahm, J.; Mattsson, M.O.; Kuster, N.; Simko, M. ROS release and Hsp70 expression after exposure to 1800 MHz radiofrequency electromagnetic fields in primary human monocytes and lymphocytes. Radiat. Environ. Biophys. 2006, 45, 55–62. [Google Scholar] [CrossRef]
- Falone, S.; Santini, S.; Cordone, V.; Cesare, P.; Bonfigli, A.; Grannonico, M.; Di Emidio, G.; Tatone, C.; Cacchio, M.; Amicarelli, F. Power frequency magnetic field promotes a more malignant phenotype in neuroblastoma cells via redox-related mechanisms. Sci. Rep. 2017, 7, 11470. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.C.; Hong, M.N.; Jung, S.H.; Kim, B.C.; Suh, Y.J.; Ko, Y.G.; Lee, Y.S.; Lee, B.Y.; Cho, Y.G.; Myung, S.H.; et al. Effect of extremely low frequency magnetic fields on cell proliferation and gene expression. Bioelectromagnetics 2015, 36, 506–516. [Google Scholar] [CrossRef]
- Finlay, C.C.; Maus, S.; Beggan, C.D.; Bondar, T.N.; Chambodut, A.; Chernova, T.A.; Chulliat, A.; Golovkov, V.P.; Hamilton, B.; Hamoudi, M.; et al. International Geomagnetic Reference Field: The eleventh generation. Geophys. J. Int. 2010, 183, 1216–1230. [Google Scholar] [CrossRef] [Green Version]
- Votis, C.I.; Tatsis, G.; Christofilakis, V.; Chronopoulos, S.K.; Kostarakis, P.; Tritakis, V.; Repapis, C. A new portable ELF Schumann resonance receiver: Design and detailed analysis of the antenna and the analog front-end. EURASIP J. Wirel. Commun. Netw. 2018, 2018, 155. [Google Scholar] [CrossRef] [Green Version]
- Akbarnejad, Z.; Eskandary, H.; Vergallo, C.; Nematollahi-Mahani, S.N.; Dini, L.; Darvishzadeh-Mahani, F.; Ahmadi, M. Effects of extremely low-frequency pulsed electromagnetic fields (ELF-PEMFs) on glioblastoma cells (U87). Electromagn. Biol. Med. 2017, 36, 238–247. [Google Scholar] [CrossRef]
- Kim, S.; Im, W.S.; Kang, L.; Lee, S.T.; Chu, K.; Kim, B.I. The application of magnets directs the orientation of neurite outgrowth in cultured human neuronal cells. J. Neurosci. Methods 2008, 174, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Koziorowska, A.; Romerowicz-Misielak, M.; Sołek, P.; Koziorowski, M. Extremely low frequency variable electromagnetic fields affect cancer and noncancerous cells in vitro differently: Preliminary study. Electromagn. Biol. Med. 2018, 37, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Dagar, M.; Singh, J.P.; Dagar, G.; Tyagi, R.K.; Bagch, G. Phosphorylation of HSP90 by protein kinase A is essential for the nuclear translocation of androgen receptor. J. Biol. Chem. 2019, 294, 8699–8710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, J.L.; Perdew, G.H. Carboxyl Terminus of hsc70-Interacting Protein (CHIP) Can Remodel Mature Aryl Hydrocarbon Receptor (AhR) Complexes and Mediate Ubiquitination of Both the AhR and the 90 kDa Heat-Shock Protein (hsp90) in Vitro. Biochemistry 2007, 46, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.P.; Le Breton, L. Hsp90: Breaking the Symetry. Mol. Cell 2015, 58, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.P. Scientific evidence contradicts findings and assumptions of Canadian Safety Panel 6: Microwaves act through voltage-gated calcium channel activation to induce biological impacts at non-thermal levels, supporting a paradigm shift for microwave/lower frequency electromagnetic field action. Rev. Environ. Health 2015, 30, 99–116. [Google Scholar] [CrossRef]
- Liboff, R.A. Geomagnetic cyclotron resonance in living cells. J. Biol. Phys. 1985, 13, 99–102. [Google Scholar] [CrossRef]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. Empirical test of an ion parametric resonance model for magnetic field interactions with PC-12 cells. Bioelectromagnetics 1994, 15, 239–260. [Google Scholar] [CrossRef]
- Lerchl, A.; Reiter, R.J.; Howes, K.A.; Nonaka, O.N.; Stokkan, K.A. Evidence that extremely low frequency Ca-cyclotron resonance depresses pineal melatonin synthesis in vitro. Neurosci. Lett. 1991, 124, 213–215. [Google Scholar] [CrossRef]
- Liboff, R.A. Ion cyclotron resonance: Geomagnetic strategy for living systems? Electromagn. Biol. Med. 2019, 38, 143–148. [Google Scholar] [CrossRef]
- Lisi, A.; Ledda, M.; de Carlo, F.; Pozzi, D.; Messina, E.; Gaetani, R.; Chimenti, I.; Barile, L.; Giacomello, A.; D’Emilia, E.; et al. Ion cyclotron resonance as a tool in regenerative medicine. Electromagn. Biol. Med. 2008, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- American Conference of Government Industrial Hygienists. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1996. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Minguillán, O.; Prous, R.; Ramirez-Castillejo, M.d.C.; Maestú, C. CT2A Cell Viability Modulated by Electromagnetic Fields at Extremely Low Frequency under No Thermal Effects. Int. J. Mol. Sci. 2020, 21, 152. https://doi.org/10.3390/ijms21010152
García-Minguillán O, Prous R, Ramirez-Castillejo MdC, Maestú C. CT2A Cell Viability Modulated by Electromagnetic Fields at Extremely Low Frequency under No Thermal Effects. International Journal of Molecular Sciences. 2020; 21(1):152. https://doi.org/10.3390/ijms21010152
Chicago/Turabian StyleGarcía-Minguillán, Olga, Raquel Prous, Maria del Carmen Ramirez-Castillejo, and Ceferino Maestú. 2020. "CT2A Cell Viability Modulated by Electromagnetic Fields at Extremely Low Frequency under No Thermal Effects" International Journal of Molecular Sciences 21, no. 1: 152. https://doi.org/10.3390/ijms21010152
APA StyleGarcía-Minguillán, O., Prous, R., Ramirez-Castillejo, M. d. C., & Maestú, C. (2020). CT2A Cell Viability Modulated by Electromagnetic Fields at Extremely Low Frequency under No Thermal Effects. International Journal of Molecular Sciences, 21(1), 152. https://doi.org/10.3390/ijms21010152





