The polypeptide GALNT6 Displays Redundant Functions upon Suppression of its Closest Homolog GALNT3 in Mediating Aberrant O-Glycosylation, Associated with Ovarian Cancer Progression
Abstract
1. Introduction
2. Results
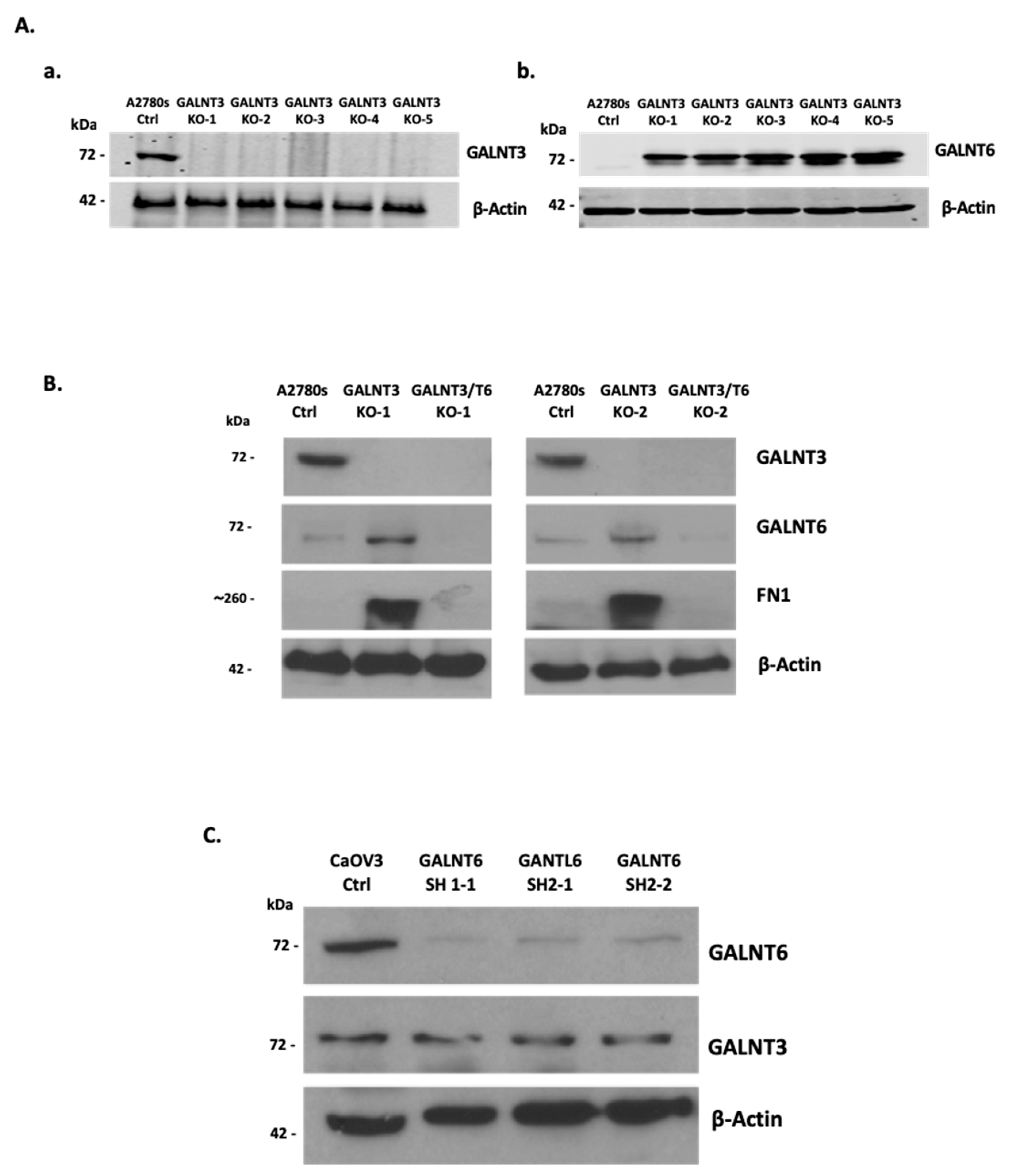
2.1. GALNT3 Gene Knockout (KO) Triggers GALNT6 Overexpression in EOC Cells
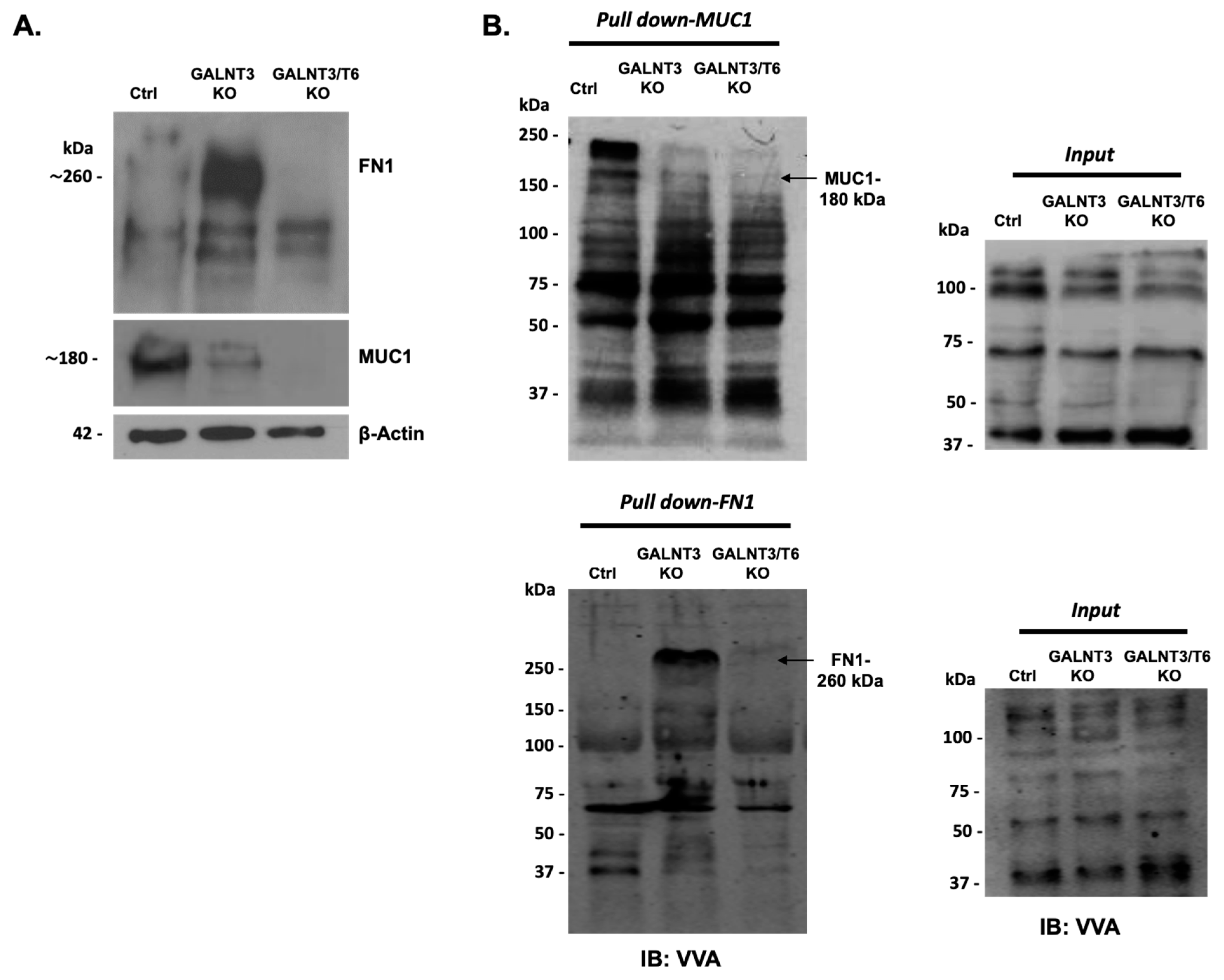
2.2. Effect of Double GALNT3/T6 KO on Protein Glycosylation in EOC Cells
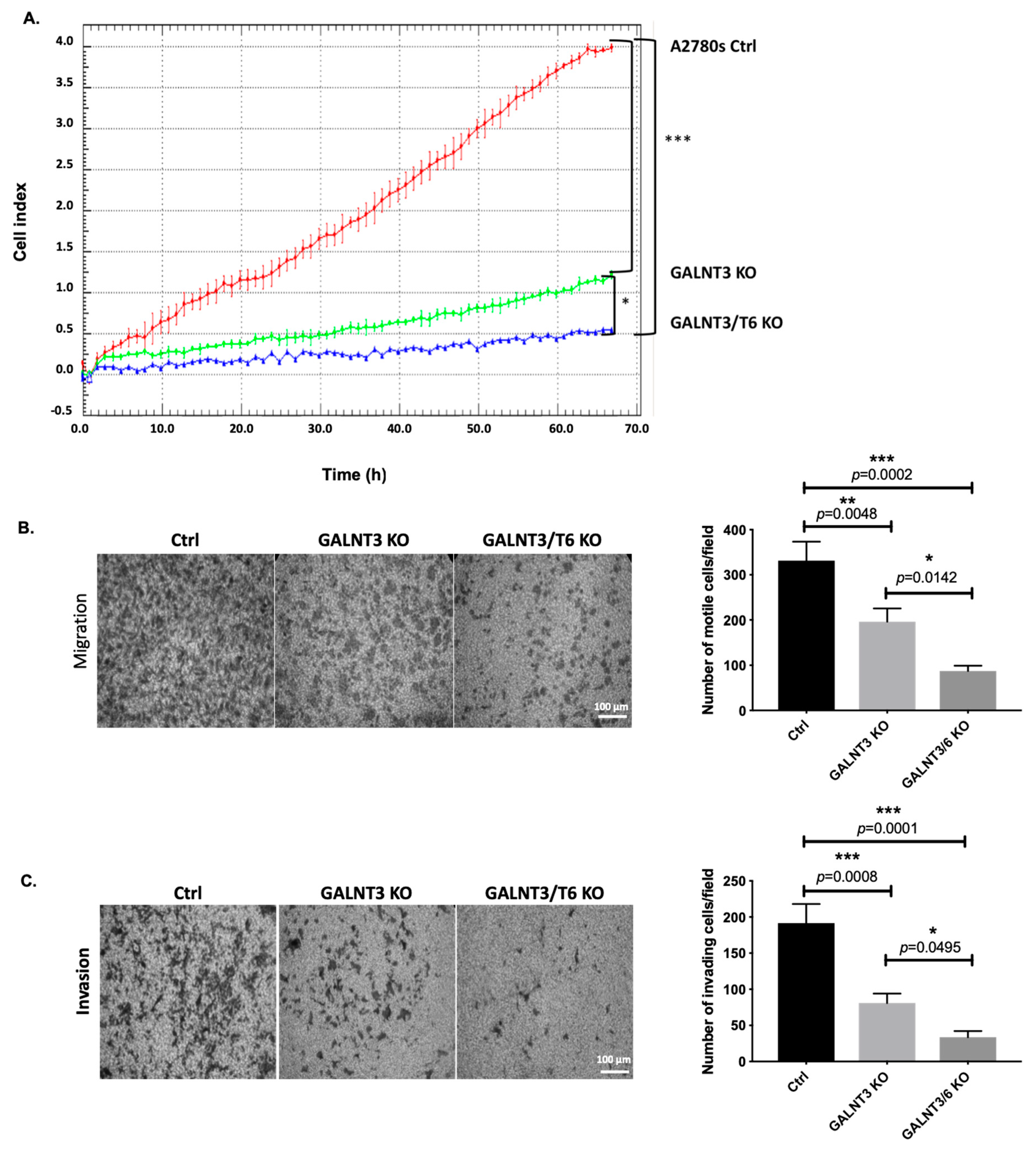
2.3. Double GALNT3/T6 KO Results in Stronger Suppression of A2780s Cellular Proliferation, Migration, and Invasion, when Compared with the Single GALNT3 KO
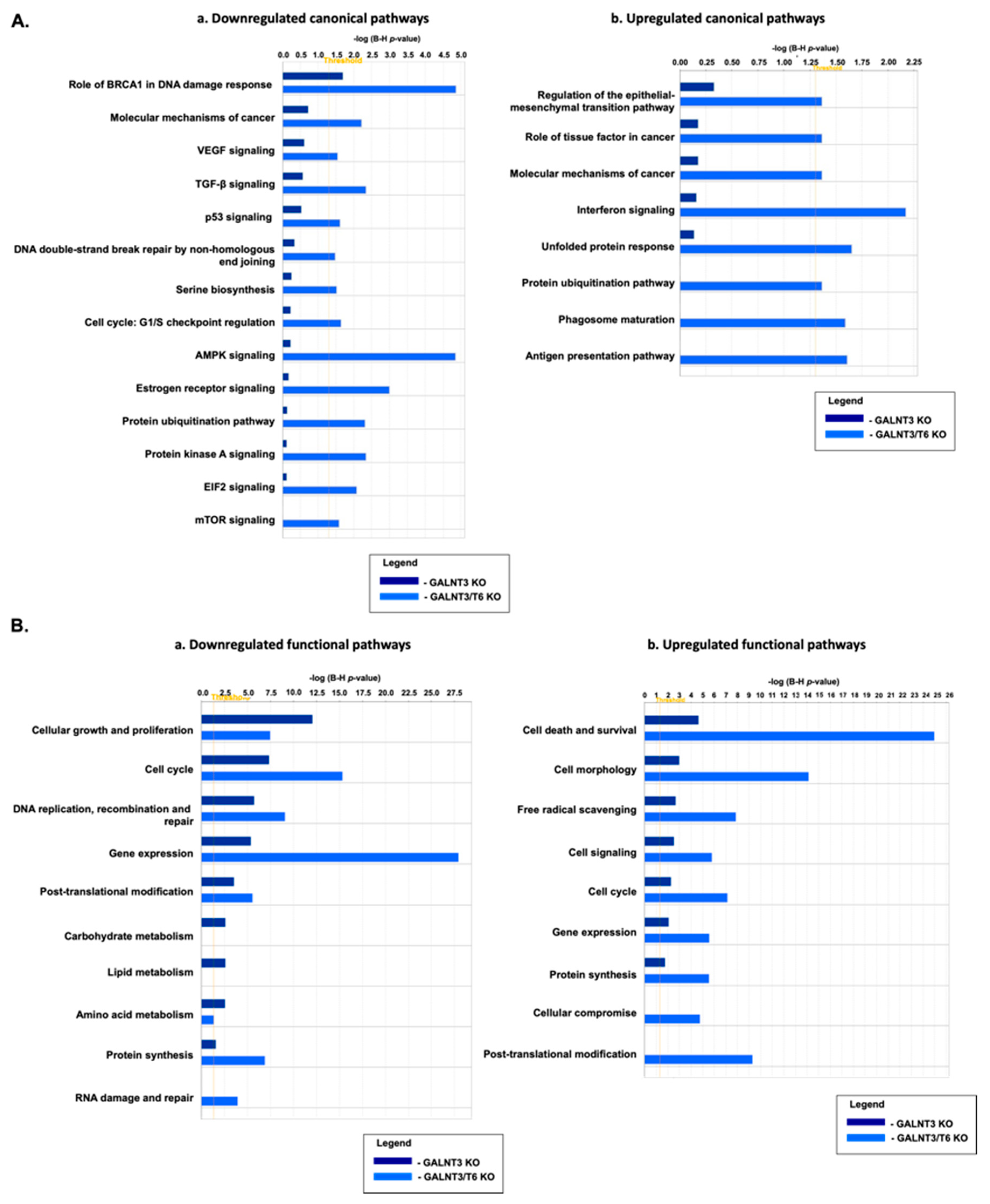
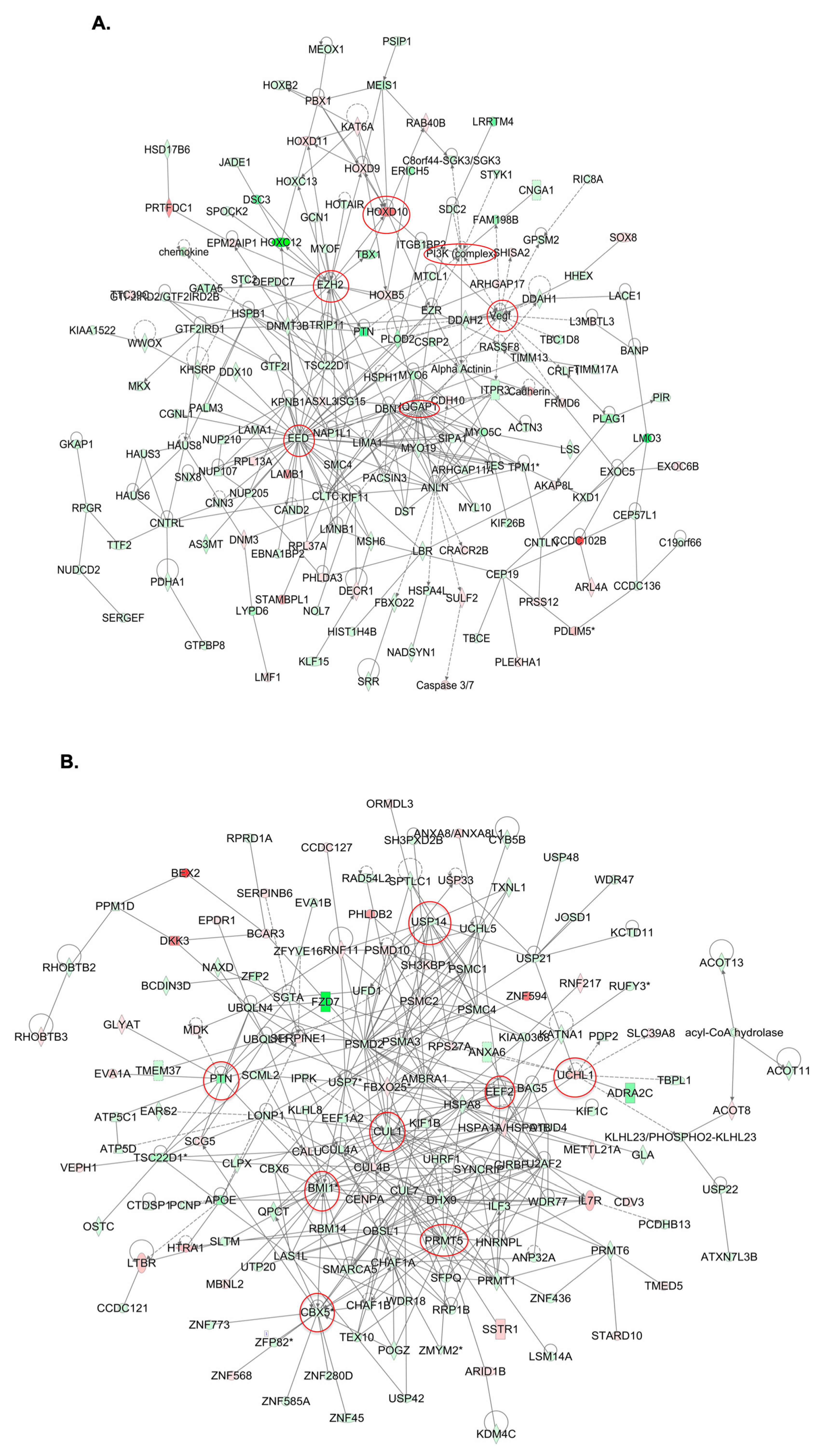
2.4. Molecular Mechanisms of GALNT3 and GALNT6 Action in EOC Cells
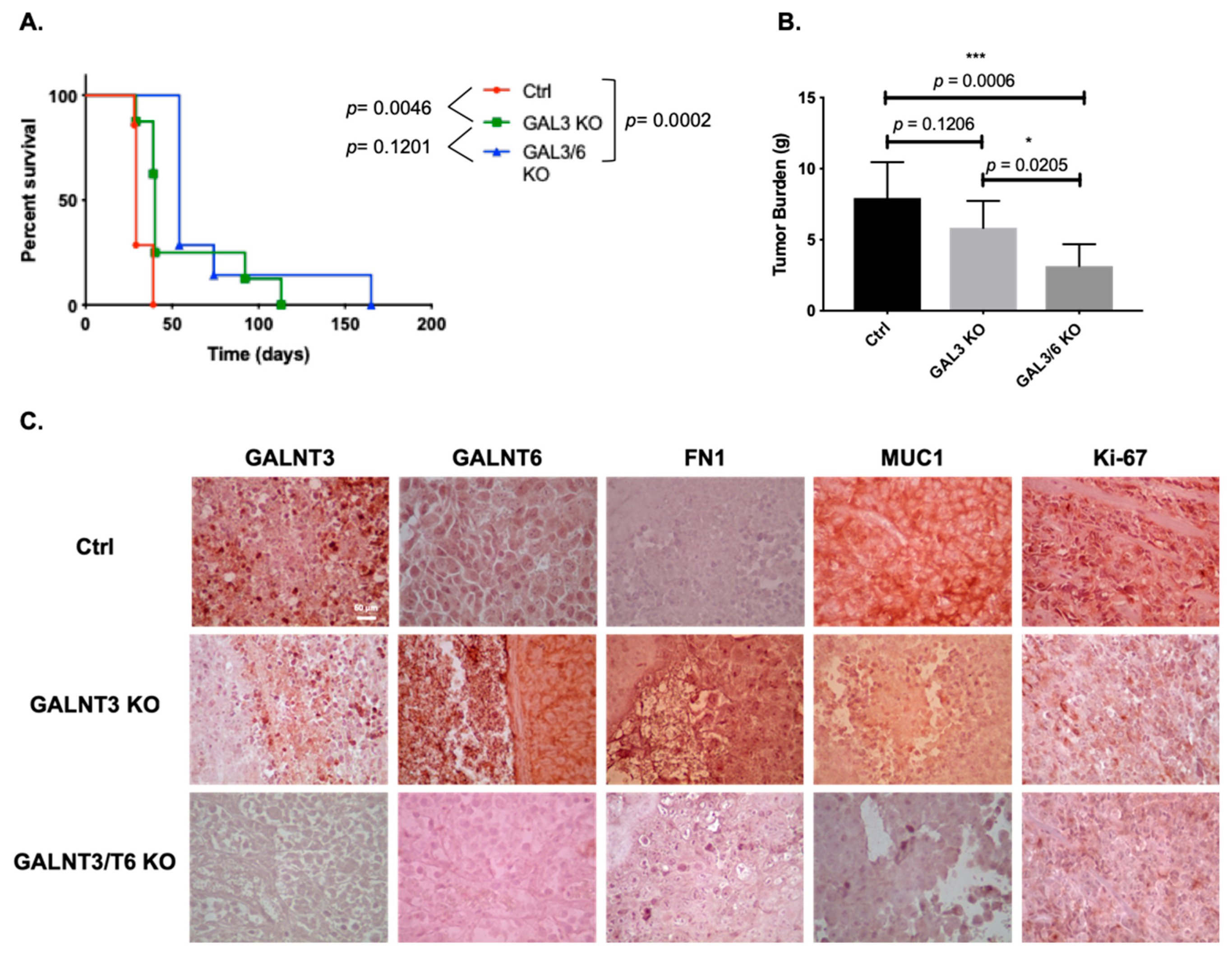
2.5. Double GALNT3/T6 Gene KO Reduces EOC Metastasis in vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. CRISPR/Cas9-Mediated GALNT3 Knockout in EOC Cells
4.3. shRNA-Mediated GALNT6 KO in EOC Cells
4.4. Western Blotting
4.5. Functional Assays
4.5.1. Cell Proliferation Assay
4.5.2. Colony Formation Assay
4.5.3. Migration Assay
4.5.4. Invasion Assay
4.5.5. Flow Cytometry-Cell Cycle Analysis
4.6. Semi-Quantitative RT-PCR (sqRT-PCR) and Quantitaive PCR (qPCR)
4.7. VVA Lectin Pull-Down Assay for O-Glycosylated (GalNAc-conjugated) Proteins
4.8. Gene Expression Profiling and Data Analysis
4.9. IP Tumor Formation in Mice, and IHC Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CRISPR | clustered regularly interspaced short palindromic repeats |
| Cas9 | CRISPR associated protein 9 |
| EMT | Epithelial-mesenchymal transition |
| EOC | Epithelial ovarian cancer |
| GalNAc-Ts | N-acetylgalactosaminyltransferases |
| GEO | Gene Expression Omnibus |
| HDR | Homology directed repair |
| IHC | Immunohistochemistry |
| IPA | Ingenuity Pathway Analysis |
| KD | Knockdown |
| KO | Knockout |
| PTM | Post-translational modification |
| qPCR | Quantitative PCR |
| RTCA | Real-Time Cell Analyzer |
| SCID | Severe combined immunodeficient |
| shRNA | Short hairpin RNA |
| sqRT-PCR | Semi-quantitative RT-PCR |
| VVA | Vicia villosa |
Appendix A
| qPCR Primers | ||||
|---|---|---|---|---|
| Gene | Primer | Primer Sequence | Product Size (bp) | Genbank Accession # |
| GALNT3 | Forward | 5′- CAATCAAGGAGGCAAACCAT -3′ | 266 | NM_004482.3 |
| Reverse | 5′- GCTCTCCATTTGCTGAAAGG -3′ | |||
| GALNT6 | Forward | 5′- CACCTTGGCGCTTACGAGA -3′ | 71 | NM_007210.3 |
| Reverse | 5′- CCTGGGAATCTGAACAACCCTC -3′ | |||
| MUC1 | Forward | 5′- ATCTCATTGCCTTGGCTGTC -3′ | 216 | NM_001018016.2 |
| Reverse | 5′- CCACTGCTGGGTTTGTGTAA -3′ | |||
| FN1 | Forward | 5′- GAGAGAAGTGGGACCGTCAG -3′ | 165 | NM_001306129.1 |
| Reverse | 5′- TGGCACCGAGATATTCCTTC -3′ | |||
| BMP2 | Forward | 5′- CCTTTTACTGCCACGGAGAA -3′ | 215 | NM_001200.3 |
| Reverse | 5′- ACAACCCTCCACAACCATGT -3′ | |||
| CFH | Forward | 5′- TGGAAGATGGGATCCAGAAG -3′ | 243 | NM_000186.3 |
| Reverse | 5′- TGAGGTGGTTGTGAACATGG -3′ | |||
| CD36 | Forward | 5′- TGGCTGTGTTTGGAGGTATTC -3′ | 349 | NM_000072.3 |
| Reverse | 5′- AAGTTGTCAGCCTCTGTTCCA -3′ | |||
| ZFHX4 | Forward | 5′- TCAAGTTTGTGCAGCACCTC -3′ | 207 | NM_024721.4 |
| Reverse | 5′- CACGGGATCCTGTCTTCACT -3′ | |||
| THSD7A | Forward | 5′- GCTGGGGCATTTGTGTTACT -3′ | 209 | NM_015204.2 |
| Reverse | 5′- ATTTGTTGTGGCCTCTGGAC -3′ | |||
| CDKN2A | Forward | 5′- ATATGCCTTCCCCCACTACC -3′ | 231 | NM_000077.4 |
| Reverse | 5′- CCCCTGAGCTTCCCTAGTTC -3′ | |||
| TIMP1 | Forward | 5′- AATTCCGACCTCGTCATCAG -3′ | 230 | NM_003254.2 |
| Reverse | 5′- TGCAGTTTTCCAGCAATGAG -3′ | |||
| HGF | Forward | 5′- CTGGTTCCCCTTCAATAGCA -3′ | 168 | NM_000601.5 |
| Reverse | 5′- CTCCAGGGCTGACATTTGAT -3′ | |||
| PCDH9 | Forward | 5′- ATGGCAACTCTGATCCCAAC -3′ | 268 | NM_001318372.1 |
| Reverse | 5′- CGGTCATTGAACTGGTTCCT -3′ | |||
| ID4 | Forward | 5′- TCCGAAGGGAGTGACTAGGA -3′ | 152 | NM_001546.3 |
| Reverse | 5′- CCGAGCCCAACAATTGAC -3′ | |||
| MMP10 | Forward | 5′- AATGAGTTCTGGGCCATCAG -3′ | 151 | NM_002425.2 |
| Reverse | 5′- ATTTGTCCGCTGCAAAGAAG -3′ | |||
| MMP3 | Forward | 5′- TCATTTTGGCCATCTCTTCC -3′ | 155 | NM_002422.4 |
| Reverse | 5′- GGGAAACCTAGGGTGTGGAT -3′ | |||
| TGFBI | Forward | 5′- CTGGTGCGGCTAAAGTCTCT -3′ | 222 | NM_000358.2 |
| Reverse | 5′- CGCTGATGCTTGTTTGAAGA -3′ | |||
| MAL2 | Forward | 5′- ATCCCTGCATGATTTGCATT -3′ | 164 | NM_052886.2 |
| Reverse | 5′- GAGTGTTACGGTCGCCATCT -3′ | |||
| GREM1 | Forward | 5′- GGTATTTGGGTTGAAAGAATTT -3′ | 424 | NM_001191322.1 |
| Reverse | 5′- AAAATACACAACAAATCACATTTTC -3′ | |||
| IFI16 | Forward | 5′- CCTTTCACACTTGTGGCTGA -3′ | 143 | NM_001206567.1 |
| Reverse | 5′- ACCTCAAACACCCCATTCAC -3′ | |||
| CASP4 | Forward | 5′- GCTTTCTGCTCTTCCACACC -3′ | 160 | NM_001225.3 |
| Reverse | 5′- CATCTGGCTGCTCAAATGAA -3′ | |||
| GUSB | Forward | 5′- ATACGTGGTTGGAGAGCTCATT -3′ | 187 | NM_000181.3 |
| Reverse | 5′- CTTGGCTACTGAGTGGGGATAC -3′ | |||
| 18S | Forward | 5′-AACCCGTTGAACCCCATT-3′ | 119 | NR_003278 |
| Reverse | 5′-CCATCCAATCGGTAGTAGCG-3′ |
References
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: A Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed]
- Varki, A.; Sharon, N. Historical Background and Overview. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor (NY): Suffolk, NY, USA, 2009. [Google Scholar]
- Spiro, R.G. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Ten Hagen, K.G.; Fritz, T.A.; Tabak, L.A. All in the family: The UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology 2003, 13, 1R–16R. [Google Scholar] [CrossRef]
- Ashkani, J.; Naidoo, K.J. Glycosyltransferase Gene Expression Profiles Classify Cancer Types and Propose Prognostic Subtypes. Sci. Rep. 2016, 6, 26451. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Bachvarova, M.; Morin, C.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Bachvarov, D. Role of the polypeptide N-acetylgalactosaminyltransferase 3 in ovarian cancer progression: Possible implications in abnormal mucin O-glycosylation. Oncotarget 2014, 5, 544–560. [Google Scholar] [CrossRef]
- Sheta, R.; Woo, C.M.; Roux-Dalvai, F.; Fournier, F.; Bourassa, S.; Droit, A.; Bertozzi, C.R.; Bachvarov, D. A metabolic labeling approach for glycoproteomic analysis reveals altered glycoprotein expression upon GALNT3 knockdown in ovarian cancer cells. J. Proteom. 2016. [Google Scholar] [CrossRef]
- Wang, R.; Yu, C.; Zhao, D.; Wu, M.; Yang, Z. The mucin-type glycosylating enzyme polypeptide N-acetylgalactosaminyltransferase 14 promotes the migration of ovarian cancer by modifying mucin 13. Oncol. Rep. 2013, 30, 667–676. [Google Scholar] [CrossRef]
- Lin, T.C.; Chen, S.T.; Huang, M.C.; Huang, J.; Hsu, C.L.; Juan, H.F.; Lin, H.H.; Chen, C.H. GALNT6 expression enhances aggressive phenotypes of ovarian cancer cells by regulating EGFR activity. Oncotarget 2017, 8, 42588–42601. [Google Scholar] [CrossRef]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef]
- Sheta, R.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Popa, I.; Bachvarov, D. Altered expression of different GalNActransferases is associated with disease progression and poor prognosis in women with high-grade serous ovarian cancer. Int. J. Oncol. 2017. [Google Scholar] [CrossRef]
- Park, J.H.; Nishidate, T.; Kijima, K.; Ohashi, T.; Takegawa, K.; Fujikane, T.; Hirata, K.; Nakamura, Y.; Katagiri, T. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010, 70, 2759–2769. [Google Scholar] [CrossRef]
- Park, J.H.; Katagiri, T.; Chung, S.; Kijima, K.; Nakamura, Y. Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin. Neoplasia 2011, 13, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Amadori, D.; Sansoni, E.; Amadori, A. Ovarian cancer: Natural history and metastatic pattern. Front. Biosci. A J. Virtual Libr. 1997, 2, g8–g10. [Google Scholar]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2004, 10, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kurita, T.; Koi, C.; Murakami, M.; Kagami, S.; Hachisuga, T.; Masanori, H.; Morimoto, Y.; Izumi, H. GalNAc-T6 in the relationship with invasion ability of endometrial carcinomas and prognostic significance. Am. J. Cancer Res. 2017, 7, 1188–1197. [Google Scholar]
- Taniuchi, K.; Cerny, R.L.; Tanouchi, A.; Kohno, K.; Kotani, N.; Honke, K.; Saibara, T.; Hollingsworth, M.A. Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene 2011, 30, 4843–4854. [Google Scholar] [CrossRef]
- Onitsuka, K.; Shibao, K.; Nakayama, Y.; Minagawa, N.; Hirata, K.; Izumi, H.; Matsuo, K.; Nagata, N.; Kitazato, K.; Kohno, K. Prognostic significance of UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-3 (GalNAc-T3) expression in patients with gastric carcinoma. Cancer Sci. 2003, 94, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Shibao, K.; Izumi, H.; Nakayama, Y.; Ohta, R.; Nagata, N.; Nomoto, M.; Matsuo, K.; Yamada, Y.; Kitazato, K.; Itoh, H. Expression of UDP-N-acetyl-alpha-D-galactosamine-polypeptide galNAc N-acetylgalactosaminyl transferase-3 in relation to differentiation and prognosis in patients with colorectal carcinoma. Cancer 2002, 94, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, Y.E.; Kato, T.; Jang, M.; Haga, Y.; Ueda, K.; Nakamura, Y.; Park, J.H. Morphological Changes, Cadherin Switching, and Growth Suppression in Pancreatic Cancer by GALNT6 Knockdown. Neoplasia 2016, 18, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shi, J.; Zhang, J.; Li, H.; Liu, B.; Guo, H. Polypeptide N-acetylgalactosaminyltransferase-6 expression in gastric cancer. OncoTargets Ther. 2017, 10, 3337–3344. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kagami, S.; Nguyen, T.T.; Koi, C.; Kurita, T.; Hachisuga, T. Expression of Polypeptide N-Acetylgalactosaminyltransferase-6 in Epithelial Ovarian Carcinoma. Anticancer Res. 2017, 37, 3911–3915. [Google Scholar] [PubMed]
- Gyorffy, B.; Lanczky, A.; Szallasi, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. -Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef]
- Li, Z.; Yamada, S.; Inenaga, S.; Imamura, T.; Wu, Y.; Wang, K.Y.; Shimajiri, S.; Nakano, R.; Izumi, H.; Kohno, K. Polypeptide N-acetylgalactosaminyltransferase 6 expression in pancreatic cancer is an independent prognostic factor indicating better overall survival. Br. J. Cancer 2011, 104, 1882–1889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freire-de-Lima, L.; Gelfenbeyn, K.; Ding, Y.; Mandel, U.; Clausen, H.; Handa, K.; Hakomori, S.I. Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc. Natl. Acad. Sci. USA 2011, 108, 17690–17695. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fei, X.; Ma, J.; Qu, Y.; Zhou, C.; Xu, K.; Lin, J. Correlation of polypeptide N-acetylgalactosamine transferases-3 and -6 to different stages of endometriosis. Arch. Gynecol. Obstet. 2017, 295, 1413–1419. [Google Scholar] [CrossRef]
- Kufe, D.W. Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther 2009, 8, 1197–1203. [Google Scholar] [CrossRef]
- Agata, N.; Ahmad, R.; Kawano, T.; Raina, D.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008, 68, 6136–6144. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res 2006, 8, R37. [Google Scholar] [CrossRef]
- Pai, P.; Rachagani, S.; Dhawan, P.; Batra, S.K. Mucins and Wnt/beta-catenin signaling in gastrointestinal cancers: An unholy nexus. Carcinogenesis 2016, 37, 223–232. [Google Scholar] [CrossRef]
- Sousa, A.M.; Rei, M.; Freitas, R.; Ricardo, S.; Caffrey, T.; David, L.; Almeida, R.; Hollingsworth, M.A.; Santos-Silva, F. Effect of MUC1/beta-catenin interaction on the tumorigenic capacity of pancreatic CD133(+) cells. Oncol Lett 2016, 12, 1811–1817. [Google Scholar] [CrossRef]
- Masoumi Moghaddam, S.; Amini, A.; Morris, D.L.; Pourgholami, M.H. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev. 2012, 31, 143–162. [Google Scholar] [CrossRef]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef]
- Rao, Z.Y.; Cai, M.Y.; Yang, G.F.; He, L.R.; Mai, S.J.; Hua, W.F.; Liao, Y.J.; Deng, H.X.; Chen, Y.C.; Guan, X.Y. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis 2010, 31, 1576–1583. [Google Scholar] [CrossRef]
- Dong, P.X.; Jia, N.; Xu, Z.J.; Liu, Y.T.; Li, D.J.; Feng, Y.J. Silencing of IQGAP1 by shRNA inhibits the invasion of ovarian carcinoma HO-8910PM cells in vitro. J. Exp. Clin. Cancer Res. CR 2008, 27, 77. [Google Scholar] [CrossRef][Green Version]
- Nakayama, I.; Shibazaki, M.; Yashima-Abo, A.; Miura, F.; Sugiyama, T.; Masuda, T.; Maesawa, C. Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int. J. Oncol. 2013, 43, 63–71. [Google Scholar] [CrossRef]
- Carlucci, A.; D’Angiolella, V. It is not all about BRCA: Cullin-Ring ubiquitin Ligases in ovarian cancer. Br. J. Cancer 2015, 112, 9–13. [Google Scholar] [CrossRef][Green Version]
- Sethi, G.; Kwon, Y.; Burkhalter, R.J.; Pathak, H.B.; Madan, R.; McHugh, S.; Atay, S.; Murthy, S.; Tawfik, O.W.; Godwin, A.K. PTN signaling: Components and mechanistic insights in human ovarian cancer. Mol. Carcinog. 2015, 54, 1772–1785. [Google Scholar] [CrossRef]
- Jin, C.; Yu, W.; Lou, X.; Zhou, F.; Han, X.; Zhao, N.; Lin, B. UCHL1 Is a Putative Tumor Suppressor in Ovarian Cancer Cells and Contributes to Cisplatin Resistance. J. Cancer 2013, 4, 662–670. [Google Scholar] [CrossRef]
- Pongas, G.; Kim, M.K.; Min, D.J.; House, C.D.; Jordan, E.; Caplen, N.; Chakka, S.; Ohiri, J.; Kruhlak, M.J.; Annunziata, C.M. BRD4 facilitates DNA damage response and represses CBX5/Heterochromatin protein 1 (HP1). Oncotarget 2017, 8, 51402–51415. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhong, J.; Deng, Y.; Xi, Q.; He, S.; Yang, S.; Jiang, L.; Huang, M.; Tang, C. Ubiquitin-specific protease 14 (USP14) regulates cellular proliferation and apoptosis in epithelial ovarian cancer. Med. Oncol. 2015, 32, 379. [Google Scholar] [CrossRef]
- Yang, G.F.; He, W.P.; Cai, M.Y.; He, L.R.; Luo, J.H.; Deng, H.X.; Guan, X.Y.; Zeng, M.S.; Zeng, Y.X.; Xie, D. Intensive expression of Bmi-1 is a new independent predictor of poor outcome in patients with ovarian carcinoma. BMC Cancer 2010, 10, 133. [Google Scholar] [CrossRef]
- Faddaoui, A.; Sheta, R.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Gobeil, S.; Morin, C.; Ghani, K. Suppression of the grainyhead transcription factor 2 gene (GRHL2) inhibits the proliferation, migration, invasion and mediates cell cycle arrest of ovarian cancer cells. Cell Cycle 2017, 16, 693–706. [Google Scholar] [CrossRef]
- Faddaoui, A.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Gobeil, S.; Morin, C.; Macdonald, E.; Vanderhyden, B. The mannose receptor LY75 (DEC205/CD205) modulates cellular phenotype and metastatic potential of ovarian cancer cells. Oncotarget 2016, 7, 14125–14142. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheta, R.; Bachvarova, M.; Macdonald, E.; Gobeil, S.; Vanderhyden, B.; Bachvarov, D. The polypeptide GALNT6 Displays Redundant Functions upon Suppression of its Closest Homolog GALNT3 in Mediating Aberrant O-Glycosylation, Associated with Ovarian Cancer Progression. Int. J. Mol. Sci. 2019, 20, 2264. https://doi.org/10.3390/ijms20092264
Sheta R, Bachvarova M, Macdonald E, Gobeil S, Vanderhyden B, Bachvarov D. The polypeptide GALNT6 Displays Redundant Functions upon Suppression of its Closest Homolog GALNT3 in Mediating Aberrant O-Glycosylation, Associated with Ovarian Cancer Progression. International Journal of Molecular Sciences. 2019; 20(9):2264. https://doi.org/10.3390/ijms20092264
Chicago/Turabian StyleSheta, Razan, Magdalena Bachvarova, Elizabeth Macdonald, Stephane Gobeil, Barbara Vanderhyden, and Dimcho Bachvarov. 2019. "The polypeptide GALNT6 Displays Redundant Functions upon Suppression of its Closest Homolog GALNT3 in Mediating Aberrant O-Glycosylation, Associated with Ovarian Cancer Progression" International Journal of Molecular Sciences 20, no. 9: 2264. https://doi.org/10.3390/ijms20092264
APA StyleSheta, R., Bachvarova, M., Macdonald, E., Gobeil, S., Vanderhyden, B., & Bachvarov, D. (2019). The polypeptide GALNT6 Displays Redundant Functions upon Suppression of its Closest Homolog GALNT3 in Mediating Aberrant O-Glycosylation, Associated with Ovarian Cancer Progression. International Journal of Molecular Sciences, 20(9), 2264. https://doi.org/10.3390/ijms20092264





