Core-Fucosylated Tetra-Antennary N-Glycan Containing A Single N-Acetyllactosamine Branch Is Associated with Poor Survival Outcome in Breast Cancer
Abstract
1. Introduction
2. Results
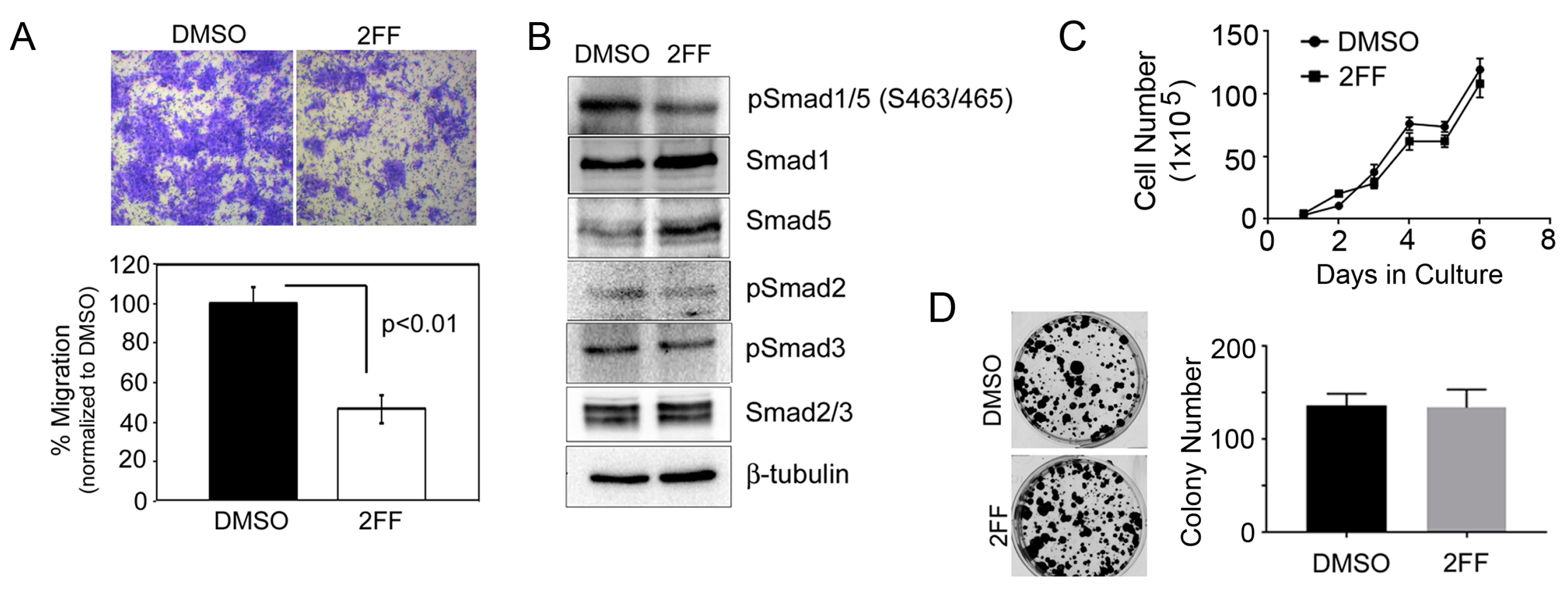
2.1. Fucosylation Regulates Cell Migration
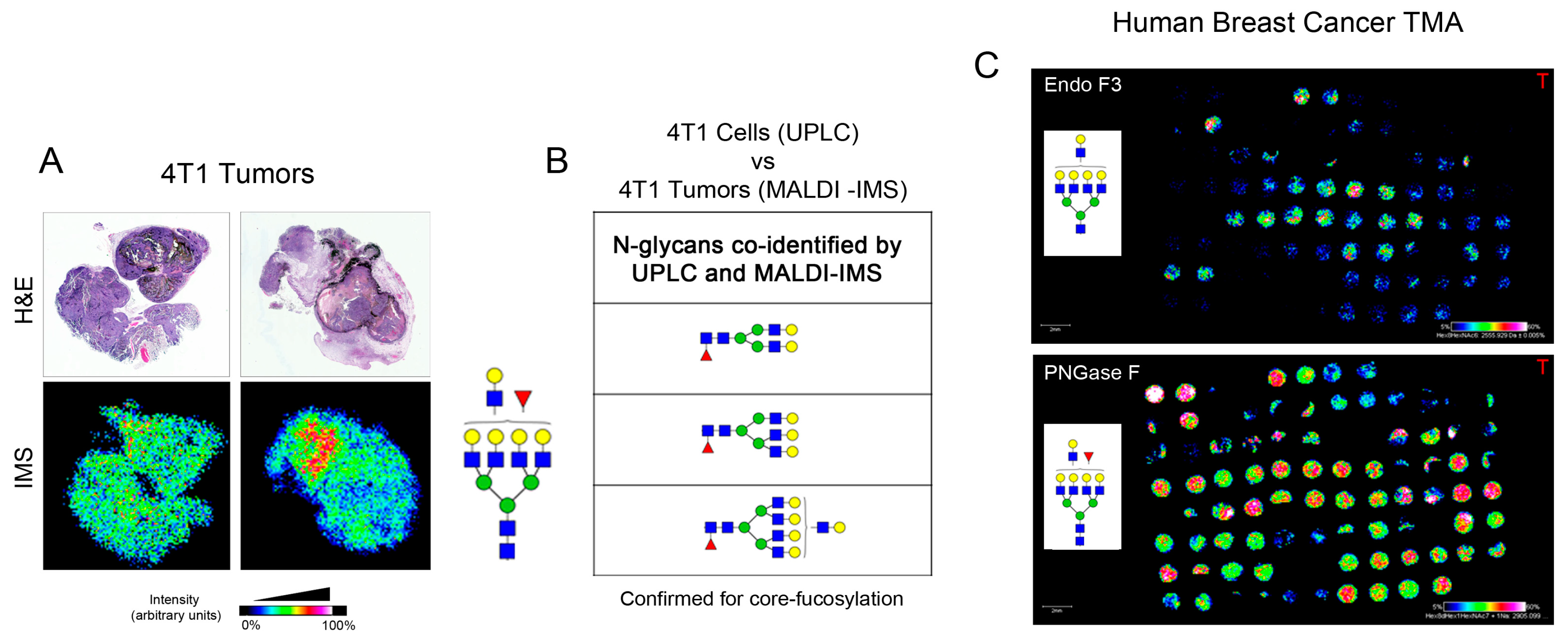
2.2. Identification of Core-Fucosylated N-Glycans in 4T1 Cells
2.3. Identification of Core-Fucosylated N-Glycans in 4T1-Derived Tumors
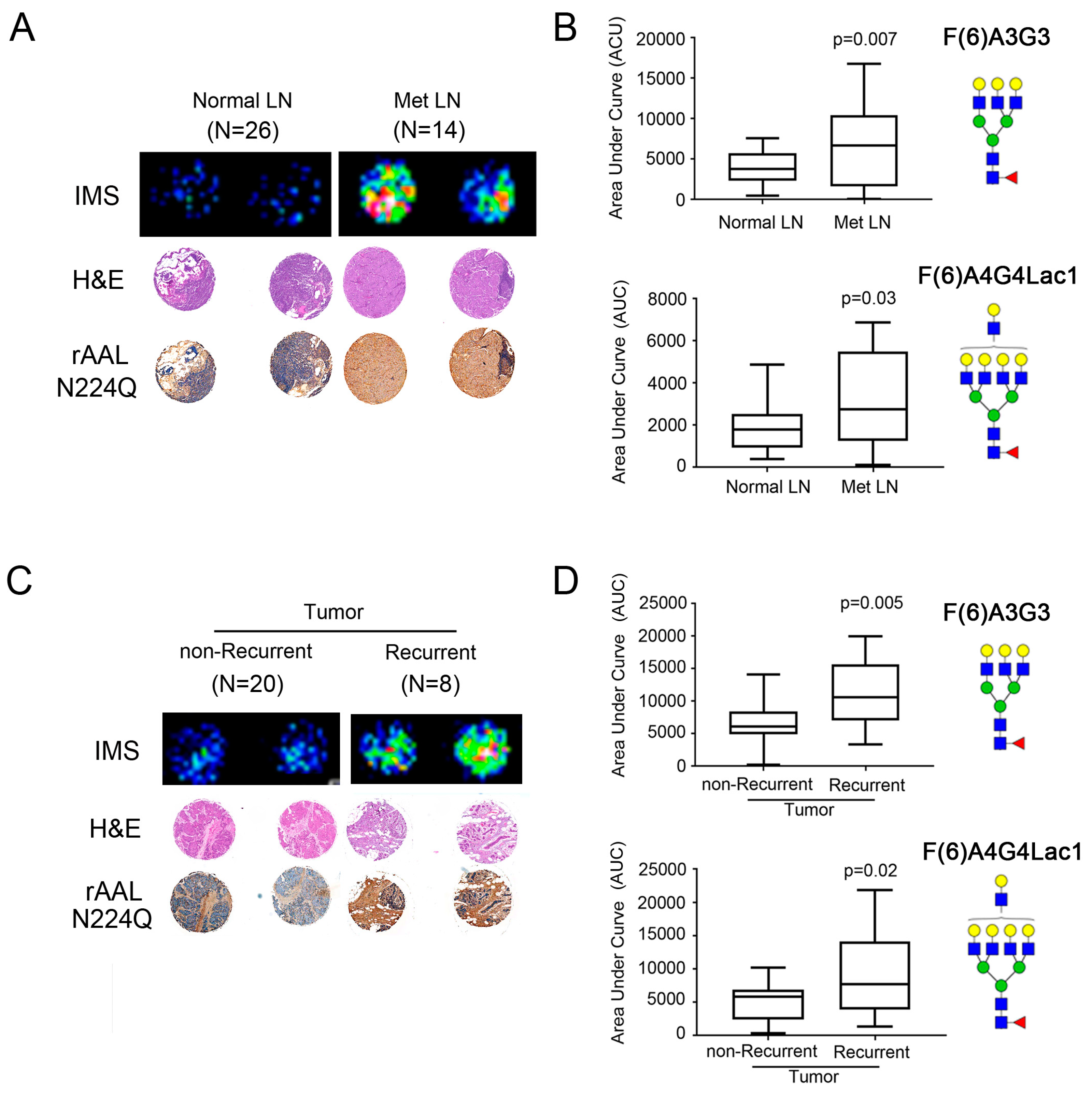
2.4. Core-Fucosylated N-Glycans Are Elevated in Lymph Node Metastasis and Tumors from Patients with Recurrence of Disease
2.5. Core-Fucosylated Tetra-Antennary Polylactosamine Is Associated with Poor Survival
3. Discussion
4. Materials and Methods
4.1. Tissue Culture
4.2. Cell-Based Mass Spectrometry Imaging
4.3. Migration Assay
4.4. Western Blotting and Lectin Blotting
4.5. Lectin Histochemistry
4.6. Proliferation and Colony Formation Assays
4.7. Exoglycosidase Digestion Coupled to UPLC
4.8. Endo F3 and PNGase F Enzymatic Digestion and α-Cyano-4-Hydroxycinnamic Acid (CHCA) Matrix Application
4.9. MALDI Imaging Mass Spectrometry of N-Glycan Released from Tissues
4.10. Animal Studies
4.11. Tumor Microarrays
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TMA | Tissue Microarray |
| H&E | hematoxylin and eosin |
| CHCA | α-cyano-4-hydroxycinnamic acid |
| Endo F3 | endoglycosidase F3 |
| PNGase F | Peptide: N-glycosidase F |
| AAL | Aleuria aurantia lectin |
| LacNAc | N-acetyllactosamine |
| AUC | Area under curve |
| SA | Sialidase A |
| NeuAc | N-acetyl neuraminic acid |
| NeuGc | N-glycolylneuraminic acid |
| AMF | Almond meal fucosidase |
| BKF | Bovine kidney fucosidase |
| FFPE | Formalin-fixed paraffin embedded |
References
- Wang, M.; Mehta, A.; Block, T.M.; Marrero, J.; Di Bisceglie, A.M.; Devarajan, K. A comparison of statistical methods for the detection of hepatocellular carcinoma based on serum biomarkers and clinical variables. BMC Med. Genom. 2013, 6, S9. [Google Scholar]
- Norton, P.; Comunale, M.A.; Herrera, H.; Wang, M.; Houser, J.; Wimmerova, M.; Romano, P.R.; Mehta, A. Development and application of a novel recombinant aleuria aurantia lectin with enhanced core fucose binding for identification of glycoprotein biomarkers of hepatocellular carcinoma. Proteomics 2016, 16, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Comunale, M.A.; Wang, M.; Hafner, J.; Krakover, J.; Rodemich, L.; Kopenhaver, B.; Long, R.E.; Junaidi, O.; Bisceglie, A.M.; Block, T.M.; et al. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J. Proteome Res. 2009, 8, 595–602. [Google Scholar] [CrossRef]
- Comunale, M.A.; Rodemich-Betesh, L.; Hafner, J.; Wang, M.; Norton, P.; Di Bisceglie, A.M.; Block, T.; Mehta, A. Linkage specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and cancer patients: Implications for a biomarker of hepatocellular carcinoma. PLoS ONE 2010, 5, e12419. [Google Scholar] [CrossRef] [PubMed]
- Norton, P.A.; Comunale, M.A.; Krakover, J.; Rodemich, L.; Pirog, N.; D’Amelio, A.; Philip, R.; Mehta, A.S.; Block, T.M. N-linked glycosylation of the liver cancer biomarker gp73. J. Cell Biochem. 2008, 104, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, E.; Kamada, Y. Application of glycoscience to the early detection of pancreatic cancer. Cancer Sci. 2016, 107, 1357–1362. [Google Scholar] [CrossRef]
- Adamczyk, B.; Tharmalingam, T.; Rudd, P.M. Glycans as cancer biomarkers. Biochim. Biophys. Acta 2012, 1820, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, E.; Nakano, M. Fucosylated haptoglobin is a novel marker for pancreatic cancer: Detailed analyses of oligosaccharide structures. Proteomics 2008, 8, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, J.; Miyoshi, E.; Honke, K.; Taniguchi, N. Phenotype changes of fut8 knockout mouse: Core fucosylation is crucial for the function of growth factor receptor(s). Methods Enzymol. 2006, 417, 11–22. [Google Scholar] [PubMed]
- Gao, C.; Maeno, T.; Ota, F.; Ueno, M.; Korekane, H.; Takamatsu, S.; Shirato, K.; Matsumoto, A.; Kobayashi, S.; Yoshida, K.; et al. Sensitivity of heterozygous alpha1,6-fucosyltransferase knock-out mice to cigarette smoke-induced emphysema: Implication of aberrant transforming growth factor-beta signaling and matrix metalloproteinase gene expression. J. Biol. Chem. 2012, 287, 16699–16708. [Google Scholar] [CrossRef]
- Shi, S.; Stanley, P. Protein o-fucosyltransferase 1 is an essential component of notch signaling pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 5234–5239. [Google Scholar] [CrossRef]
- Okamura, Y.; Saga, Y. Pofut1 is required for the proper localization of the notch receptor during mouse development. Mech. Dev. 2008, 125, 663–673. [Google Scholar] [CrossRef]
- Du, J.; Takeuchi, H.; Leonhard-Melief, C.; Shroyer, K.R.; Dlugosz, M.; Haltiwanger, R.S.; Holdener, B.C. O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (emt) and maintains epiblast pluripotency during mouse gastrulation. Dev. Biol. 2010, 346, 25–38. [Google Scholar] [CrossRef]
- Yuan, K.; Kucik, D.; Singh, R.K.; Listinsky, C.M.; Listinsky, J.J.; Siegal, G.P. Alterations in human breast cancer adhesion-motility in response to changes in cell surface glycoproteins displaying alpha-l-fucose moieties. Int. J. Oncol. 2008, 32, 797–807. [Google Scholar]
- Yuan, K.; Listinsky, C.M.; Singh, R.K.; Listinsky, J.J.; Siegal, G.P. Cell surface associated alpha-l-fucose moieties modulate human breast cancer neoplastic progression. Pathol. Oncol. Res. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Listinsky, J.J.; Listinsky, C.M.; Alapati, V.; Siegal, G.P. Cell surface fucose ablation as a therapeutic strategy for malignant neoplasms. Adv. Anat. Pathol. 2001, 8, 330–337. [Google Scholar] [CrossRef]
- Listinsky, J.J.; Siegal, G.P.; Listinsky, C.M. The emerging importance of alpha-l-fucose in human breast cancer: A review. Am. J. Transl. Res. 2011, 3, 292–322. [Google Scholar] [PubMed]
- Kyselova, Z.; Mechref, Y.; Kang, P.; Goetz, J.A.; Dobrolecki, L.E.; Sledge, G.W.; Schnaper, L.; Hickey, R.J.; Malkas, L.H.; Novotny, M.V. Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin. Chem. 2008, 54, 1166–1175. [Google Scholar] [CrossRef]
- Scott, D.A.; Casadonte, R.; Cardinali, B.; Spruill, L.; Mehta, A.S.; Carli, F.; Simone, N.; Kriegsmann, M.; Mastro, L.D.; Kriegsmann, J.; et al. Increases in tumor n-glycan polylactosamines associated with advanced her2 positive and triple negative breast cancer tissues. Proteomics. Clin. Appl. 2018, 13, e1800014. [Google Scholar] [CrossRef]
- Milde-Langosch, K.; Karn, T.; Schmidt, M.; Zu Eulenburg, C.; Oliveira-Ferrer, L.; Wirtz, R.M.; Schumacher, U.; Witzel, I.; Schutze, D.; Muller, V. Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res. Treat. 2014, 145, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Han, C.; Li, Z.; Li, X.; Liu, D.; Liu, S.; Yu, H. Fucosyltransferase 8 expression in breast cancer patients: A high throughput tissue microarray analysis. Histol. Histopathol. 2016, 31, 547–555. [Google Scholar]
- Andres, S.A.; Brock, G.N.; Wittliff, J.L. Interrogating differences in expression of targeted gene sets to predict breast cancer outcome. BMC Cancer 2013, 13, 326. [Google Scholar] [CrossRef]
- Tu, C.F.; Wu, M.Y.; Lin, Y.C.; Kannagi, R.; Yang, R.B. Fut8 promotes breast cancer cell invasiveness by remodeling tgf-beta receptor core fucosylation. Breast Cancer Res. 2017, 19, 111. [Google Scholar] [CrossRef]
- Okeley, N.M.; Alley, S.C.; Anderson, M.E.; Boursalian, T.E.; Burke, P.J.; Emmerton, K.M.; Jeffrey, S.C.; Klussman, K.; Law, C.L.; Sussman, D.; et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. USA 2013, 110, 5404–5409. [Google Scholar] [CrossRef]
- Trimble, R.B.; Tarentino, A.L. Identification of distinct endoglycosidase (endo) activities in flavobacterium meningosepticum: Endo f1, endo f2, and endo f3. Endo f1 and endo h hydrolyze only high mannose and hybrid glycans. J. Biol. Chem. 1991, 266, 1646–1651. [Google Scholar]
- Angel, P.M.; Mehta, A.; Norris-Caneda, K.; Drake, R.R. Maldi imaging mass spectrometry of n-glycans and tryptic peptides from the same formalin-fixed, paraffin-embedded tissue section. Methods Mol. Biol. 2018, 1788, 225–241. [Google Scholar]
- Romano, P.R.; Mackay, A.; Vong, M.; DeSa, J.; Lamontagne, A.; Comunale, M.A.; Hafner, J.; Block, T.; Lec, R.; Mehta, A. Development of recombinant aleuria aurantia lectins with altered binding specificities to fucosylated glycans. Biochem. Biophys. Res. Commun. 2011, 414, 84–89. [Google Scholar] [CrossRef]
- Zhou, Y.; Fukuda, T.; Hang, Q.; Hou, S.; Isaji, T.; Kameyama, A.; Gu, J. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer hepg2 cell proliferation and migration as well as tumor formation. Sci. Rep. 2017, 7, 11563. [Google Scholar] [CrossRef]
- Varki, A.; Kannagi, R.; Toole, B.; Stanley, P. Glycosylation changes in cancer. In Essentials of glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Eds.; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 2015; pp. 597–609. [Google Scholar]
- Ni, J.; Jiang, Z.; Shen, L.; Gao, L.; Yu, M.; Xu, X.; Zou, S.; Hua, D.; Wu, S. Beta3gnt8 regulates the metastatic potential of colorectal carcinoma cells by altering the glycosylation of cd147. Oncol. Rep. 2014, 31, 1795–1801. [Google Scholar] [CrossRef][Green Version]
- Togayachi, A.; Kozono, Y.; Ishida, H.; Abe, S.; Suzuki, N.; Tsunoda, Y.; Hagiwara, K.; Kuno, A.; Ohkura, T.; Sato, N.; et al. Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc. Natl. Acad. Sci. USA 2007, 104, 15829–15834. [Google Scholar] [CrossRef]
- Dange, M.C.; Srinivasan, N.; More, S.K.; Bane, S.M.; Upadhya, A.; Ingle, A.D.; Gude, R.P.; Mukhopadhyaya, R.; Kalraiya, R.D. Galectin-3 expressed on different lung compartments promotes organ specific metastasis by facilitating arrest, extravasation and organ colonization via high affinity ligands on melanoma cells. Clin. Exp. Metastasis 2014, 31, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R.; Powers, T.W.; Norris-Caneda, K.; Mehta, A.S.; Angel, P.M. In situ imaging of n-glycans by maldi imaging mass spectrometry of fresh or formalin-fixed paraffin-embedded tissue. Curr. Protoc. Protein Sci. 2018, 94, e68. [Google Scholar] [CrossRef]
- Powers, T.W.; Jones, E.E.; Betesh, L.R.; Romano, P.R.; Gao, P.; Copland, J.A.; Mehta, A.S.; Drake, R.R. Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of n-linked glycan expression in tissues. Anal. Chem. 2013, 85, 9799–9806. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Comunale, M.A.; Rawat, S.; Casciano, J.C.; Lamontagne, J.; Herrera, H.; Ramanathan, A.; Betesh, L.; Wang, M.; Norton, P.; et al. Intrinsic hepatocyte dedifferentiation is accompanied by upregulation of mesenchymal markers, protein sialylation and core alpha 1,6 linked fucosylation. Sci. Rep. 2016, 6, 27965. [Google Scholar] [CrossRef]
- Comunale, M.A.; Wang, M.; Anbarasan, N.; Betesh, L.; Karabudak, A.; Moritz, E.; Devarajan, K.; Marrero, J.; Block, T.M.; Mehta, A. Total serum glycan analysis is superior to lectin-flisa for the early detection of hepatocellular carcinoma. Proteom. Clin. Appl. 2013, 7, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Powers, T.W.; Holst, S.; Wuhrer, M.; Mehta, A.S.; Drake, R.R. Two-dimensional n-glycan distribution mapping of hepatocellular carcinoma tissues by maldi-imaging mass spectrometry. Biomolecules 2015, 5, 2554–2572. [Google Scholar] [CrossRef] [PubMed]
- West, C.A.; Wang, M.; Herrera, H.; Liang, H.; Black, A.; Angel, P.M.; Drake, R.R.; Mehta, A.S. N-linked glycan branching and fucosylation are increased directly in hepatocellular carcinoma tissue as determined through in situ glycan imaging. J. Proteome Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. Glycoworkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, H.; Dilday, T.; Uber, A.; Scott, D.; Zambrano, J.N.; Wang, M.; Angel, P.M.; Mehta, A.S.; Drake, R.R.; Hill, E.G.; et al. Core-Fucosylated Tetra-Antennary N-Glycan Containing A Single N-Acetyllactosamine Branch Is Associated with Poor Survival Outcome in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 2528. https://doi.org/10.3390/ijms20102528
Herrera H, Dilday T, Uber A, Scott D, Zambrano JN, Wang M, Angel PM, Mehta AS, Drake RR, Hill EG, et al. Core-Fucosylated Tetra-Antennary N-Glycan Containing A Single N-Acetyllactosamine Branch Is Associated with Poor Survival Outcome in Breast Cancer. International Journal of Molecular Sciences. 2019; 20(10):2528. https://doi.org/10.3390/ijms20102528
Chicago/Turabian StyleHerrera, Harmin, Tinslee Dilday, Allison Uber, Danielle Scott, Joelle N. Zambrano, Mengjun Wang, Peggi M. Angel, Anand S. Mehta, Richard R. Drake, Elizabeth G. Hill, and et al. 2019. "Core-Fucosylated Tetra-Antennary N-Glycan Containing A Single N-Acetyllactosamine Branch Is Associated with Poor Survival Outcome in Breast Cancer" International Journal of Molecular Sciences 20, no. 10: 2528. https://doi.org/10.3390/ijms20102528
APA StyleHerrera, H., Dilday, T., Uber, A., Scott, D., Zambrano, J. N., Wang, M., Angel, P. M., Mehta, A. S., Drake, R. R., Hill, E. G., & Yeh, E. S. (2019). Core-Fucosylated Tetra-Antennary N-Glycan Containing A Single N-Acetyllactosamine Branch Is Associated with Poor Survival Outcome in Breast Cancer. International Journal of Molecular Sciences, 20(10), 2528. https://doi.org/10.3390/ijms20102528





