Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons
Abstract
1. Introduction
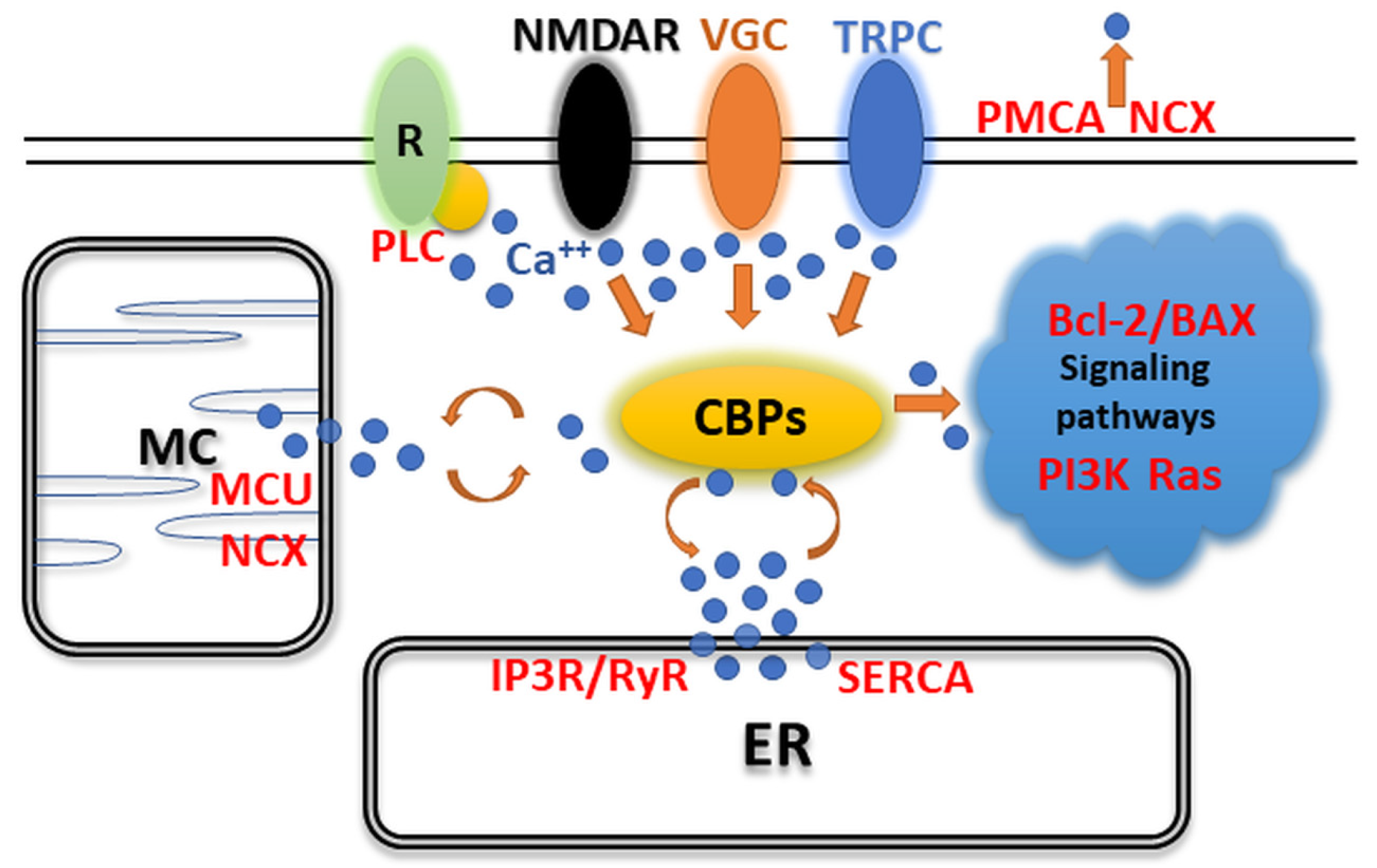
2. Expression of CaBPs May Alter Neuronal Activity in the CNS
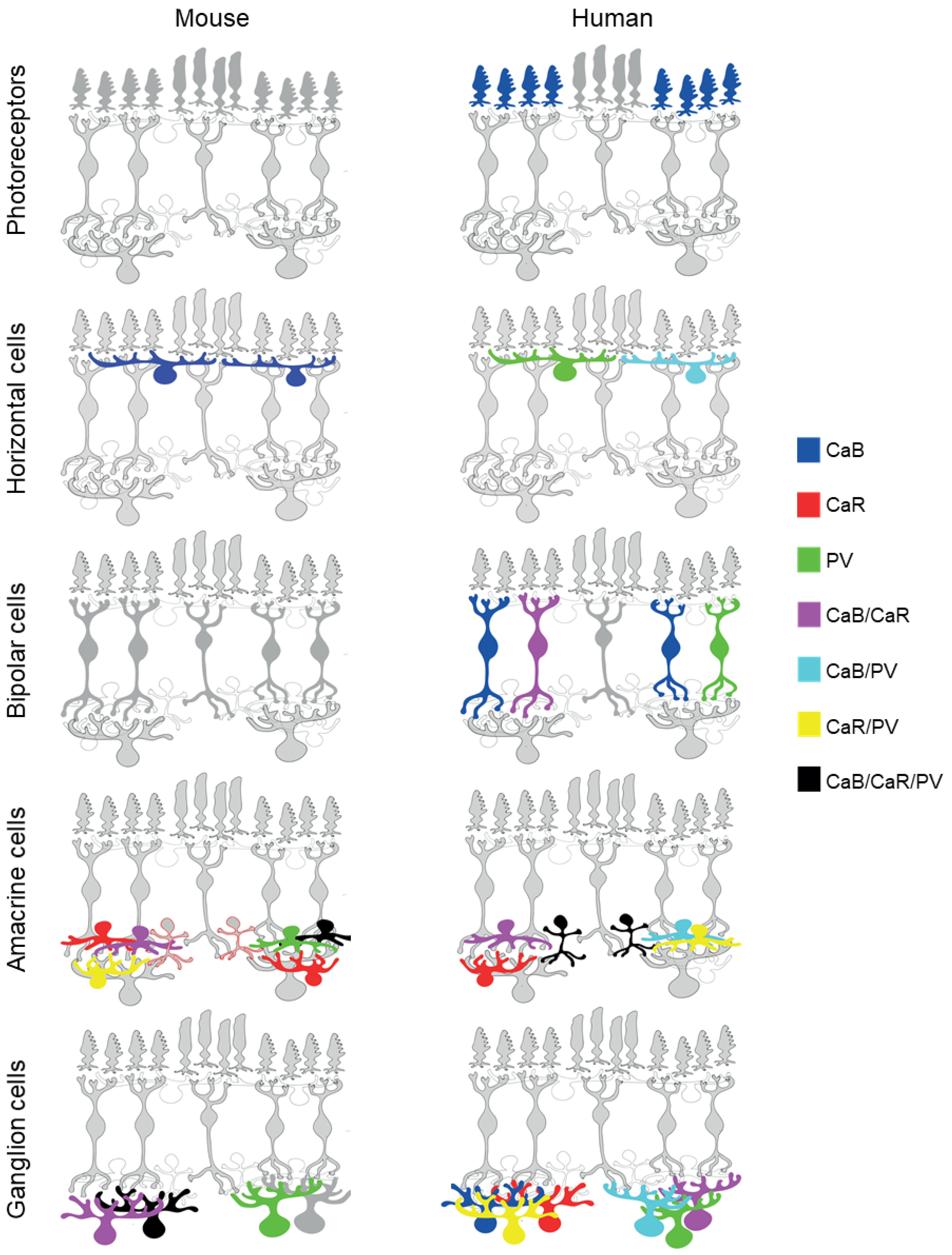
3. CaBP Expression in PRs
4. CaBP Expression in HCs
5. CaBP Expression in BCs
6. CaBP Expression in ACs
7. CaBP Expression in GCs
8. CaBP Expressional Changes in Retinal Neurons
9. Concluding Remarks on CaBP Expression in Retinal Neurons
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | amacrine cell |
| BB | blue cone-specific bipolar cell |
| BC | bipolar cell |
| CaB | calbindin |
| CaBP | Ca2+-binding protein |
| CaM | calmodulin |
| CaMKII | calmodulin kinase II |
| CaR | calretinin |
| CNS | central nervous system |
| dAC | displaced amacrine cell |
| DB | diffuse bipolar |
| DS | direction selective |
| FMB | flat midget bipolar |
| GABA | gamma-Aminobutyric acid |
| GC | ganglion cell |
| GCL | ganglion cell layer |
| GFP | green fluorescent protein |
| HC | horizontal cell |
| IMB | invaginating midget bipolar |
| INL | inner nuclear layer |
| IPL | inner plexiform layer |
| L (PR) | long |
| LGN | lateral geniculate nucleus |
| LTP | long-term potentiation |
| M (GC) | magnocellular |
| M (PR) | medium |
| ONL | outer nuclear layer |
| OPL | outer plexiform layer |
| P | parvocellular |
| PKC | protein kinase C |
| PR | photoreceptor |
| PV | parvalbumin |
| REC | recoverin |
| RGC | retinal ganglion cell |
| S (PR) | short |
| SCGN | secretagogin |
| SCN | suprachiasmatic nucleus |
| Stb | starburst |
| TH | tyrosine-hydroxilase |
| UI | unidentified |
| WA | wild field amacrine |
| ZT | zeitgeber time |
References
- Schwaller, B. Cytosolic Ca2+ Buffers. Cold Spring Harb. Perspect. Biol. 2010, 2, a004051. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Boil. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Mitchell, S.A.; Randers-Pehrson, G.; Brenner, D.J.; Hall, E.J. The Bystander Response in C3H 10T½ Cells: The Influence of Cell-to-Cell Contact. Radiat. Res. 2004, 161, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Tetenborg, S.; Yadav, S.C.; Hormuzdi, S.G.; Monyer, H.; Janssen-Bienhold, U.; Dedek, K. Differential Distribution of Retinal Ca2+/Calmodulin-Dependent Kinase II (CaMKII) Isoforms Indicates CaMKII-β and -δ as Specific Elements of Electrical Synapses Made of Connexin36 (Cx36). Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef]
- Xu, X.-Z.S.; Wes, P.D.; Chen, H.; Li, H.-S.; Yu, M.; Morgan, S.; Liu, Y.; Montell, C. Retinal Targets for Calmodulin Include Proteins Implicated in Synaptic Transmission. J. Boil. Chem. 1998, 273, 31297–31307. [Google Scholar] [CrossRef]
- Pochet, R.; Pasteels, B.; Seto-Ohshima, A.; Bastianelli, E.; Kitajima, S.; Van Eldik, L.J.; Seto-Ohshima, A. Calmodulin and calbindin localization in retina from six vertebrate species. J. Comp. Neurol. 1991, 314, 750–762. [Google Scholar] [CrossRef]
- Noble, J.W.; Almalki, R.; Roe, S.M.; Wagner, A.; Duman, R.; Atack, J.R. The X-ray structure of human calbindin-D28K: An improved model. Acta Crystallogr. Sect. D Struct. Boil. 2018, 74, 1008–1014. [Google Scholar] [CrossRef]
- Giacomello, M.; Oliveros, J.; Naranjo, J.; Carafoli, E. Neuronal Ca2+ dyshomeostasis in Huntington disease. Prion 2013, 7, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kolobkova, Y.A.; Vigont, V.A.; Shalygin, A.V.; Kaznacheyeva, E.V. Huntington’s Disease: Calcium Dyshomeostasis and Pathology Models. Acta Nat. 2017, 9, 34–46. [Google Scholar]
- Koch, E.T.; Woodard, C.L.; Raymond, L.A. Direct assessment of presynaptic modulation of cortico-striatal glutamate release in a Huntington’s disease mouse model. J. Neurophysiol. 2018, 120, 3077–3084. [Google Scholar] [CrossRef]
- Birkholz, T.M.; Beligala, D.H.; De, A.; Geusz, M.E. Expression pattern of the calcium-binding protein calreticulin in a mammalian circadian clock. Ohio J. Sci. 2018, 118, A47. [Google Scholar]
- Wen, X.-H.; Duda, T.; Pertzev, A.; Venkataraman, V.; Makino, C.L.; Sharma, R.K. S100B Serves as a Ca2+ Sensor for ROS-GC1 Guanylate Cyclase in Cones but not in Rods of the Murine Retina. Cell. Physiol. Biochem. 2012, 29, 417–430. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Kuja-Panula, J.; Sorci, G.; Donato, R.; Rauvala, H.; Agneletti, A.L. Coregulation of Neurite Outgrowth and Cell Survival by Amphoterin and S100 Proteins through Receptor for Advanced Glycation End Products (RAGE) Activation. J. Boil. Chem. 2000, 275, 40096–40105. [Google Scholar] [CrossRef]
- Inglese, J.; Chen, C.-K.; Lefkowitz, R.J.; Hurley, J.B. Ca[IMAGE]-dependent Interaction of Recoverin with Rhodopsin Kinase. J. Boil. Chem. 1995, 270, 18060–18066. [Google Scholar]
- Bindreither, D.; Lackner, P. Structural diversity of calcium binding sites. Gen. Physiol. Biophys. 2009, 28, F82–F88. [Google Scholar]
- Gifford, J.L.; Walsh, M.P.; Vogel, H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix–loop–helix EF-hand motifs. Biochem. J. 2007, 405, 199–221. [Google Scholar] [CrossRef]
- Blatow, M.; Caputi, A.; Burnashev, N.; Monyer, H.; Rozov, A. Ca2+ Buffer Saturation Underlies Paired Pulse Facilitation in Calbindin-D28k-Containing Terminals. Neuron 2003, 38, 79–88. [Google Scholar] [CrossRef]
- Schiffmann, S.N.; Cheron, G.; Lohof, A.; D’Alcantara, P.; Meyer, M.; Parmentier, M.; Schurmans, S. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. Proc. Natl. Acad. Sci. USA 1999, 96, 5257–5262. [Google Scholar] [CrossRef]
- Cheron, G.; Gall, D.; Servais, L.; Dan, B.; Maex, R.; Schiffmann, S.N. Inactivation of Calcium-Binding Protein Genes Induces 160 Hz Oscillations in the Cerebellar Cortex of Alert Mice. J. Neurosci. 2004, 24, 434–441. [Google Scholar] [CrossRef]
- Schurmans, S.; Schiffmann, S.N.; Gurden, H.; Lemaire, M.; Lipp, H.-P.; Schwam, V.; Pochet, R.; Imperato, A.; Böhme, G.A.; Parmentier, M. Impaired long-term potentiation induction in dentate gyrus of calretinin-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 10415–10420. [Google Scholar] [CrossRef]
- Gurden, H.; Schiffmann, S.N.; Lemaire, M.; Böhme, G.A.; Parmentier, M.; Schurmans, S. Calretinin expression as a critical component in the control of dentate gyrus long-term potentiation induction in mice. Eur. J. Neurosci. 1998, 10, 3029–3033. [Google Scholar] [CrossRef]
- Gall, C.; Pinkstaff, J.; Lauterborn, J.; Xie, Y.; Lynch, G. Integrins regulate neuronal neurotrophin gene expression through effects on voltage-sensitive calcium channels. Neuroscience 2003, 118, 925–940. [Google Scholar] [CrossRef]
- Collin, T.; Marty, A.; Llano, I. Presynaptic calcium stores and synaptic transmission. Curr. Opin. Neurobiol. 2005, 15, 275–281. [Google Scholar] [CrossRef]
- Lee, S.-H.; Schwaller, B.; Neher, E. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: Implications for [Ca2+] transients of neuronal dendrites. J. Physiol. 2000, 525, 419–432. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Gan, L.; Forsythe, I.D.; Kaczmarek, L.K. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J. Physiol. 1998, 509, 183–194. [Google Scholar] [CrossRef]
- Erisir, A.; Lau, D.; Rudy, B.; Leonard, C.S. Function of Specific K+ Channels in Sustained High-Frequency Firing of Fast-Spiking Neocortical Interneurons. J. Neurophysiol. 1999, 82, 2476–2489. [Google Scholar] [CrossRef]
- Rudy, B.; McBain, C.J. Kv3 channels: Voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001, 24, 517–526. [Google Scholar] [CrossRef]
- Lien, C.-C.; Jonas, P. Kv3 Potassium Conductance is Necessary and Kinetically Optimized for High-Frequency Action Potential Generation in Hippocampal Interneurons. J. Neurosci. 2003, 23, 2058–2068. [Google Scholar] [CrossRef]
- Aponte, Y.; Bischofberger, J.; Jonas, P. Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus. J. Physiol. 2008, 586, 2061–2075. [Google Scholar] [CrossRef]
- Orduz, D.; Bischop, D.P.; Schwaller, B.; Schiffmann, S.N.; Gall, D. Parvalbumin tunes spike-timing and efferent short-term plasticity in striatal fast spiking interneurons. J. Physiol. 2013, 591, 3215–3232. [Google Scholar] [CrossRef]
- McGinnis, K.M.; Whitton, M.M.; Gnegy, M.E.; Wang, K.K.W. Calcium/Calmodulin-dependent Protein Kinase IV Is Cleaved by Caspase-3 and Calpain in SH-SY5Y Human Neuroblastoma Cells Undergoing Apoptosis. J. Boil. Chem. 1998, 273, 19993–20000. [Google Scholar] [CrossRef]
- Haverkamp, S.; Ghosh, K.K.; Hirano, A.A.; Wässle, H. Immunocytochemical description of five bipolar cell types of the mouse retina. J. Comp. Neurol. 2003, 455, 463–476. [Google Scholar] [CrossRef]
- Chiquet, C.; Dkhissi-Benyahya, O.; Cooper, H.M. Calcium-binding protein distribution in the retina of strepsirhine and haplorhine primates. Brain Res. Bull. 2005, 68, 185–194. [Google Scholar] [CrossRef]
- Kántor, O.; Benkő, Z.; Énzsöly, A.; Dávid, C.; Naumann, A.; Nitschke, R.; Szabó, A.; Emese, P.; Orbán, J.; Nyitrai, M.; et al. Characterization of connexin36 gap junctions in the human outer retina. Brain Struct. Funct. 2016, 221, 2963–2984. [Google Scholar] [CrossRef]
- Kántor, O.; Varga, A.; Nitschke, R.; Naumann, A.; Énzsöly, A.; Lukáts, Á.; Szabó, A.; Németh, J.; Völgyi, B. Bipolar cell gap junctions serve major signaling pathways in the human retina. Brain Struct. Funct. 2017, 222, 2603–2624. [Google Scholar]
- Haverkamp, S.; Wässle, H. Immunocytochemical analysis of the mouse retina. J. Comp. Neurol. 2000, 424, 1–23. [Google Scholar] [CrossRef]
- Tanaka, T.; Amest, J.B.; Harvey, T.S.; Stryer, L.; Lkura, M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature 1995, 376, 444–447. [Google Scholar] [CrossRef]
- Hamano, K.; Kiyama, H.; Emson, P.C.; Manabe, R.; Nakauchi, M.; Tohyama, M. Localization of two calcium binding proteins, calbindin (28 kD) and parvalbumin (12 kD), in the vertebrate retina. J. Comp. Neurol. 1990, 302, 417–424. [Google Scholar] [CrossRef]
- Massey, S.C.; Mills, S.L. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J. Comp. Neurol. 1996, 366, 15–33. [Google Scholar] [CrossRef]
- Völgyi, B.; Pollák, E.; Buzás, P.; Gábriel, R. Calretinin in neurochemically well-defined cell populations of rabbit retina. Brain Res. 1997, 763, 79–86. [Google Scholar] [CrossRef]
- Grünert, U.; Martin, P.R.; Wässle, H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J. Comp. Neurol. 1994, 348, 607–627. [Google Scholar] [CrossRef]
- Haverkamp, S.; Haeseleer, F.; Hendrickson, A. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Vis. Neurosci. 2003, 20, 589–600. [Google Scholar] [CrossRef]
- Hendrickson, A.; Yan, Y.-H.; Erickson, A.; Possin, D.; Pow, D. Expression patterns of calretinin, calbindin and parvalbumin and their colocalization in neurons during development of Macaca monkey retina. Exp. Eye 2007, 85, 587–601. [Google Scholar] [CrossRef]
- Kántor, O.; Mezey, S.; Adeghate, J.; Naumann, A.; Nitschke, R.; Énzsöly, A.; Szabó, A.; Lukáts, Á.; Németh, J.; Somogyvári, Z.; et al. Calcium buffer proteins are specific markers of human retinal neurons. Cell Tissue Res. 2016, 365, 29–50. [Google Scholar] [CrossRef]
- Pasteels, B.; Rogers, J.; Blacher, F.; Pochet, R. Calbindin and calretinin localiyation in retina from different species. Vis. Neurosci. 1990, 5, 1–16. [Google Scholar] [CrossRef]
- Nag, T.; Wadhwa, S.; Nag, T. Developmental expression of calretinin immunoreactivity in the human retina and a comparison with two other EF-hand calcium-binding proteins. Neuroscience 1999, 91, 41–50. [Google Scholar] [CrossRef]
- Ramon y Cajal, S. La retine des vertebres. La Cell. 1893, 9, 119–257. [Google Scholar]
- Kolb, H. The connections between horizontal cells and photoreceptors in the retina of the cat: Electron microscopy of Golgi preparations. J. Comp. Neurol. 1974, 155, 1–14. [Google Scholar] [CrossRef]
- Massey, S.C.; Redburn, D.A. Transmitter circuits in the vertebrate retina. Prog. Neurobiol. 1987, 28, 55–96. [Google Scholar] [CrossRef]
- Fisher, S.K.; Boycott, B.B. Synaptic Connexions Made by Horizontal Cells within the Outer Plexiform Layer of the Retina of the Cat and the Rabbit. Proc. R. Soc. B Biol. Sci. 1974, 186, 317–331. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Miller, R.F. A physiological and morphological study of the horizontal cell types of the rabbit retina. J. Comp. Neurol. 1982, 208, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, R.; Raviola, E. Horizontal cells in the retina of the rabbit. J. Neurosci. 1982, 2, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Boije, H.; Fard, S.S.; Edqvist, P.-H.; Hallböök, F. Horizontal Cells, the Odd Ones Out in the Retina, Give Insights into Development and Disease. Front. Neuroanat. 2016, 10, 77. [Google Scholar] [CrossRef]
- Kolb, H.; Linberg, K.A.; Fisher, S.K. Neurons of the human retina: A Golgi study. J. Comp. Neurol. 1992, 318, 147–187. [Google Scholar] [CrossRef]
- Kolb, H.; Nelson, R. The organization of photoreceptor to bipolar synapses in the outer plexiform layer. In Neurobiology and Clinical Aspects of the Outer Retina; Djamgoz, M.B.A., Archer, S.N., Vallerga, S., Eds.; Chapman and Hall: London, UK, 1995; pp. 273–296. [Google Scholar]
- Peichl, L.; Gonzalez-Soriano, J. Morphological types of horizontal cell in rodent retinae: A comparison of rat, mouse, gerbil, and guinea pig. Vis. Neurosci. 1994, 11, 501. [Google Scholar] [CrossRef]
- Marquardt, T.; Ashery-Padan, R.; Andrejewski, N.; Scardigli, R.; Guillemot, F.; Gruss, P. Pax6 Is Required for the Multipotent State of Retinal Progenitor Cells. Cell 2001, 105, 43–55. [Google Scholar] [CrossRef]
- Dyer, M.A.; Livesey, F.J.; Cepko, C.L.; Oliver, G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat. Genet. 2003, 34, 53–58. [Google Scholar] [CrossRef]
- Hombach, S.; Janssen-Bienhold, U.; Söhl, G.; Schubert, T.; Büssow, H.; Ott, T.; Weiler, R.; Willecke, K.; Janssen-Bienhold, U. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur. J. Neurosci. 2004, 19, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Elshatory, Y.; Everhart, D.; Deng, M.; Xie, X.; Barlow, R.B.; Gan, L. Islet-1 Controls the Differentiation of Retinal Bipolar and Cholinergic Amacrine Cells. J. Neurosci. 2007, 27, 12707–12720. [Google Scholar] [CrossRef]
- Boycott, B.B.; Dowling, J.E.; Kolb, H. Organization of the Primate Retina: Light Microscopy. Philos. Trans. R. Soc. B Biol. Sci. 1969, 255, 109–184. [Google Scholar] [CrossRef]
- Kolb, H. Organization of the Outer Plexiform Layer of the Primate Retina: Electron Microscopy of Golgi-Impregnated Cells. Philos. Trans. R. Soc. B Boil. Sci. 1970, 258, 261–283. [Google Scholar] [CrossRef]
- Boycott, B.B.; Hopkins, J.M.; Sperling, H.G. Cone connections of the horizontal cells of the rhesus monkey’s retina. Proc. R. Soc. Lond. Ser. B Boil. Sci. 1987, 229, 345–379. [Google Scholar]
- Wässle, H.; Röhrenbeck, J.; Boycott, B.B. Horizontal Cells in the Monkey Retina: Cone connections and dendritic network. Eur. J. Neurosci. 1989, 1, 421–435. [Google Scholar] [CrossRef]
- Boycott, B.B.; Wässle, H. Morphological Classification of Bipolar Cells of the Primate Retina. Eur. J. Neurosci. 1991, 3, 1069–1088. [Google Scholar] [CrossRef]
- Peichl, L.; Airaksinen, M.S.; Wässle, H.; Meyer, M. Calcium-binding proteins in the retina of a calbindin-null mutant mouse. Cell Tissue Res. 1998, 292, 211–218. [Google Scholar]
- Telkes, I.; Kóbor, P.; Orbán, J.; Kovács-Öller, T.; Völgyi, B.; Buzás, P. Connexin-36 Distribution and Layer-specific Topography in the Cat Retina. Brain Struct. Funct. 2019, in press. [Google Scholar]
- Münch, T.A.; Da Silveira, R.A.; Siegert, S.; Viney, T.J.; Awatramani, G.B.; Roska, B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat. Neurosci. 2009, 12, 1308–1316. [Google Scholar] [CrossRef]
- Barasso, A.P.; Wang, S.; Tong, X.; Christianse, A.E.; Larina, I.V.; Poché, R.A. Live imaging of developing mouse retinal slice. Neural Dev. 2018, 13. [Google Scholar] [CrossRef]
- Cuenca, N.; Deng, P.; Linberg, K.A.; Lewis, G.P.; Fisher, S.K.; Kolb, H. The neurons of the ground squirrel retina as revealed by immunostains for calcium binding proteins and neurotransmitters. J. Neurocytol. 2002, 31, 649–666. [Google Scholar] [CrossRef]
- Wassle, H.; Grunert, U.; Martin, P.R.; Boycotts, B.B. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vis. Res. 1994, 34, 561–579. [Google Scholar] [CrossRef]
- Milam, A.H.; Dacey, D.M.; Dizhoor, A.M. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis. Neurosci. 1993, 10, 1. [Google Scholar] [CrossRef]
- Chun, M.H.; Kim, I.B.; Oh, S.J.; Chung, J.W. Synaptic connectivity of two types of recoverin-labeled cone bipolar cells and glutamic andi decarboxylase immunoreactive ACs in the inner plexiform layer of the rat retina. Vis Neruosci. 1999, 16, 791–800. [Google Scholar] [CrossRef]
- Jacoby, R.A.; Wiechmann, A.F.; Amara, S.G.; Leighton, B.H.; Marshak, D.W. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. J. Comp. Neurol. 2000, 416, 6–18. [Google Scholar] [CrossRef]
- Chan, T.L.; Grünert, U.; Martin, P.R. Immunocytochemical identification and analysis of the diffuse bipolar cell type DB6 in macaque monkey retina. Eur. J. Neurosci. 2001, 13, 829–832. [Google Scholar] [CrossRef]
- Weltzien, F.; Percival, K.A.; Martin, P.R.; Grünert, U. Analysis of bipolar and amacrine populations in marmoset retina. J. Comp. Neurol. 2014, 523, 313–334. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Bujan, S.; Haverkamp, S.; Feigenspan, A.; Wässle, H. Types of bipolar cells in the mouse retina. J. Comp. Neurol. 2003, 469, 70–82. [Google Scholar] [CrossRef]
- Veruki, M.L.; Wässle, H. Immunohistochemical Localization of Dopamine D Receptors in Rat Retina. Eur. J. Neurosci. 1996, 8, 2286–2297. [Google Scholar] [CrossRef]
- Kim, I.-J.; Zhang, Y.; Meister, M.; Sanes, J.R. Laminar Restriction of Retinal Ganglion Cell Dendrites and Axons: Subtype-Specific Developmental Patterns Revealed with Transgenic Markers. J. Neurosci. 2010, 30, 1452–1462. [Google Scholar] [CrossRef]
- Kim, S.A.; Jung, C.K.; Kang, T.-H.; Jeon, J.H.; Cha, J.; Kim, I.-B.; Chun, M.-H. Synaptic connections of calbindin-immunoreactive cone bipolar cells in the inner plexiform layer of rabbit retina. Cell Tissue Res. 2009, 339, 311–320. [Google Scholar] [CrossRef]
- Puthussery, T.; Taylor, W.R.; Gayet-Primo, J.; Gayet-Primo, J. Localization of the Calcium-binding Protein Secretagogin in Cone Bipolar Cells of the Mammalian Retina. J. Comp. Neurol. 2010, 518, 513–525. [Google Scholar] [CrossRef]
- Puthussery, T.; Taylor, W.R.; Haverkamp, S.; Gayet-Primo, J.; Gayet-Primo, J. Immunohistochemical Identification and Synaptic Inputs to the Diffuse Bipolar Cell Type DB1 in Macaque Retina. J. Comp. Neurol. 2011, 519, 3640–3656. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Eilers, J.; Garaschuk, O.; Thoenen, H.; Konnerth, A.; Meyer, M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc. Natl. Acad. Sci. USA 1997, 94, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Stiefel, K.M.; Racay, P.; Schwaller, B.; Eilers, J. Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: Role of parvalbumin and calbindin D28k. J. Physiol. 2003, 551, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Nägerl, U.V.; Mody, I. Calcium-dependent inactivation of high-threshold calcium currents in human dentate gyrus granule cells. J. Physiol. 1998, 509, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Klapstein, G.; Vietla, S.; Lieberman, D.; Gray, P.; Airaksinen, M.; Thoenen, H.; Meyer, M.; Mody, I. Calbindin-D28k fails to protect hippocampal neurons against ischemia in spite of its cytoplasmic calcium buffering properties: Evidence from calbindin-D28k knockout mice. Neuroscience 1998, 85, 361–373. [Google Scholar] [CrossRef]
- Haeseleer, F.; Sokal, I.; Verlinde, C.L.M.J.; Erdjument-Bromage, H.; Tempst, P.; Pronin, A.N.; Benovic, J.L.; Fariss, R.N.; Palczewski, K. Five Members of a Novel Ca++-binding Protein (CABP) Subfamily with Similarity to Calmodulin. J. Biol. Chem. 2000, 275, 1247–1260. [Google Scholar] [CrossRef]
- Haverkamp, S.; Specht, D.; Majumdar, S.; Zaidi, N.F.; Brandstätter, J.H.; Wasco, W.; Wassle, H.; tom Dieck, S. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J. Comp. Neurol. 2007, 507, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Percival, K.A.; Koizumi, A.; Masri, R.A.; Buzás, P.; Martin, P.R.; Grünert, U. Identification of a Pathway from the Retina to Koniocellular Layer K1 in the Lateral Geniculate Nucleus of Marmoset. J. Neurosci. 2014, 34, 3821–3825. [Google Scholar] [CrossRef]
- Vaney, D.I.; Whitington, G.E.; Young, H.M. The Morphology and Topographic Distribution of Substance-P-Like Immunoreactive Amacrine Cells in the Cat Retina. Proc. R. Soc. B Biol. Sci. 1989, 237, 471–488. [Google Scholar] [CrossRef]
- Helmstaedter, M.; Briggman, K.L.; Turaga, S.C.; Jain, V.; Seung, H.S.; Denk, W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 2013, 500, 168–174. [Google Scholar] [CrossRef]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu. Rev. Vis. Sci. 2017, 3, 1–24. [Google Scholar] [CrossRef]
- Barlow, H.B.; Hill, R.M. Selective Sensitivity to Direction of Movement in Ganglion Cells of the Rabbit Retina. Science 1963, 139, 412. [Google Scholar] [CrossRef]
- Barlow, H.B.; Levick, W.R. The mechanism of directionally selective units in rabbit’s retina. J. Physiol. 1965, 178, 477–504. [Google Scholar] [CrossRef]
- Taylor, W.; Vaney, D.I. New directions in retinal research. Trends Neurosci. 2003, 26, 379–385. [Google Scholar] [CrossRef]
- Fried, S.I.; Munch, T.A.; Werblin, F.S. Directional Selectivity Is Formed at Multiple Levels by Laterally Offset Inhibition in the Rabbit Retina. Neuron 2005, 46, 117–127. [Google Scholar] [CrossRef]
- Knop, G.C.; Pottek, M.; Monyer, H.; Weiler, R.; Dedek, K. Morphological and physiological properties of enhanced green fluorescent protein (EGFP)-expressing wide-field amacrine cells in the ChAT-EGFP mouse line. Eur. J. Neurosci. 2013, 39, 800–810. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, J.M.; Shin, Y.-J.; Chun, M.-H.; Oh, S.-J. Immunochemical changes of calbindin, calretinin and SMI32 in ischemic retinas induced by increase of intraocular pressure and by middle cerebral artery occlusion. Anat. Cell Boil. 2011, 44, 25–34. [Google Scholar] [CrossRef]
- Kovács-Öller, T.; Debertin, G.; Balogh, M.; Ganczer, A.; Orbán, J.; Nyitrai, M.; Balogh, L.; Kántor, O.; Völgyi, B. Connexin36 Expression in the Mammalian Retina: A Multiple-Species Comparison. Front. Cell. Neurosci. 2017, 11, 220. [Google Scholar] [CrossRef]
- Massey, S.C.; Mills, S.L. Antibody to calretinin stains AII amacrine cells in the rabbit retina: Double-label and confocal analyses. J. Comp. Neurol. 1999, 411, 3–18. [Google Scholar] [CrossRef]
- Gábriel, R.; Völgyi, B.; Pollák, E. Most calretinin-containing amacrine cells in the rabbit retina co-localize glycine. Vis. Neurosci. 1999, 16, 983–990. [Google Scholar] [CrossRef]
- Deans, M.R.; Volgyi, B.; Goodenough, D.A.; Bloomfield, S.A.; Paul, D.L. Connexin36 Is Essential for Transmission of Rod-Mediated Visual Signals in the Mammalian Retina. Neuron 2002, 36, 703–712. [Google Scholar] [CrossRef]
- Sanna, P.P.; Keyser, K.T.; Battenberg, E.; Bloom, F.E. Parvalbumin immunoreactivity in the rat retina. Neurosci. Lett. 1990, 118, 136–139. [Google Scholar] [CrossRef]
- Gábriel, R.; Straznicky, C. Immunocytochemical localization of parvalbumin- and neurofilament triplet protein immunoreactivity in the cat retina: Colocalization in a subpopulation of AII amacrine cells. Brain Res. 1992, 595, 133–136. [Google Scholar] [CrossRef]
- Wäussle, H.; Grüunert, U.; Röhrenbeck, J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J. Comp. Neurol. 1993, 332, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Casini, G.; Rickman, D.W.; Brecha, N.C. AII amacrine cell population in the rabbit retina: Identification by parvalbumin immunoreactivity. J. Comp. Neurol. 1995, 356, 132–142. [Google Scholar] [CrossRef]
- Huberman, A.D.; Manu, M.; Koch, S.M.; Susman, M.W.; Lutz, A.B.; Ullian, E.M.; Baccus, S.A.; Barres, B.A. Architecture and Activity-Mediated Refinement of Axonal Projections from a Mosaic of Genetically Identified Retinal Ganglion Cells. Neuron 2008, 59, 425–438. [Google Scholar] [CrossRef]
- Völgyi, B.; Kovács-Oller, T.; Atlasz, T.; Wilhelm, M.; Gábriel, R. Gap junctional coupling in the vertebrate retina: Variations on one theme? Prog. Retin. Eye Res. 2013, 34, 1–18. [Google Scholar] [CrossRef]
- Park, H.-S.; Park, S.-J.; Park, S.-H.; Chun, M.-H.; Oh, S.-J. Shifting of parvalbumin expression in the rat retina in experimentally induced diabetes. Acta Neuropathol. 2007, 115, 241–248. [Google Scholar] [CrossRef]
- Debertin, G.; Kántor, O.; Kovács-Öller, T.; Balogh, L.; Szabó-Meleg, E.; Orbán, J.; Nyitrai, M.; Völgyi, B.; Kovács-Öller, T.; Szabó-Meleg, E. Tyrosine Hydroxylase Positive Perisomatic Rings are Formed around Various Amacrine Cell Types in the Mammalian Retina. J. Neurochem. 2015, 134, 416–428. [Google Scholar] [CrossRef]
- Stafford, D.K.; Dacey, D.M. Physiology of the A1 amacrine: A spiking, axon-bearing interneuron of the macaque monkey retina. Vis. Neurosci. 1997, 14, 507. [Google Scholar] [CrossRef]
- Famiglietti, E.V. Polyaxonal amacrine cells of rabbit retina: Morphology and stratification of PA1 cells. J. Comp. Neurol. 1992, 316, 391–405. [Google Scholar] [CrossRef]
- Famiglietti, E.V. Polyaxonal amacrine cells of rabbit retina: PA2, PA3, and PA4 cells. Light and electron microscopic studies with a functional interpretation. J. Comp. Neurol. 1992, 316, 422–446. [Google Scholar] [CrossRef]
- Völgyi, B.; Xin, D.; Amarillo, Y.; Bloomfield, S.A. Morphology and physiology of the polyaxonal amacrine cells in the rabbit retina. J. Comp. Neurol. 2001, 440, 109–125. [Google Scholar] [CrossRef]
- Voigt, T.; Wässle, H. Dopaminergic innervation of A II amacrine cells in mammalian retina. J. Neurosci. 1987, 7, 4115–4128. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. 2004, 108, 17–39. [Google Scholar] [CrossRef]
- Masland, R.H. Neuronal cell types. Curr. Boil. 2004, 14, R497–R500. [Google Scholar] [CrossRef]
- Rodieck, R.W. The First Steps in Seeing; Sinauer Associates: Sunderland, NA, USA, 1998. [Google Scholar]
- Yamada, E.S.; Bordt, A.S.; Marshak, D.W. Wide-field ganglion cells in macaque retinas. Vis. Neurosci. 2005, 22, 383–393. [Google Scholar] [CrossRef]
- Boycott, B.B.; Wässle, H. The morphological types of ganglion cells of the domestic cat’s retina. J. Physiol. 1974, 240, 397–419. [Google Scholar] [CrossRef]
- Pu, M.; Berson, D.; Pan, T. Structure and function of retinal ganglion cells innervating the cat’s geniculate wing: An in vitro study. J. Neurosci. 1994, 14, 4338–4358. [Google Scholar] [CrossRef]
- Berson, D.; Pu, M.; Famiglietti, E. The zeta cell: A new ganglion cell type in cat retina. J. Comp. Neurol. 1998, 399, 269–288. [Google Scholar] [CrossRef]
- Berson, D.; Isayama, T.; Pu, M. The eta ganglion cell type of cat retina. J. Comp. Neurol. 1999, 408, 204–219. [Google Scholar] [CrossRef]
- Isayama, T.; Berson, D.; Pu, M. Theta ganglion cell type of cat retina. J. Comp. Neurol. 2000, 417, 32–48. [Google Scholar] [CrossRef]
- Rockhill, R.L.; Daly, F.J.; MacNeil, M.A.; Brown, S.P.; Masland, R.H. The Diversity of Ganglion Cells in a Mammalian Retina. J. Neurosci. 2002, 22, 3831–3843. [Google Scholar] [CrossRef]
- Sun, W.; Li, N.; He, S. Large-scale morphological survey of mouse retinal ganglion cells. J. Comp. Neurol. 2002, 451, 115–126. [Google Scholar] [CrossRef]
- Badea, T.C.; Nathans, J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J. Comp. Neurol. 2004, 480, 331–351. [Google Scholar] [CrossRef]
- Coombs, J.; Van Der List, D.; Wang, G.-Y.; Chalupa, L. Morphological properties of mouse retinal ganglion cells. Neuroscience 2006, 140, 123–136. [Google Scholar] [CrossRef]
- Völgyi, B.; Chheda, S.; Bloomfield, S.A. Tracer Coupling Patterns of the Ganglion Cell Subtypes in the Mouse Retina. J. Comp. Neurol. 2009, 512, 664–687. [Google Scholar] [CrossRef]
- Baden, T.; Berens, P.; Franke, K.; Rosón, M.R.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef]
- Roski, C.; Langrock, C.; Körber, N.; Habermann, G.; Buse, E.; Reichenbach, A.; Pannicke, T.; Francke, M. Comparison of cellular localisation of the Ca2+ -binding proteins calbindin, calretinin and parvalbumin in the retina of four different Macaca species. Anat. Histol. Embryol. 2018, 47, 573–582. [Google Scholar] [CrossRef]
- Yan, Y.-H.; Van Brederode, J.F.M.; Hendrickson, A.E.; Brederode, J.F.M. Transient co-localization of calretinin, parvalbumin, and calbindin-D28k in developing visual cortex of monkey. J. Neurocytol. 1995, 24, 825–837. [Google Scholar] [CrossRef]
- Kim, T.-J.; Jeon, C.-J. Morphological Classification of Parvalbumin-Containing Retinal Ganglion Cells in Mouse: Single-Cell Injection after Immunocytochemistry. Investig. Opthalmol. Vis. Sci. 2006, 47, 2757–2764. [Google Scholar] [CrossRef]
- Lee, E.-S.; Kim, T.-J.; Jeon, C.-J. Identification of parvalbumin-containing retinal ganglion cells in rabbit. Exp. Eye Res. 2013, 110, 113–124. [Google Scholar] [CrossRef]
- Vugler, A.A.; Semo, M.; Joseph, A.; Jeffery, G. Survival and remodeling of melanopsin cells during retinal dystrophy. Vis. Neurosci. 2008, 25, 125. [Google Scholar] [CrossRef]
- Lee, E.-S.; Lee, J.-Y.; Jeon, C.-J. Types and density of calretinin-containing retinal ganglion cells in mouse. Neurosci. Res. 2010, 66, 141–150. [Google Scholar] [CrossRef]
- Lee, E.-S.; Lee, J.-Y.; Kim, G.H.; Jeon, C.-J. Identification of calretinin-expressing retinal ganglion cells projecting to the mouse superior colliculus. Cell Tissue Res. 2018, 376, 153–163. [Google Scholar] [CrossRef]
- Gu, Y.-N.; Lee, E.-S.; Jeon, C.-J. Types and density of calbindin D28k-immunoreactive ganglion cells in mouse retina. Exp. Eye Res. 2016, 145, 327–336. [Google Scholar] [CrossRef]
- Hu, H.; Gan, J.; Jonas, P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. [Google Scholar] [CrossRef]
- Gunn, D.J.; Gole, G.A.; Barnett, N.L. Specific amacrine cell changes in an induced mouse model of glaucoma. Clin. Exp. Ophthalmol. 2011, 39, 555–563. [Google Scholar] [CrossRef]
- Huang, J.-F.; Shang, L.; Zhang, M.-Q.; Wang, H.; Chen, D.; Tong, J.-B.; Huang, H.; Yan, X.-X.; Zeng, L.-P.; Xiong, K. Differential neuronal expression of receptor interacting protein 3 in rat retina: Involvement in ischemic stress response. BMC Neurosci. 2013, 14, 16. [Google Scholar] [CrossRef]
- Hernandez, M.; Rodriguez, F.D.; Sharma, S.; Vecino, E. Immunohistochemical changes in rat retinas at various time periods of elevated intraocular pressure. Mol. Vis. 2009, 15, 2696–2709. [Google Scholar]
- Heizmann, C.W.; Braun, K. Changes in Ca2+-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992, 15, 259–264. [Google Scholar] [CrossRef]
- Schäfer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef]
- Leuba, G.; Kraftsik, R.; Saini, K. Quantitative Distribution of Parvalbumin, Calretinin, and Calbindin D-28k Immunoreactive Neurons in the Visual Cortex of Normal and Alzheimer Cases. Exp. Neurol. 1998, 152, 278–291. [Google Scholar] [CrossRef]
- Cicchetti, F.; Prensa, L.; Wu, Y.; Parent, A. Chemical anatomy of striatal interneurons in normal individuals and in patients with Huntington’s disease. Brain Res. Rev. 2000, 34, 80–101. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Bartlett, S.E.; Morgans, C. The distribution of neuronal calcium sensor-1 protein in the developing and adult rat retina. Neuroreport 2001, 12, 725–728. [Google Scholar] [CrossRef]
- Eyles, D.; McGrath, J.; Reynolds, G.; McGrath, J.; Reynolds, G. Neuronal calcium-binding proteins and schizophrenia. Schizophr. Res. 2002, 57, 27–34. [Google Scholar] [CrossRef]
- Hong, C.J.H.; Siddiqui, A.M.; Sabljic, T.F.; Ball, A.K. Changes in parvalbumin immunoreactive retinal ganglion cells and amacrine cells after optic nerve injury. Exp. Eye Res. 2016, 145, 363–372. [Google Scholar] [CrossRef]
- D’Orlando, C.; Fellay, B.; Schwaller, B.; Salicio, V.; Bloc, A.; Gotzos, V.; Celio, M.R. Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 2001, 909, 145–158. [Google Scholar] [CrossRef]
- Lukas, W.; Jones, K. Cortical neurons containing calretinin are selectively resistant to calcium overload and excitotoxicity in vitro. Neuroscience 1994, 61, 307–316. [Google Scholar] [CrossRef]
- Pike, C.J.; Cotman, C.W. Calretinin-immunoreactive neurons are resistant to β-amyloid toxicity in vitro. Brain Res. 1995, 671, 293–298. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Kim, J.-Y.; Kim, S.-Y.; Jeon, C.-J. Alterations in the localization of calbindin D28K-, calretinin-, and parvalbumin-immunoreactive neurons of rabbit retinal ganglion cell layer from ischemia and reperfusion. Mol. Cells 2005, 19, 382–390. [Google Scholar]
- Fan, Y.; Shi, L.; Gu, Y.; Zhao, Y.; Xie, J.; Qiao, J.; Yang, G.-Y.; Wang, Y.; Lu, C.-Z. Pretreatment with PTD-Calbindin D 28k Alleviates Rat Brain Injury Induced by Ischemia and Reperfusion. J. Cereb. Blood Flow Metab. 2006, 27, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.A.; Minami, M.; Sun, G.H.; Meier, T.J.; Kunis, D.M.; McLaughlin, J.R.; Ho, D.Y.; Sapolsky, R.M.; Steinberg, G.K. Calbindin D28K Overexpression Protects Striatal Neurons from Transient Focal Cerebral Ischemia. Stroke 2001, 32, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Gábriel, R.; Lesauter, J.; Bánvölgyi, T.; Petrovics, G.; Silver, R.; Witkovsky, P. AII amacrine neurons of the rat retina show diurnal and circadian rhythms of parvalbumin immunoreactivity. Cell Tissue Res. 2004, 315, 181–186. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Calbindin | Calretinin | Parvalbumin | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | HC | BC | AC | GC | PR | HC | BC | AC | GC | PR | HC | BC | AC | GC | |
| Mouse | 1. B type | 1. Stb-a 2. Stb-b 3. WA type 2/3 | 1. A2 2. B1 3. B2 4. B3 5. B4 6. C2 7. C3 8. C4 9. C5 10. D2 | 1. Stb-a 2. Stb-b 3. WAtype 2/3 4. AII | 1. PV5/Off alpha/A2 3. B1 3. B2 4. B3 5. B4 6. C2 7. C3 8. C4 9. C5 10. D2/ON-OFF DS | 1. B type (?) | 1. UI | 1. PV1 2. PV2/ON-OFF DS/D2 3. PV3 4. PV4 5. PV-5/Off alpha 6. PV6 7. PV7 8. A1 9. B3 10. B4 11. C1 12. C2 13. C4 14. C5 | |||||||
| Human | 1. S 2. L/M | 1. HII | 1. DB3 2. DB4 3. DB5 4. DB6 5. IMB (?) | 1. Semilunar 2. Stellate 3. AII 4. UI | 1. UI | 1. IMB or DB4 | 1. Semi-lunar 2. Stellate 3. type1 TH AC 4. Star-shaped middle-field AC 5. AII 6. UI | 1. bistratified 2. UI | 1. HI 2. HII | 1. giant diffuse or giant bistrati-fied | 1. star-shaped 2. middle-field 3. AII 4. UI | 1. parasol 2. UI | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács-Öller, T.; Szarka, G.; Ganczer, A.; Tengölics, Á.; Balogh, B.; Völgyi, B. Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons. Int. J. Mol. Sci. 2019, 20, 2229. https://doi.org/10.3390/ijms20092229
Kovács-Öller T, Szarka G, Ganczer A, Tengölics Á, Balogh B, Völgyi B. Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons. International Journal of Molecular Sciences. 2019; 20(9):2229. https://doi.org/10.3390/ijms20092229
Chicago/Turabian StyleKovács-Öller, Tamás, Gergely Szarka, Alma Ganczer, Ádám Tengölics, Boglárka Balogh, and Béla Völgyi. 2019. "Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons" International Journal of Molecular Sciences 20, no. 9: 2229. https://doi.org/10.3390/ijms20092229
APA StyleKovács-Öller, T., Szarka, G., Ganczer, A., Tengölics, Á., Balogh, B., & Völgyi, B. (2019). Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons. International Journal of Molecular Sciences, 20(9), 2229. https://doi.org/10.3390/ijms20092229






