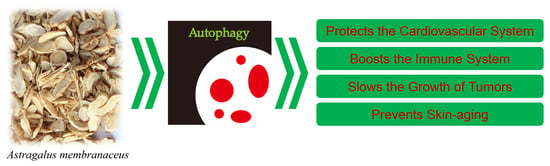
The Effects of Astragalus membranaceus Active Extracts on Autophagy-Related Diseases
Abstract
:1. Introduction
2. Autophagic Pathways and Human Diseases
3. AM and AM Extracts
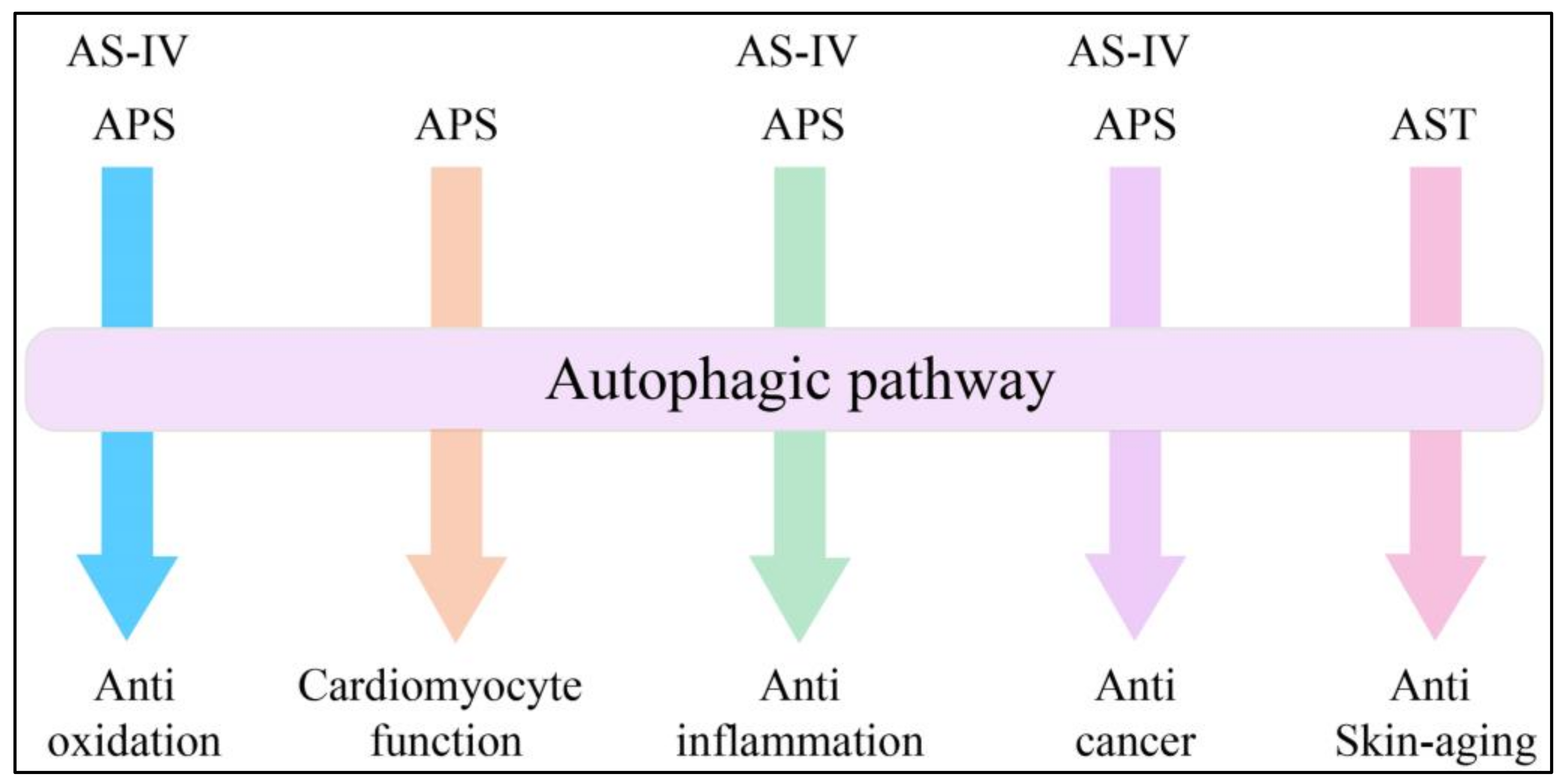
4. Expanding Mechanisms of Autophagy in AM Extracts
4.1. Advances in Anti-Oxidation
4.2. Advances in Cardiomyocyte Function
4.3. Advances in Anti-Inflammation
4.4. Advances in Anticancer Treatment
4.5. Advances in Skin Aging
5. Application of AM Extracts through Regulation of Autophagy: A Nexus for Protection or Therapeutics?
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, H.; Luo, Y. Anti-Aging Implications of Astragalus Membranaceus (Huangqi): A Well-Known Chinese Tonic. Aging Dis. 2017, 8, 868–886. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef]
- Oku, M.; Sakai, Y. Three Distinct Types of Microautophagy Based on Membrane Dynamics and Molecular Machineries. Bioessays 2018, 40, e1800008. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Kroemer, G. Therapeutic modulation of autophagy: Which disease comes first? Cell Death Differ. 2019. [Google Scholar] [CrossRef]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef]
- Man-xiu, L.I.; Xiao-qin, L.I.U. Determination of Eight Metal Elements in Astragalus by Microwave Digestion-Flame Atomic Absorption Spectrometry. J. Anal. Sci. 2009, 25, 605–608. [Google Scholar]
- Song, J.-Z.; Mo, S.-F.; Yip, Y.-K.; Qiao, C.-F.; Han, Q.-B.; Xu, H.-X. Development of microwave assisted extraction for the simultaneous determination of isoflavonoids and saponins in Radix Astragali by high performance liquid chromatography. J. Sep. Sci. 2007, 30, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-W.; Yu, Q.-T.; Yi, L.; Ren, M.-T.; Wen, X.-D.; Wang, Y.-X.; Li, P. Simultaneous determination of 15 marker constituents in various Radix Astragaii preparations by solid-phase extraction and high-performance liquid chromatography. J. Sep. Sci. 2008, 31, 97–106. [Google Scholar] [CrossRef]
- Matkowski, A.; Wozniak, D.; Lamer-Zarawska, E.; Oszmianski, J.; Leszczynska, A. Flavonoids and phenol carboxylic acids in the oriental medicinal plant Astragalus membranaceus acclimated in Poland. Z. Naturforschung C 2003, 58, 602–604. [Google Scholar] [CrossRef]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the Genus Astragalus: Phytochemistry and Biological Activity. Pharmacogn. Rev. 2016, 10, 11–32. [Google Scholar] [CrossRef]
- Kiyohara, H.; Uchida, T.; Takakiwa, M.; Matsuzaki, T.; Hada, N.; Takeda, T.; Shibata, T.; Yamada, H. Different contributions of side-chains in beta-D-(1→3,6)-galactans on intestinal Peyer’s patch-immunomodulation by polysaccharides from Astragalus mongholics Bunge. Phytochemistry 2010, 71, 280–293. [Google Scholar] [CrossRef]
- Wang, P.C.; Zhang, Z.Y.; Ma, X.F.; Yu, H.; Liu, X.W.; Tu, P.F.; Tong, T.J. HDTIC-1 and HDTIC-2, two compounds extracted from Astragali Radix, delay replicative senescence of human diploid fibroblasts. Mech. Ageing Dev. 2003, 124, 1025–1034. [Google Scholar] [CrossRef]
- Xie, D.; Wu, B.; Sun, H.; Guo, X.; Zhang, L.; Qin, X. Correlation between the Flavor and Quality of Radix Astragali: The Extraction and Characterization of Lipoxygenase in Radix Astragali. World Sci. Technol. 2009, 11, 375–381. [Google Scholar]
- Yu, Q.-T.; Qi, L.-W.; Li, P.; Yi, L.; Zhao, J.; Bi, Z. Determination of seventeen main flavonoids and saponins in the medicinal plant Huang-qi (Radix Astragali) by HPLC-DAD-ELSD. J. Sep. Sci. 2007, 30, 1292–1299. [Google Scholar] [CrossRef]
- Chu, C.; Cai, H.-X.; Ren, M.-T.; Liu, E.H.; Li, B.; Qi, L.-W.; Li, P. Characterization of novel astragaloside malonates from Radix Astragali by HPLC with ESI quadrupole TOF MS. J. Sep. Sci. 2010, 33, 570–581. [Google Scholar] [CrossRef]
- He, Z.Q.; Wang, B.Q. Isolation and identification of chemical constituents of astragalus root. Acta Pharm. Sin. 1990, 25, 694–698. [Google Scholar]
- Lin, L.Z.; He, X.G.; Lindenmaier, M.; Nolan, G.; Yang, J.; Cleary, M.; Qiu, S.X.; Cordell, G.A. Liquid chromatography-electrospray ionization mass spectrometry study of the flavonoids of the roots of Astragalus mongholicus and A-membranaceus. J. Chromatogr. A 2000, 876, 87–95. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.; Wang, W.; Tian, W.; Zhang, X. Extraction, characterization of Astragalus polysaccharides and its immune modulating activities in rats with gastric cancer. Carbohydr. Polym. 2009, 78, 738–742. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohydr. Polym. 2009, 78, 343–348. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Chan, B.C.-L.; Yu, H.; Lau, I.Y.-K.; Han, X.-Q.; Cheng, S.-W.; Wong, C.-K.; Lau, C.B.-S.; Xie, M.-Y.; Fung, K.-P.; et al. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr. Polym. 2012, 87, 667–675. [Google Scholar] [CrossRef]
- Subarnas, A.; Oshima, Y.; Hikino, H. New constituents of astragalus-mongholicus. Planta Med. 1991, 57, 590. [Google Scholar] [CrossRef]
- Hikino, H.; Funayama, S.; Endo, K. Hypotensive principle of astragalus and hedysarum roots. Planta Med. 1976, 30, 297–302. [Google Scholar] [CrossRef]
- Koyama-Honda, I.; Itakura, E.; Fujiwara, T.K.; Mizushima, N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy 2013, 9, 1491–1499. [Google Scholar] [CrossRef]
- Hu, J.Y.; Han, J.; Chu, Z.G.; Song, H.P.; Zhang, D.X.; Zhang, Q.; Huang, Y.S. Astragaloside IV attenuates hypoxia-induced cardiomyocyte damage in rats by upregulating superoxide dismutase-1 levels. Clin. Exp. Pharmacol. Physiol. 2009, 36, 351–357. [Google Scholar] [CrossRef]
- Qiu, L.H.; Xie, X.J.; Zhang, B.Q. Astragaloside IV improves homocysteine-induced acute phase endothelial dysfunction via antioxidation. Biol. Pharm. Bull. 2010, 33, 641–646. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, N.; Wang, W.; Jin, H.; Xu, J.; Hu, H. Protective effects of astragaloside IV against amyloid beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLoS ONE 2014, 9, e98866. [Google Scholar] [CrossRef]
- Ko, J.K.S.; Lam, F.Y.L.; Cheung, A.P.L. Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World J. Gastroenterol. 2005, 11, 5787–5794. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Gustafsson, A.B.; Dorn, G.W. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol. Rev. 2019, 99, 853–892. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Wu, H.; Bian, Z.; Xu, J.; Gu, C.; Chen, X.; Yang, D. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int. J. Mol. Med. 2015, 36, 1223–1232. [Google Scholar] [CrossRef]
- Kageyama, Y.; Hoshijima, M.; Seo, K.; Bedja, D.; Sysa-Shah, P.; Andrabi, S.A.; Chen, W.; Hoke, A.; Dawson, V.L.; Dawson, T.M.; et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014, 33, 2798–2813. [Google Scholar] [CrossRef]
- Durcan, T.M.; Fon, E.A. The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Gene Dev. 2015, 29, 989–999. [Google Scholar] [CrossRef]
- Wang, Y.; Nartiss, Y.; Steipe, B.; McQuibban, G.A.; Kim, P.K. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 2012, 8, 1462–1476. [Google Scholar] [CrossRef]
- Yin, Y.; Lu, L.; Wang, D.; Shi, Y.; Wang, M.; Huang, Y.; Chen, D.; Deng, C.; Chen, J.; Lv, P.; et al. Astragalus Polysaccharide Inhibits Autophagy and Apoptosis from Peroxide-Induced Injury in C2C12 Myoblasts. Cell Biochem. Biophys. 2015, 73, 433–439. [Google Scholar] [CrossRef]
- Sachdev, S.; Davies, K.J.A. Production, detection, and adaptive responses to free radicals in exercise. Free Radic. Boil. Med. 2008, 44, 215–223. [Google Scholar] [CrossRef]
- Sim, M.-K.; Wong, Y.-C.; Xu, X.-G.; Loke, W.-K. Des-aspartate-angiotensin I attenuates ICAM-1 formation in hydrogen peroxide-treated L6 skeletal muscle cells and soleus muscle of mice subjected to eccentric exercise. Regul. Pept. 2014, 188, 40–45. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef]
- Glass, D.J. Molecular mechanisms modulating muscle mass. Trends Mol. Med. 2003, 9, 344–350. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lu, L.; Zhu, D.J.; Wang, M.; Yin, Y.; Chen, D.X.; Wei, L.B. Effects of Astragalus Polysaccharides on Dysfunction of Mitochondrial Dynamics Induced by Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 9573291. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Yang, Y.; Li, D.; Lv, J.; Ou, Y.; Sun, F.; Chen, J.; Shi, Y.; Xia, P. Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. Int. J. Biol. Macromol. 2014, 68, 209–214. [Google Scholar] [CrossRef]
- Lu, L.; Huang, Y.F.; Chen, D.X.; Wang, M.; Zou, Y.C.; Wan, H.; Wei, L.B. Astragalus polysaccharides decrease muscle wasting through Akt/mTOR, ubiquitin proteasome and autophagy signalling in 5/6 nephrectomised rats. J. Ethnopharmacol. 2016, 186, 125–135. [Google Scholar] [CrossRef]
- Liu, D.; Xu, J.; Qian, G.; Hamid, M.; Gan, F.; Chen, X.; Huang, K. Selenizing astragalus polysaccharide attenuates PCV2 replication promotion caused by oxidative stress through autophagy inhibition via PI3K/AKT activation. Int. J. Biol. Macromol. 2018, 108, 350–359. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, W.; Chen, D.; Feng, C.; Zhang, L.; Wang, X.; Lv, X.; Zheng, N.; Jin, Y.; Wu, Z. Enterovirus 71 induces autophagy by regulating has-miR-30a expression to promote viral replication. Antivir. Res. 2015, 124, 43–53. [Google Scholar] [CrossRef]
- Pei, J.; Zhao, M.; Ye, Z.; Gou, H.; Wang, J.; Yi, L.; Dong, X.; Liu, W.; Luo, Y.; Liao, M.; et al. Autophagy enhances the replication of classical swine fever virus in vitro. Autophagy 2014, 10, 93–110. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid. Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, T.; Huang, X.; Lin, Y.; Chen, B.; Pang, J.; Li, G.; Wang, Q.; Zohrabian, S.; Duan, C.; et al. Astragalus polysaccharide restores autophagic flux and improves cardiomyocyte function in doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 4837–4848. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Bhuiyan, M.A.N.; Panchatcharam, M.; Pattillo, C.B.; Orr, A.W.; Sadoshima, J.; Hill, J.A.; et al. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Sci. Rep. 2019, 9, 2002. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, J.; Zhang, Y.; Chen, Y.; Yang, Z.; Huang, G.; Chen, Y.; Yuan, Z.; Peng, Y.; Cao, T. Astragaloside-IV Alleviates Heat-Induced Inflammation by Inhibiting Endoplasmic Reticulum Stress and Autophagy. Cell. Physiol. Biochem. 2017, 42, 824–837. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L.; Tan, J.W.; Chen, X.Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.; Liu, D.; Abdulrahim, Y.; Khan, A.; Qian, G.; Huang, K. Inactivation of Kupffer Cells by Selenizing Astragalus Polysaccharides Prevents CCl4-Induced Hepatocellular Necrosis in the Male Wistar Rat. Biol. Trace Elem. Res. 2017, 179, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Du, X.; Wang, H.; Gu, H.; Zhan, J.; Zhou, Z. Astragalus polysaccharides inhibits cell growth and pro-inflammatory response in IL-1beta-stimulated fibroblast-like synoviocytes by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Apoptosis 2017, 22, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Zhang, C.; Zhao, Z.; Li, S.; Cai, H.; Chen, X.; Cai, D.; Liu, W.; Yan, Y.; Xie, K.; et al. The gastric mucosal protective effects of astragaloside IV in mnng-induced GPL rats. Biomed. Pharmacother. 2018, 104, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Su, Q.; Ding, W.; Li, T.; Yu, J.; Cao, B. Traditional Chinese medicine Astragalus polysaccharide enhanced antitumor effects of the angiogenesis inhibitor apatinib in pancreatic cancer cells on proliferation, invasiveness, and apoptosis. Onco Targets Ther. 2018, 11, 2685–2698. [Google Scholar] [CrossRef]
- Wu, J.; Yu, J.; Wang, J.; Zhang, C.; Shang, K.; Yao, X.; Cao, B. Astragalus polysaccharide enhanced antitumor effects of Apatinib in gastric cancer AGS cells by inhibiting AKT signalling pathway. Biomed. Pharmacother. 2018, 100, 176–183. [Google Scholar] [CrossRef]
- Zhai, Q.L.; Hu, X.D.; Xiao, J.; Yu, D.Q. Astragalus polysaccharide may increase sensitivity of cervical cancer HeLa cells to cisplatin by regulating cell autophagy. Zhongguo Zhong Yao Za Zhi 2018, 43, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Izumiyama-Shimomura, N.; Sawabe, M.; Arai, T.; Aoyagi, Y.; Fujiwara, M.; Tsuchiya, E.; Kobayashi, Y.; Kato, M.; Oshimura, M.; et al. Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. J. Investig. Dermatol. 2002, 119, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kosmadaki, M.G.; Gilchrest, B.A. The role of telomeres in skin aging/photoaging. Micron 2004, 35, 155–159. [Google Scholar] [CrossRef]
- Montagna, W.; Kirchner, S.; Carlisle, K. Histology of sun-damaged human skin. J. Am. Acad. Dermatol. 1989, 21, 907–918. [Google Scholar] [CrossRef]
- Landau, M. Exogenous factors in skin aging. Curr. Probl. Dermatol. 2007, 35, 1–13. [Google Scholar] [CrossRef]
- Nassour, J.; Radford, R.; Correia, A.; Fuste, J.M.; Schoell, B.; Jauch, A.; Shaw, R.J.; Karlseder, J. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 2019, 565, 659–663. [Google Scholar] [CrossRef]
- Addor, F.A.S. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Investig. Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. UV-induced immune suppression and photocarcinogenesis: Chemoprevention by dietary botanical agents. Cancer Lett. 2007, 255, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Palacky Univ. Olomouc 2006, 150, 25. [Google Scholar] [CrossRef]
- Wen, W.; Chen, J.; Ding, L.; Luo, X.; Zheng, X.; Dai, Q.; Gu, Q.; Liu, C.; Liang, M.; Guo, X.; et al. Astragaloside exerts anti-photoaging effects in UVB-induced premature senescence of rat dermal fibroblasts through enhanced autophagy. Arch. Biochem. Biophys. 2018, 657, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, H.; Kim, M.; Xu, L.; Li, Y.; Zhang, L.; Cai, Y.; Norberg, H.V.; Zhang, T.; Furuya, T.; et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 2011, 147, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hong, L.; Xu, J.; Zhong, G.; Gu, Q.; Gu, Q.; Guan, Y.; Zheng, X.; Dai, Q.; Luo, X.; et al. Discovery of a small molecule targeting autophagy via ATG4B inhibition and cell death of colorectal cancer cells in vitro and in vivo. Autophagy 2019, 15, 295–311. [Google Scholar] [CrossRef]
- Choi, Y.K.; Cho, S.G.; Choi, Y.J.; Yun, Y.J.; Lee, K.M.; Lee, K.; Yoo, H.H.; Shin, Y.C.; Ko, S.G. SH003 suppresses breast cancer growth by accumulating p62 in autolysosomes. Oncotarget 2017, 8, 88386–88400. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Chen, H.; Callow, D.; Li, S.; Wang, L.; Li, S.; Chen, L.; Ding, J.; Gao, W.; Xu, H.; et al. Multifaceted effects of astragaloside IV on promotion of random pattern skin flap survival in rats. Am. J. Transl. Res. 2017, 9, 4161–4172. [Google Scholar]

| Categories | Bioactive Chemical Constituents |
|---|---|
| Triterpene saponins [13,14,20,21] | Astragalosides Acetylastragaloside Isoastragaloside Astramembrannin Cycloastragenol Cyclosieversigenis Soyasaponin Soyasapogenol Lupeol |
| Flavonoids [13,15,16,22,23] | Isoflavonones Isoflavans Pterocarpans Flavonones Chalcones |
| Polysaccharides [17,24,25,26] | Glucans Heteropolysaccharide |
Other components [18,27,28] | Phytosterols Volatile oil Fatty acids Amino acids Trace elements |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, H.; Zheng, X.; Li, M. The Effects of Astragalus membranaceus Active Extracts on Autophagy-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1904. https://doi.org/10.3390/ijms20081904
Shan H, Zheng X, Li M. The Effects of Astragalus membranaceus Active Extracts on Autophagy-Related Diseases. International Journal of Molecular Sciences. 2019; 20(8):1904. https://doi.org/10.3390/ijms20081904
Chicago/Turabian StyleShan, Hao, Xueping Zheng, and Min Li. 2019. "The Effects of Astragalus membranaceus Active Extracts on Autophagy-Related Diseases" International Journal of Molecular Sciences 20, no. 8: 1904. https://doi.org/10.3390/ijms20081904
APA StyleShan, H., Zheng, X., & Li, M. (2019). The Effects of Astragalus membranaceus Active Extracts on Autophagy-Related Diseases. International Journal of Molecular Sciences, 20(8), 1904. https://doi.org/10.3390/ijms20081904