Green Ferrate(VI) for Multiple Treatments of Fracturing Wastewater: Demulsification, Visbreaking, and Chemical Oxygen Demand Removal
Abstract
1. Introduction
2. Results
2.1. Analysis of Fracturing Wastewater
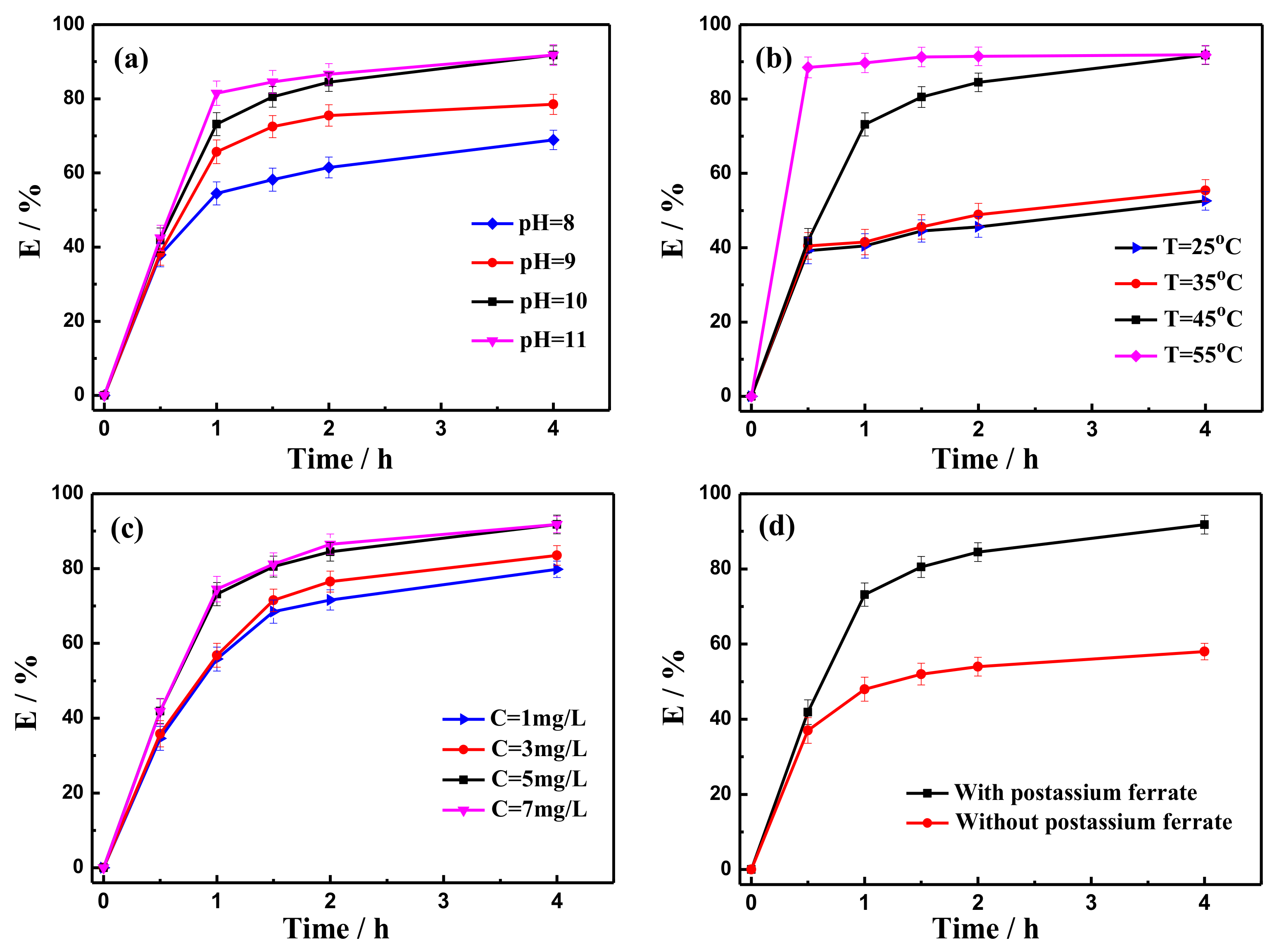
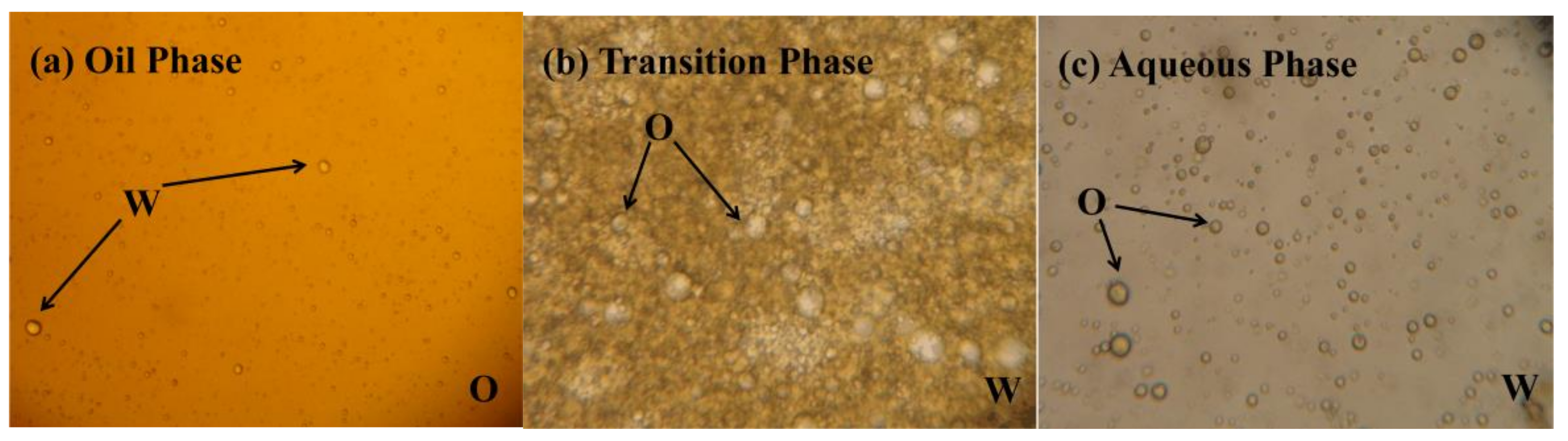
2.2. Demulsification Efficiency by Potassium Ferrate Oxidation
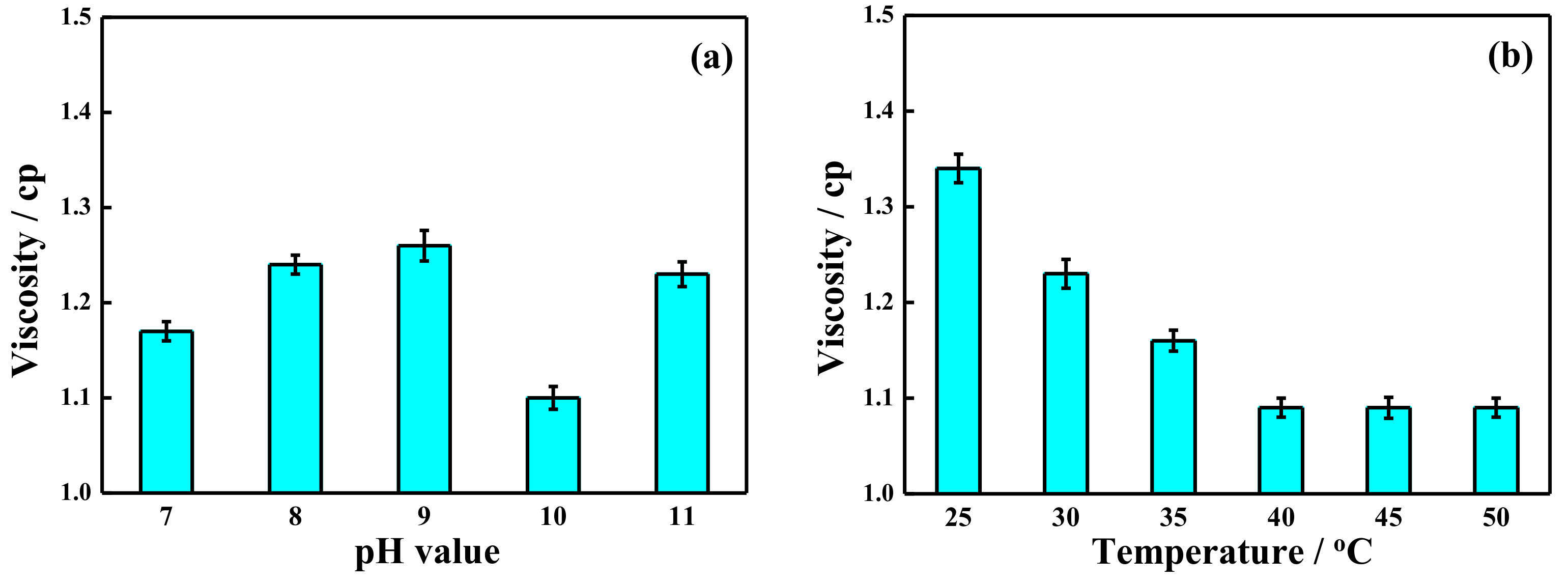
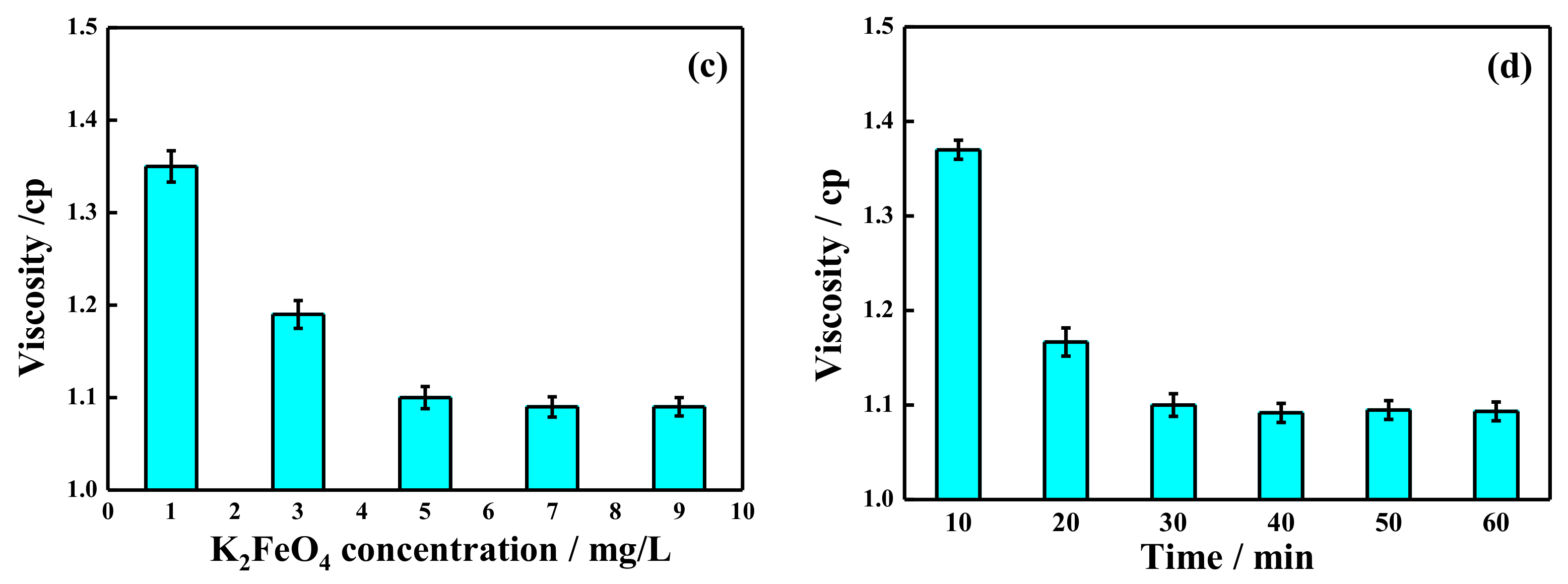
2.3. Visbreaking by Potassium Ferrate Oxidation after Demulsification
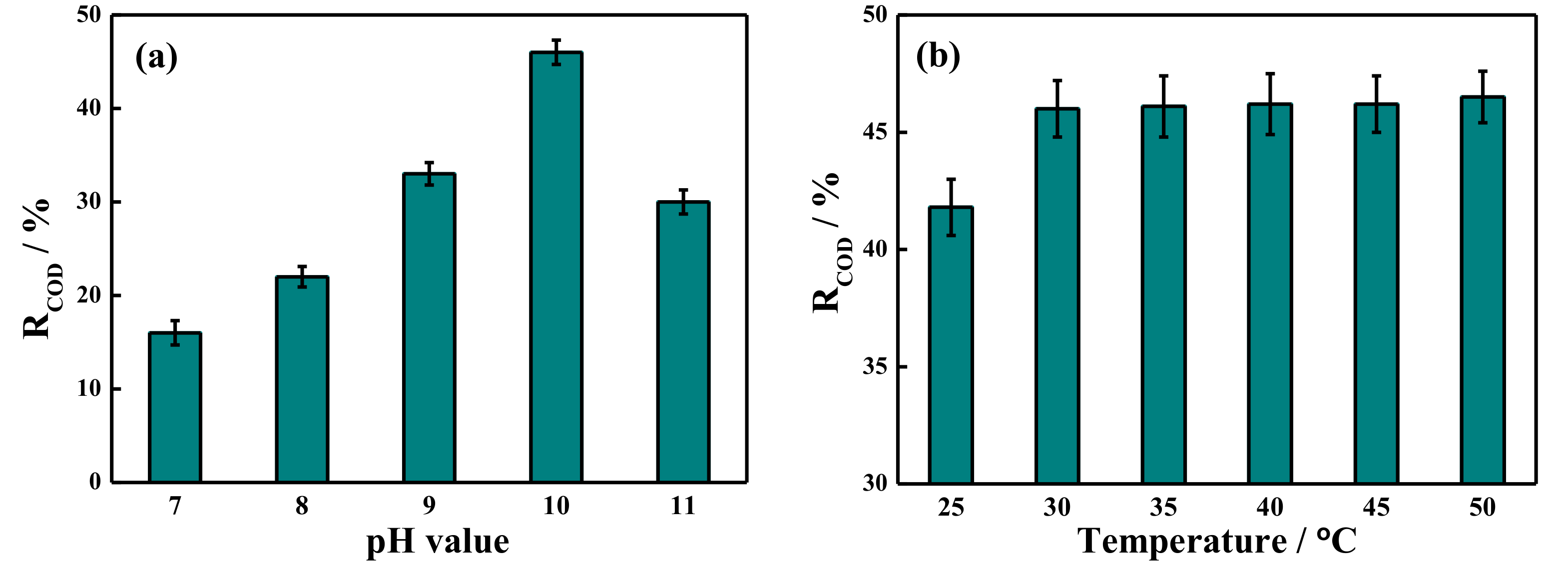
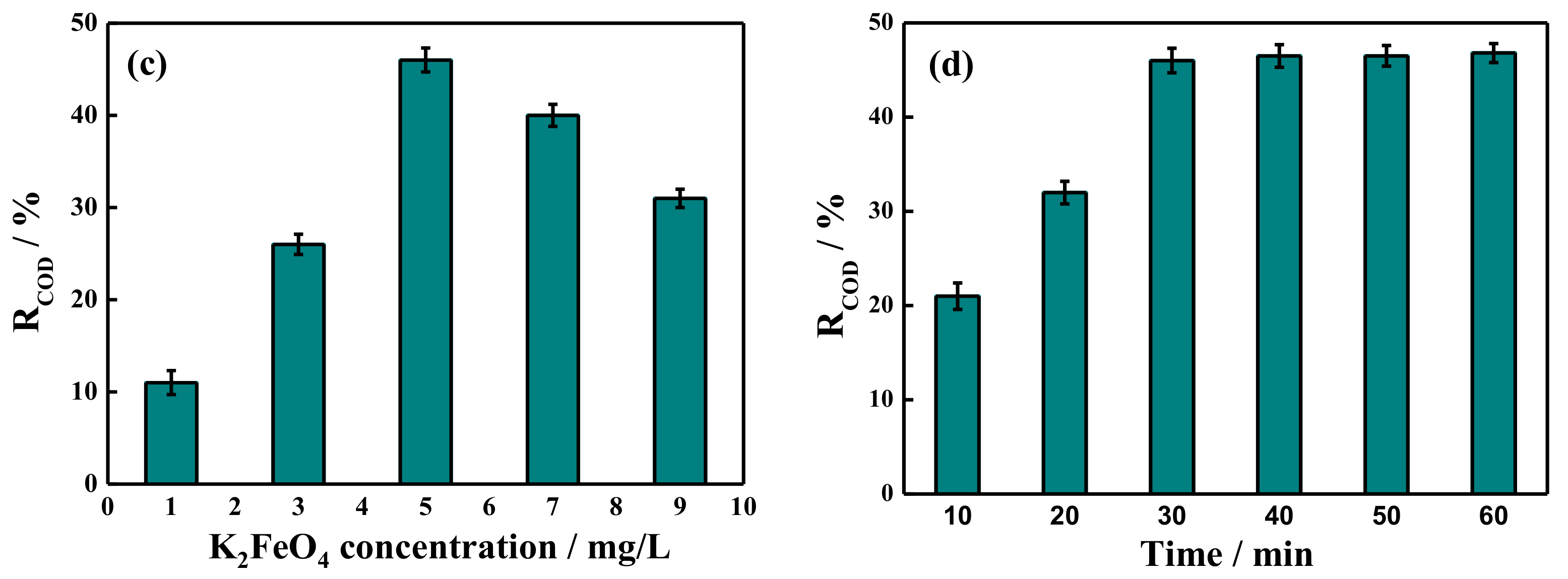
2.4. COD Removal Rate by Potassium Ferrate Oxidation after Demulsification
3. Discussion
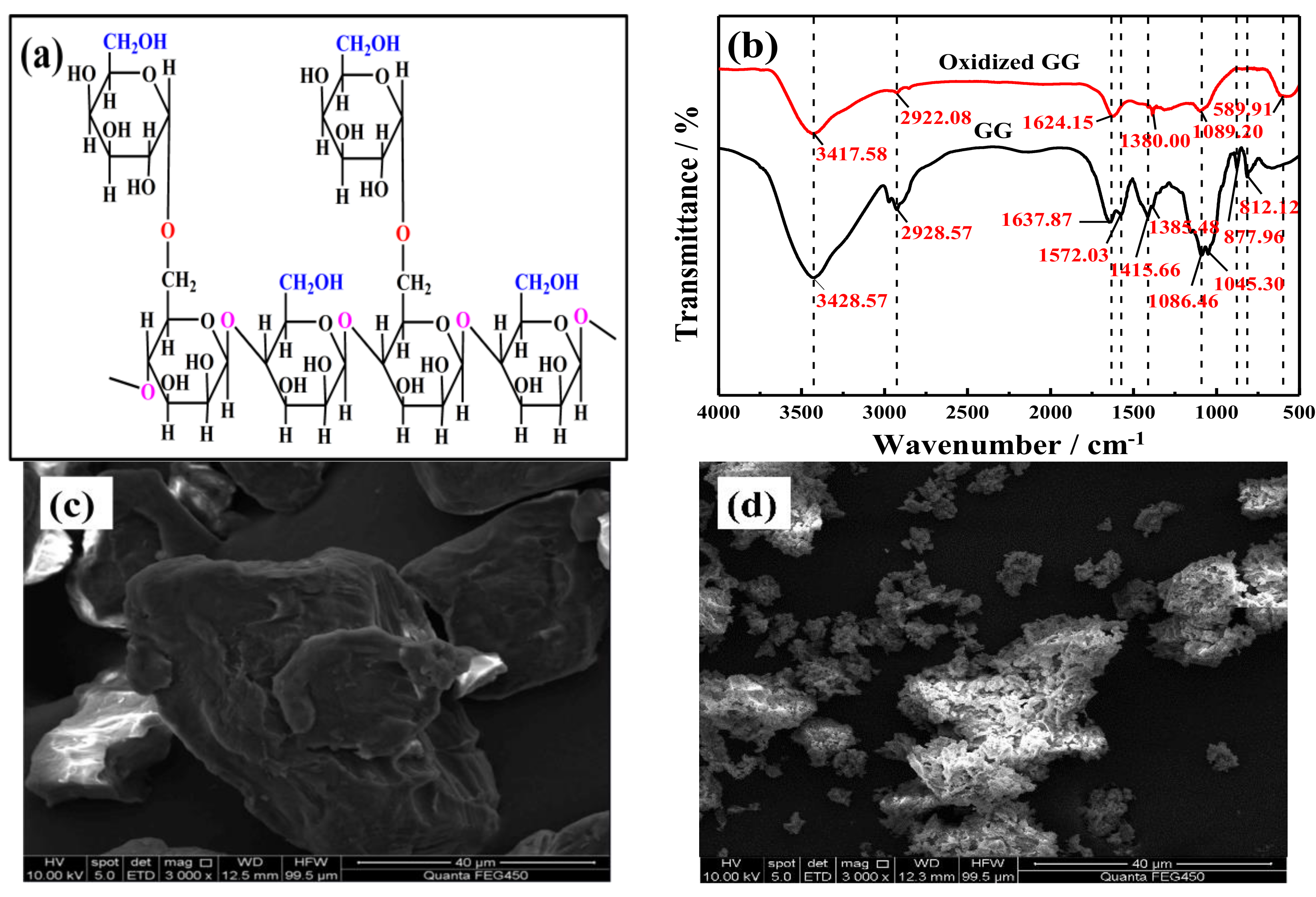
3.1. Characterization of GG and Oxidized GG
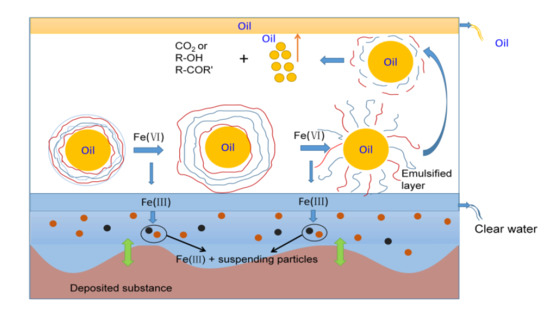
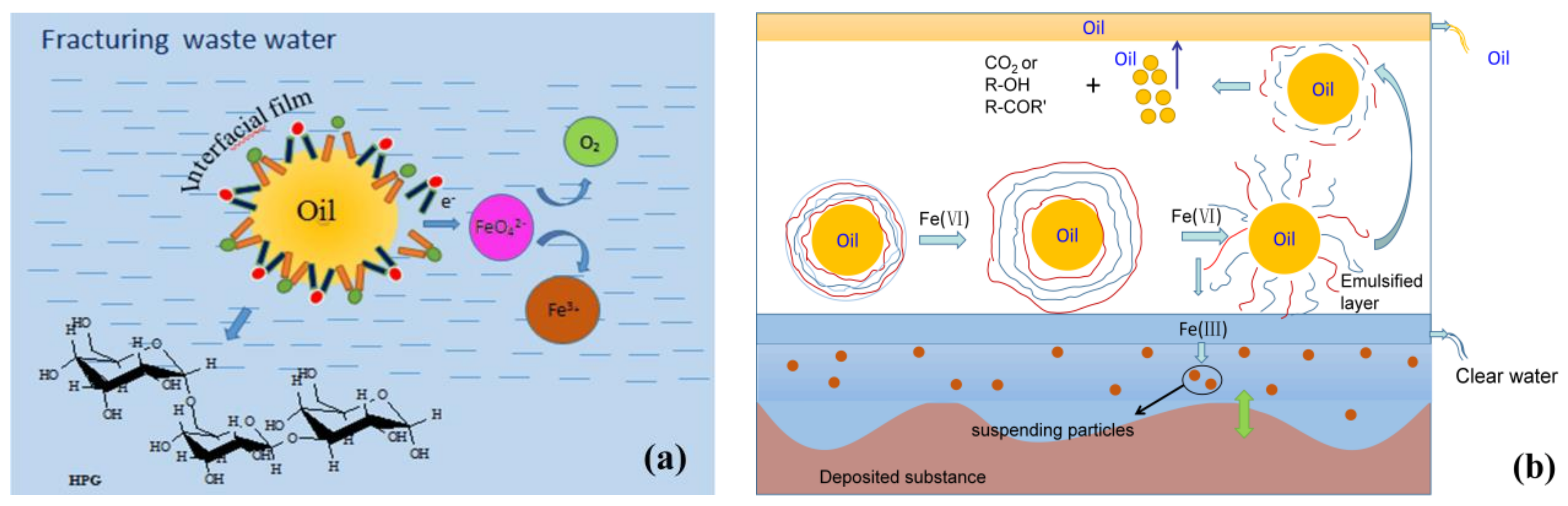
3.2. Possible Oxidation Mechanism for the Demulsification, Visbreaking and COD Removal
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Sampling of the Fracturing Wastewater
4.3. Experimental Methods
4.3.1. The Demulsification Test
4.3.2. The Visbreaking Test
4.3.3. The Measurement of COD
4.4. Characterization
5. Conclusions
- (1)
- Ferrate was highly efficient at demulsification. At 45 °C, a potassium ferrate concentration of 5 mg/L, a pH of 10, and a demulsification time of 4 h, the demulsification efficiency was 91.8%, and the COD decreased by 52.3% (from 5190 mg/L to 2476 mg/L), the viscosity decreased from 1.45 cp to 1.38 cp, and the content of the suspended substances declined from 93 mg/L to 47 mg/L.
- (2)
- Ferrate oxidation was used for visbreaking and COD removal of fracturing wastewater after demulsification. The optimal conditions for treatment were determined to be 40 °C, a pH of 10, a potassium ferrate concentration of 5 mg/L, and a time of 30 min. The viscosity was reduced from 1.38 cp to 1.10 cp, the COD removal rate increased 46% (from 2476 mg/L to 1337 mg/L), and the quality of wastewater after treatment met the standard for produced water reinjection.
- (3)
- According to the FT-IR and SEM analyses, a possible mechanism was introduced. Through the demulsification and the strong oxidation of ferrate, polymer chains in the oil-water interface films were broken, and the generated organic compounds were further degraded in the emulsified solution, which reduced the viscosity and COD value of the fracturing wastewater.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warner, N.R.; Darrah, T.H.; Jackson, R.B.; Millot, R.; Kloppmann, W.; Vengosh, A. New tracers identify hydraulic fracturing fluids and accidental releases from oil and gas operations. Environ. Sci. Technol. 2014, 48, 12552–12560. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, L.; Keer, L.M. Pore pressure cohesive zone modeling of hydraulic fracture in quasi-brittle rocks. Mech. Mater. 2015, 83, 17–29. [Google Scholar] [CrossRef]
- Birdsell, D.T.; Rajaram, H.; Dempsey, D.; Viswanathan, H.S. Hydraulic fracturing fluid migration in the subsurface: A review and expanded modeling results. Water Resour. Res. 2015, 51, 7159–7188. [Google Scholar] [CrossRef]
- Getzinger, G.J.; O’Connor, M.P.; Hoelzer, K.; Drollette, B.D.; Karatum, O.; Deshusses, M.A.; Ferguson, P.L.; Elsner, M.; Plata, D.L. Natural gas residual fluids: Sources, endpoints, and organic chemical composition after centralized waste treatment in pennsylvania. Environ. Sci. Technol. 2015, 49, 8347–8355. [Google Scholar] [CrossRef]
- Weaver, J.D.; Liang, F.; Schultheiss, N.C. Assessment of fracturing-fluid cleanup by use of a rapid-gel-damage method. SPE Prod. Oper. 2015, 30, 69–75. [Google Scholar] [CrossRef]
- Drollette, B.D.; Hoelzer, K.; Warner, N.R.; Darrah, T.H.; Karatum, O.; O’Connor, M.P.; Nelson, R.K.; Fernandez, L.A.; Reddy, C.M.; Vengosh, A. Elevated levels of diesel range organic compounds in groundwater near Marcellus gas operations are derived from surface activities. Proc. Natl. Acad. Sci. USA 2015, 112, 13184–13189. [Google Scholar] [CrossRef]
- Adgate, J.L.; Goldstein, B.D.; McKenzie, L.M. Potential public health hazards, exposures and health effects from unconventional natural gas development. Environ. Sci. Technol. 2014, 48, 8307–8320. [Google Scholar] [CrossRef]
- Estrada, J.M.; Rao, B. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; Thomas, H.Y. Evolution of water chemistry during marcellus shale gas development: A case study in West Virginia. Chemosphere 2015, 134, 224–231. [Google Scholar] [CrossRef]
- Hao, H.; Xing, H.; Gao, C.; Gao, X. Application of an integrated system of coagulation and electrodialysis for treatment of wastewater produced by fracturing. Desalin. Water Treat. 2014, 55, 2034–2043. [Google Scholar] [CrossRef]
- Rosenblum, J.S.; Sitterley, K.A.; Thurman, E.M.; Ferrer, I.; Linden, K.G. Hydraulic fracturing wastewater treatment by coagulation-adsorption for removal of organic compounds and turbidity. J. Environ. Chem. Eng. 2016, 4, 1978–1984. [Google Scholar] [CrossRef]
- Coday, B.D.; Almaraz, N.; Cath, T.Y. Forward osmosis desalination of oil and gas wastewater: Impacts of membrane selection and operating conditions on process performance. J. Membr. Sci. 2015, 488, 40–55. [Google Scholar] [CrossRef]
- Sari, M.A.; Chellam, S. Mechanisms of boron removal from hydraulic fracturing wastewater by aluminum electrocoagulation. J. Colloid Interface Sci. 2015, 458, 103–111. [Google Scholar] [CrossRef]
- Gregory, K.; Mohan, A.M. Current perspective on produced water management challenges during hydraulic fracturing for oil and gas recovery. Environ. Chem. 2015, 12, 261–266. [Google Scholar] [CrossRef]
- Huerta, N.J.; Hesse, M.A.; Bryant, S.L.; Strazisar, B.R.; Lopano, C. Reactive transport of CO2-saturated water in a cement fracture: Application to wellbore leakage during geologic CO2 storage. Int. J. Greenh. Gas Control 2016, 44, 276–289. [Google Scholar] [CrossRef]
- Lester, Y.; Ferrer, I.; Thurman, E.M.; Sitterley, K.A.; Korak, J.A.; Aiken, G.; Linden, K.G. Characterization of hydraulic fracturing flowback water in Colorado: Implications for water treatment. Phys. Procedia 2015, 512–513, 637–644. [Google Scholar] [CrossRef]
- Mancebo, U.; Hettiaratchi, P.; Jayasinghe, P.; Rao, S. Determination of water retention capacity of granular media of methane biofilters: A simplified approach. Environ. Earth Sci. 2016, 75, 74. [Google Scholar] [CrossRef]
- Sun, M.; Lowry, G.V.; Gregory, K.B. Selective oxidation of bromide in wastewater brines from hydraulic fracturing. Water Res. 2013, 47, 3723–3731. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Stanford, C.; Petri, M. Practical application of ferrate(VI) for water and wastewater treatment—Site study’s approach. Water-Energy Nexus 2018, 1, 42–46. [Google Scholar] [CrossRef]
- Licht, S.; Wang, B.; Ghosh, S. Energetic iron(VI) chemistry: The super-iron battery. Science 1999, 285, 1039–1042. [Google Scholar] [CrossRef]
- Licht, S.; Yang, L.; Wang, B. Synthesis and analysis of AgFeO Fe(VI) ferrate super-iron cathodes. Electrochem. Commun. 2005, 7, 931–936. [Google Scholar] [CrossRef]
- Tang, G.; Pei, L.; Yang, X.; Yun, C. Advanced treatment of wastewater generated by traditional chinese medicine by coagulating sedimentation after oxidation with potassium ferrate. J. Chin. Inst. Eng. 2014, 37, 784–792. [Google Scholar] [CrossRef]
- Hua, S.; Wang, B. Green synthetic oxidant ferrate. Chemistry 2003, 66, 252–257. [Google Scholar]
- Jiang, J.Q.; Lloyd, B. Progress in the development and use of ferrate(VI) salt as an oxidant and coagulant for water and wastewater treatment. Water Res. 2002, 36, 1397–1408. [Google Scholar] [CrossRef]
- Manoli, K.; Morrison, L.M.; Sumarah, M.W.; Nakhla, G.; Ray, A.K.; Sharma, V.K. Pharmaceuticals and pesticides in secondary effluent wastewater: Identification and enhanced removal by acid-activated ferrate(VI). Water Res. 2019, 148, 272–280. [Google Scholar] [CrossRef]
- Feng, M.; Jinadatha, C.; McDonald, T.J.; Sharma, V.K. Accelerated oxidation of organic contaminants by ferrate(VI): The overlooked role of reducing additives. Environ. Sci. Technol. 2018, 52, 11319–11327. [Google Scholar] [CrossRef]
- Kolařík, J.; Prucek, R.; Tuček, J.; Filip, J.; Sharma, V.K.; Zbořil, R. Impact of inorganic ions and natural organic matter on arsenates removal by ferrate(VI): Understanding a complex effect of phosphates ions. Water Res. 2018, 141, 357–365. [Google Scholar] [CrossRef]
- Feng, M.; Sharma, V.K. Enhanced oxidation of antibiotics by ferrate(VI)-sulfur(IV) system: Elucidating multi-oxidant mechanism. Chem. Eng. J. 2018, 341, 137–145. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G.G.; Zhang, L.J.; Zhou, L.J.; Liu, S.; Fang, Y.X. Kinetics modeling and reaction mechanism of ferrate(VI) oxidation of benzotriazoles. Water Res. 2011, 45, 2261–2269. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Manoli, K.; Nakhla, G.; Ray, A.K.; Sharma, V.K. Enhanced Oxidative transformation of organic contaminants by activation of ferrate(VI): Possible involvement of Fe(V)/Fe(IV) species. Chem. Eng. J. 2017, 307, 513–517. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Kailasa, S.K.; Kwon, E.E.; Tsang, Y.F.; Ok, Y.S.; Kim, K.H. A critical review of ferrate(VI)-based remediation of soil and groundwater. Environ. Res. 2018, 160, 420–448. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Goodwill, J.E.; Tobiason, J.E.; Reckhow, D.A. Impacts of ferrate oxidation on natural organic matter and disinfection byproduct precursors. Water Res. 2016, 96, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Jia-Qian, J.; Alex, P.; Mike, B.; Pete, P. The application of potassium ferrate for sewage treatment. J. Environ. Manag. 2006, 79, 215–220. [Google Scholar]
- Graham, N.J.D.; Khoi, T.T.; Jiang, J.-Q. Oxidation and coagulation of humic substances by potassium ferrate. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2010, 62, 929–936. [Google Scholar] [CrossRef]
- Elliott, E.G.; Ettinger, A.S.; Leaderer, B.P.; Bracken, M.B.; Deziel, N.C. A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity. J. Expo. Sci. Environ. Epidemiol. 2016, 27, 90–99. [Google Scholar] [CrossRef]
- Sharma, V.K. Disinfection performance of Fe(VI) in water and wastewater: A review. Water Sci. Technol. 2007, 55, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Yunho, L.; Saskia Gisela, Z.; Anh Trung, K.; Urs, V.G. Ferrate (Fe(VI)) application for Municipal wastewater treatment: A novel process for simultaneous micropollutant oxidation and phosphate removal. Environ. Sci. Technol. 2009, 43, 3831–3838. [Google Scholar]
- Yanjun, J.; Goodwill, J.E.; Tobiason, J.E.; Reckhow, D.A. Effect of different solutes, natural organic matter, and particulate Fe(III) on ferrate(VI) decomposition in aqueous solutions. Environ. Sci. Technol. 2015, 49, 2841–2848. [Google Scholar]
- Ma, J.; Liu, W. Effectiveness of ferrate(VI) preoxidation in enhancing the coagulation of surface waters. Water Res. 2002, 36, 4959–4962. [Google Scholar] [CrossRef]
- Sharma, V.K. Potassium ferrate(VI): An environmentally friendly oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
| Time (h) | 0 | 0.5 | 1 | 1.5 | 2 | 4 |
| Suspended substance (mg/L) | 93 | 69.4 | 58.6 | 48.0 | 50.0 | 47.0 |
| Characteristic Group | Wave Number (GG) | Wave Number (Oxidized GG) |
|---|---|---|
| O-H stretching vibration | 3428.57 | 3417.58 |
| C-H stretching of the CH2 group | 2928.57 | – |
| Ring stretching | 1637.87 | 1624.15 |
| C=O stretching vibration of COO− group | 1572.03/1415.66 | – |
| Symmetrical deformations of the CH2 group | 1385.48/1341.59 | 1380.00/1336.59 |
| CH2OH primary alcoholic stretching mode | 1086.46 | 1089.20 |
| CH2 twisting vibration | 1045.30 | – |
| Galactose and mannose | 877.96 | – |
| The (1–4) and (1–6) linkages of galactose and mannose | 960.26/812.12 | – |
| Crystallinity of polymer | 500~700 | 500~700 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Li, J.; Ge, Q.; Wang, Y.; Chen, Y.; Wang, B. Green Ferrate(VI) for Multiple Treatments of Fracturing Wastewater: Demulsification, Visbreaking, and Chemical Oxygen Demand Removal. Int. J. Mol. Sci. 2019, 20, 1857. https://doi.org/10.3390/ijms20081857
Han H, Li J, Ge Q, Wang Y, Chen Y, Wang B. Green Ferrate(VI) for Multiple Treatments of Fracturing Wastewater: Demulsification, Visbreaking, and Chemical Oxygen Demand Removal. International Journal of Molecular Sciences. 2019; 20(8):1857. https://doi.org/10.3390/ijms20081857
Chicago/Turabian StyleHan, Hongjing, Jinxin Li, Qin Ge, Yizhen Wang, Yanguang Chen, and Baohui Wang. 2019. "Green Ferrate(VI) for Multiple Treatments of Fracturing Wastewater: Demulsification, Visbreaking, and Chemical Oxygen Demand Removal" International Journal of Molecular Sciences 20, no. 8: 1857. https://doi.org/10.3390/ijms20081857
APA StyleHan, H., Li, J., Ge, Q., Wang, Y., Chen, Y., & Wang, B. (2019). Green Ferrate(VI) for Multiple Treatments of Fracturing Wastewater: Demulsification, Visbreaking, and Chemical Oxygen Demand Removal. International Journal of Molecular Sciences, 20(8), 1857. https://doi.org/10.3390/ijms20081857