Evaluation of Milk Colostrum Derived Lactoferrin of Sahiwal (Bos indicus) and Karan Fries (Cross-Bred) Cows for Its Anti-Cancerous Potential
Abstract
1. Introduction
2. Results
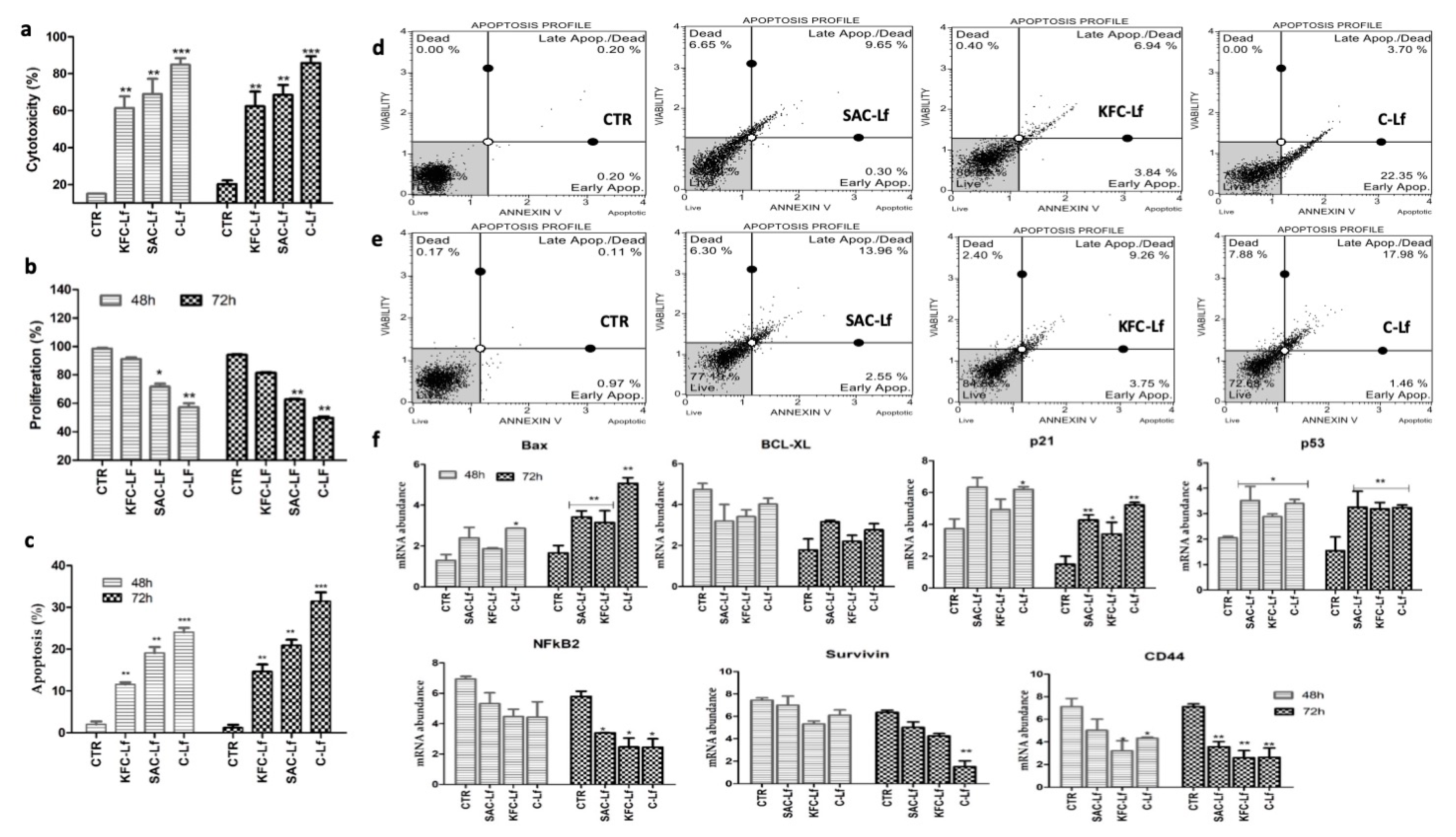
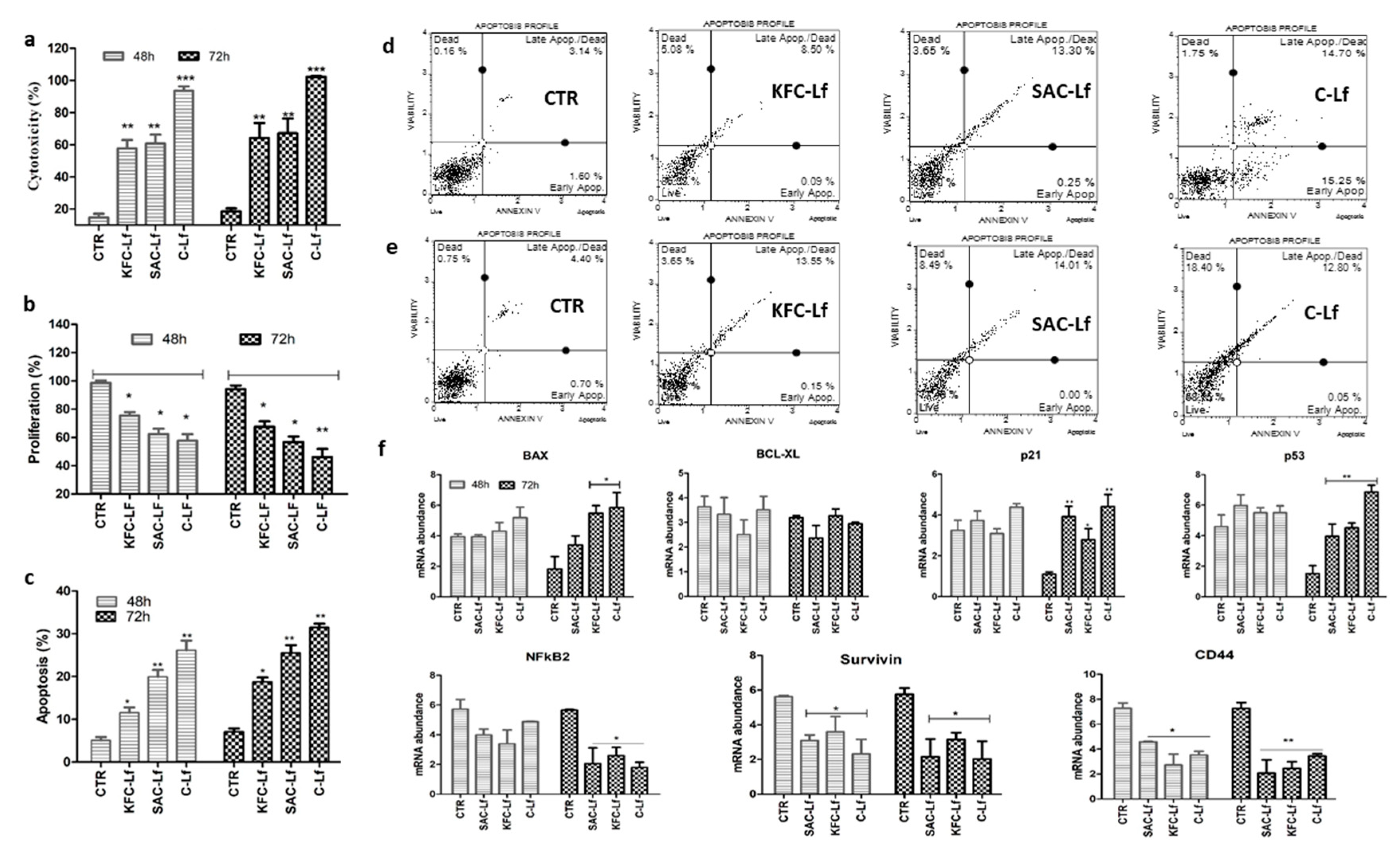
2.1. Cytotoxicity Induced by Lf from Different Sources
2.2. Reduction in Cell Proliferation Treated with Lf from Different Sources
2.3. Apoptosis Induced by Lf from Different Sources
2.4. Changes at Transcriptional Level
3. Discussion
4. Materials and Methods
4.1. Lf Purification
4.2. In-Vitro Treatment of Purified Lf on Cancerous Cells
4.3. Cytotoxicity Assay
4.4. Cell Proliferation Assay
4.5. Cell Viability and Apoptosis Assay
4.6. Gene Expression Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Lf | Lactoferrin |
| SAC | Sahiwal cattle |
| KFC | Karan Fries cattle |
| C-Lf | Commercial Lactoferrin |
| HPLC | High Performance Liquid Chromatography |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| ACTB | ß-actin |
| RS18 | Ribosomal Protein S18 |
References
- Yang, T.X.; Li, H.; Wang, F.; Liu, X.L.; Li, Q.Y. Effect of cattle breeds on milk composition and technological characteristics in china. Asian-Austr. J. Anim. Sci. 2013, 26, 896. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.A.; Gustavsson, F.; Glantz, M.; Paulsson, M.; Larsen, L.B.; Larsen, M.K. The influence of feed and herd on fatty acid composition in 3 dairy breeds [Danish Holstein, Danish Jersey, and Swedish Red]. J. Dairy Sci. 2012, 95, 6362–6371. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.; Nagaraj, S.H.; Reverter, A. The evolution of tropical adaptation: Comparing taurine and zebu cattle. Anim. Genet. 2010, 41, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Pramod, R.K.; Velayutham, D.; Zachariah, A.; Zachariah, A.; Santhosh, S.; Iype, S.; Dhinoth Kumar, B.; Gupta, R.; Thomas, G. The complete mitochondrial genome of Indian cattle [Bos indicus]. Mitochondrial DNA Part B 2018, 3, 207–208. [Google Scholar] [CrossRef]
- Porto-Neto, L.R.; Sonstegard, T.S.; Liu, G.E.; Bickhart, D.M.; Da Silva, M.V.; Machado, M.A.; Utsunomiya, Y.T.; Garcia, J.F.; Gondro, C.; Van Tassell, C.P. Genomic divergence of zebu and taurine cattle identified through high-density SNP genotyping. BMC Genom. 2013, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.G.; Loftus, R.T.; Cunningham, P.; MacHugh, D.E. Genetics and domestic cattle origins. Evol. Anthropol. Issues News Rev. Issues News Rev. 1998, 6, 79–86. [Google Scholar] [CrossRef]
- Sodhi, M.; Mukesh, M.; Kataria, R.S.; Mishra, B.P.; Joshii, B.K. Milk proteins and human health: A1/A2 milk hypothesis. Indian J. Endocrinol. Metab. 2012, 16, 856. [Google Scholar] [CrossRef]
- Mishra, B.P.; Mukesh, M.; Prakash, B.; Sodhi, M.; Kapila, R.; Kishore, A.; Kataria, R.R.; Joshi, B.K.; Bhasin, V.; Rasool, T.J.; et al. Status of milk protein, b-casein variants among Indian milch animals. Indian J. Anim. Sci. 2009, 79, 722–725. [Google Scholar]
- Le Parc, A.; Dallas, D.C.; Duaut, S.; Leonil, J.; Martin, P.; Barile, D. Characterization of goat milk lactoferrin n-glycans and comparison with the N-glycomes of human and bovine milk. Electrophoresis 2014, 35, 1560–1570. [Google Scholar] [CrossRef]
- EL fakharany, E.M.; Tabll, A.; ABD, E.W.A.; Haroun, B.M.; Redwan, E.R.M. Potential activity of camel milk-amylase and lactoferrin against hepatitis C virus infectivity in HepG2 and lymphocytes. Hepat. Mon. 2008, 8, 101–109. [Google Scholar]
- Mohamad, R.H.; Zekry, Z.K.; Al-Mehdar, H.A.; Salama, O.; El-Shaieb, S.E.; El-Basmy, A.A.; Al-said, M.G.A.M.; Sharawy, S.M. Camel milk as an adjuvant therapy for the treatment of type 1 diabetes: Verification of a traditional ethnomedical practice. J. Med. Food 2009, 12, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Mahidhara, G.; Roy, K.; Sasidharan, S.; Krishnakumar, S.; Prasad, N.; Sehgal, R.; Kanwar, R.K. Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine 2015, 10, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Tenore, G.C.; Mastrocinque, R.; Stusio, P.; Campiglia, P. Potential anticarcinogenic peptides from bovine milk. J. Amino Acids 2013, 2013, e939804. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.R.; Chen, P.W.; Chen, Y.L.; Hsu, H.C.; Lin, C.C.; Chen, W.J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013, 96, 7511–7520. [Google Scholar] [CrossRef]
- Małaczewska, J.; Rotkiewicz, Z. Laktoferyna–białko, multipotencjalne. Medycyna Wet. 2007, 63, 136. [Google Scholar]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agent. 2009, 33, 301. [Google Scholar] [CrossRef]
- Duarte, D.C.; Nicolau, A.; Teixeira, J.A.; Rodrigues, L.R. The effect of bovine milk lactoferrin on human breast cancer cell lines. J. Dairy Sci. 2011, 94, 66–76. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, J.R.; Kanwar, R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 2015, 15, 425. [Google Scholar] [CrossRef]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef]
- Adlerova, L.; Bartoskova, A.; Faldyna, M. Lactoferrin: A review. Lactoferrin A Rev. Veterinarni Med. 2008, 53, 457–468. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, H.L.; Yen, C.C.; Lee, P.Y.; Tsai, H.C.; Lin, M.F.; Chen, C.M. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Regulation of iron acquisition and iron distribution in mammals. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Farziyan, M.A.; Moradian, F.; Rafiei, A.R. Anticancer effect of bovine lactoferrin on human esophagus cancer cell line. Res. Mol. Med. 2016, 4, 18–23. [Google Scholar]
- Velliyagounder, K.; Kaplan, J.B.; Furgang, D.; Legarda, D.; Diamond, G.; Parkin, R.E.; Fine, D.H. One of two human lactoferrin variants exhibits increased antibacterial and transcriptional activation activities and is associated with localized juvenile periodontitis. Infect. Immun. 2003, 71, 6141–6147. [Google Scholar] [CrossRef]
- Daly, M.; Ross, P.; Giblin, L.; Buckley, F. Polymorphisms within the lactoferrin gene promoter in various cattle breeds. Anim. Biotechnol. 2006, 17, 33–42. [Google Scholar] [CrossRef]
- Shashidharan, A.; Singh, R.; Bhasker, S.; Mohankumar, C. Physicochemical characterization and functional site analysis of lactoferrin gene of Vechur cow. Bioinformation 2011, 6, 275. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Swami, S.K.; Kumar, M.; Sodhi, M.; Kataria, R.S.; Jain, P.; Bhatia, A.K.; Mohanty, A.K.; Niranjan, S.K.; Shandilya, U.K.; et al. Analysis of sequence variability and expression pattern of lactoferrin gene in Sahiwal cows (Bos indicus) and Murrah buffaloes (Bubalus bubalis). J. Livest. Biodivers. 2017, 7, 36–45. [Google Scholar]
- O’Halloran, F.; Berry, D.P.; Bahar, B.; Howard, D.J.; Sweeney, T.; Giblin, L. Polymorphisms in the bovine lactoferrin promoter are associated with reproductive performance and somatic cell count. J. Dairy Sci. 2010, 93, 1253–1259. [Google Scholar] [CrossRef]
- Chopra, A.; Gupta, I.D.; Verma, A.; Soumya, N.P.; Vohra, V. Identification of Lactoferrin gene Polymorphism and its association with Mastitis incidence. J. Anim. Res. 2013, 3, 103. [Google Scholar]
- Chopra, A.; Gupta, I.D.; Verma, A.; Chakravarty, A.K.; Vohra, V. Lactoferrin gene promoter variants and their association with clinical and subclinical mastitis in indigenous and crossbred cattle. Polish J. Vet. Sci. 2015, 18, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Traina, G.; Tomasello, G.; Casagrande-Proietti, P.; Leonardi, L.; Barbato, O.; Brecchia, G. Potential benefits of colostrum in gastrointestinal diseases. Front. Biosci. 2016, 8, 331–351. [Google Scholar]
- Otto, W.; Najnigier, B.; Stelmasiak, T.; Robins-Browne, R.M. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand. J. Gastroenterol. 2011, 46, 862–868. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; Mckechnie, S.; Donkor, O.N. Identification of anticancer peptides from bovine milk proteins and their potential roles in management of cancer: A critical review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 123–138. [Google Scholar] [CrossRef]
- Frislev, H.S.; Jessen, C.M.; Oliveira, C.L.; Pedersen, J.S.; Otzen, D.E. Liprotides made of α-lactalbumin and cis fatty acids form core–shell and multi-layer structures with a common membrane-targeting mechanism. Biochim. Et Biophys. Acta [Bba]-Proteins Proteom. 2016, 1864, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Abeykoon, C.D.; Rathnayake, R.M.C.; Johansson, M.; Silva, G.L.L.P.; Ranadheera, C.S.; Lundh, Å.; Vidanarachchi, J.K. Milk coagulation properties and milk protein genetic variants of three cattle breeds/types in Sri Lanka. Procedia Food Sci. 2016, 6, 348–351. [Google Scholar] [CrossRef]
- Litwińczuk, Z.; Barłowska, J.; Chabuz, W.; Brodziak, A. Nutritional value and technological suitability of milk from cows of three Polish breeds included in the genetic resources conservation programme. Ann. Anim. Sci. 2012, 12, 423–432. [Google Scholar] [CrossRef]
- Zhang, Y.; Nicolau, A.; Lima, C.F.; Rodrigues, L.R. Bovine lactoferrin induces cell cycle arrest and inhibits mTOR signaling in breast cancer cells. Nutr. Cancer 2014, 66, 1371–1385. [Google Scholar] [CrossRef]
- Ebrahim, F.; Shankaranarayanan, J.S.; Kanwar, J.R.; Gurudevan, S.; Krishnan, U.M.; Kanwar, R.K. Identification of unprecedented anticancer properties of high molecular weight biomacromolecular complex containing bovine lactoferrin [HMW-bLf]. PLoS ONE 2014, 9, e106568. [Google Scholar] [CrossRef]
- Anand, N.; Kanwar, R.K.; Mohan Lal Dubey, R.K.; Sehgal, R.; Verma, A.K.; Kanwar, J.R. Effect of lactoferrin protein on red blood cells and macrophages: Mechanism of parasite–host interaction. Drug Des. Dev. 2015, 9, 3821. [Google Scholar]
- Fujita, K.I.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin enhances Fas expression and apoptosis in the colon mucosa of azoxymethane-treated rats. Carcinogenesis 2004, 25, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Sah, N.K.; Khan, Z.; Khan, G.J.; Bisen, P.S. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006, 244, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Targeting survivin in cancer. Cancer Lett. 2013, 332, 225. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Torigoe, T.; Kamiguchi, K.; Hirohashi, Y.; Ohmura, T.; Hirata, K.; Sato, M.; Sato, N. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005, 65, 11018–11025. [Google Scholar] [CrossRef]
- Xie, T.X.; Xia, Z.; Zhang, N.U.; Gong, W.; Huang, S. Constitutive NF-κB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol. Rep. 2010, 23, 725–732. [Google Scholar]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013, 12, 86. [Google Scholar] [CrossRef]
- Sharma, A.; Shandilya, U.K.; Sodhi, M.; Jatav, P.; Mohanty, A.; Jain, P.; Verma, P.; Kataria, R.S.; Kumari, P.; Mukesh, M. Milk-derived mammary epithelial cells as non-invasive source to define stage-specific abundance of milk protein and fat synthesis transcripts in native Sahiwal cows and Murrah buffaloes. 3 Biotech 2019, 9, 106. [Google Scholar] [CrossRef]
- Bathla, S.; Rawat, P.; Baithalu, R.; Yadav, M.L.; Naru, J.; Tiwari, A.; Kumar, S.; Balhara, A.K.; Singh, S.; Chaudhary, S.; et al. Profiling of urinary proteins in Karan Fries cows reveals more than 1550 proteins. J. Proteom. 2015, 127, 193–201. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhao, H.; Ma, T.F.; Ge, F.; Chen, C.S.; Zhang, Y.P. Identification of valid reference genes for the normalization of RT-qPCR expression studies in human breast cancer cell lines treated with and without transient transfection. PLoS ONE 2015, 10, e0117058. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
| Genes | Primer Sequence | Annealing Temperature | Slope | Efficiency | R2 |
|---|---|---|---|---|---|
| RS18 | GGATGTAAAGGATGGAAAATACA TCCAGGTCTTCACGGAGCTTGTT | 60 °C | −3.191 | 104.62 | 0.996 |
| ACTB | GGCGGCACCACCATGTACCCT AGGGGCCGGACTCGTCATACT | 60 °C | −3.085 | 94.85 | 0.989 |
| p21 | TGGAGACTCTCAGGGTCGAAA CGGCGTTTGGAGTGGTAGA | 60 °C | −3.306 | 100.67 | 0.998 |
| p53 | ATCTACTGGGACGGAACAGC GTGAGGCTCCCCTTTCTTG | 60 °C | −3.021 | 111.86 | 0.992 |
| Bax | CCTTTTCTACTTTGCCAGCAAAC GAGGCCGTCCCAACCAC | 60 °C | −3.131 | 105.77 | 0.981 |
| BCL-xl | GATCCCCATGGCAGCAGTAAAGCAAG CCCCATCCCGGAAGAGTTCATTCACT | 60 °C | −3.265 | 102.44 | 0.988 |
| Survivin | AGAACTGGCCCTTCTTGGAGG CTTTTTATGTTCCTCTATGGGGTC | 60 °C | −2.984 | 94.62 | 0.856 |
| NFkβ2 | ATGGAGAGTTGCTACAACCCA CTGTTCCACGATCACCAGGTA | 60 °C | −3.091 | 110.75 | 0.991 |
| CD44 | TGCCGCTTTGCAGGTGTATT CCGATGCTCAGAGCTTTCTCC | 60 °C | −3.28 | 101.78 | 0.989 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Shandilya, U.K.; Sodhi, M.; Mohanty, A.K.; Jain, P.; Mukesh, M. Evaluation of Milk Colostrum Derived Lactoferrin of Sahiwal (Bos indicus) and Karan Fries (Cross-Bred) Cows for Its Anti-Cancerous Potential. Int. J. Mol. Sci. 2019, 20, 6318. https://doi.org/10.3390/ijms20246318
Sharma A, Shandilya UK, Sodhi M, Mohanty AK, Jain P, Mukesh M. Evaluation of Milk Colostrum Derived Lactoferrin of Sahiwal (Bos indicus) and Karan Fries (Cross-Bred) Cows for Its Anti-Cancerous Potential. International Journal of Molecular Sciences. 2019; 20(24):6318. https://doi.org/10.3390/ijms20246318
Chicago/Turabian StyleSharma, Ankita, Umesh K Shandilya, Monika Sodhi, Ashok K Mohanty, Pranay Jain, and Manishi Mukesh. 2019. "Evaluation of Milk Colostrum Derived Lactoferrin of Sahiwal (Bos indicus) and Karan Fries (Cross-Bred) Cows for Its Anti-Cancerous Potential" International Journal of Molecular Sciences 20, no. 24: 6318. https://doi.org/10.3390/ijms20246318
APA StyleSharma, A., Shandilya, U. K., Sodhi, M., Mohanty, A. K., Jain, P., & Mukesh, M. (2019). Evaluation of Milk Colostrum Derived Lactoferrin of Sahiwal (Bos indicus) and Karan Fries (Cross-Bred) Cows for Its Anti-Cancerous Potential. International Journal of Molecular Sciences, 20(24), 6318. https://doi.org/10.3390/ijms20246318





