Correlations of Ion Composition and Power Efficiency in a Reverse Electrodialysis Heat Engine
Abstract
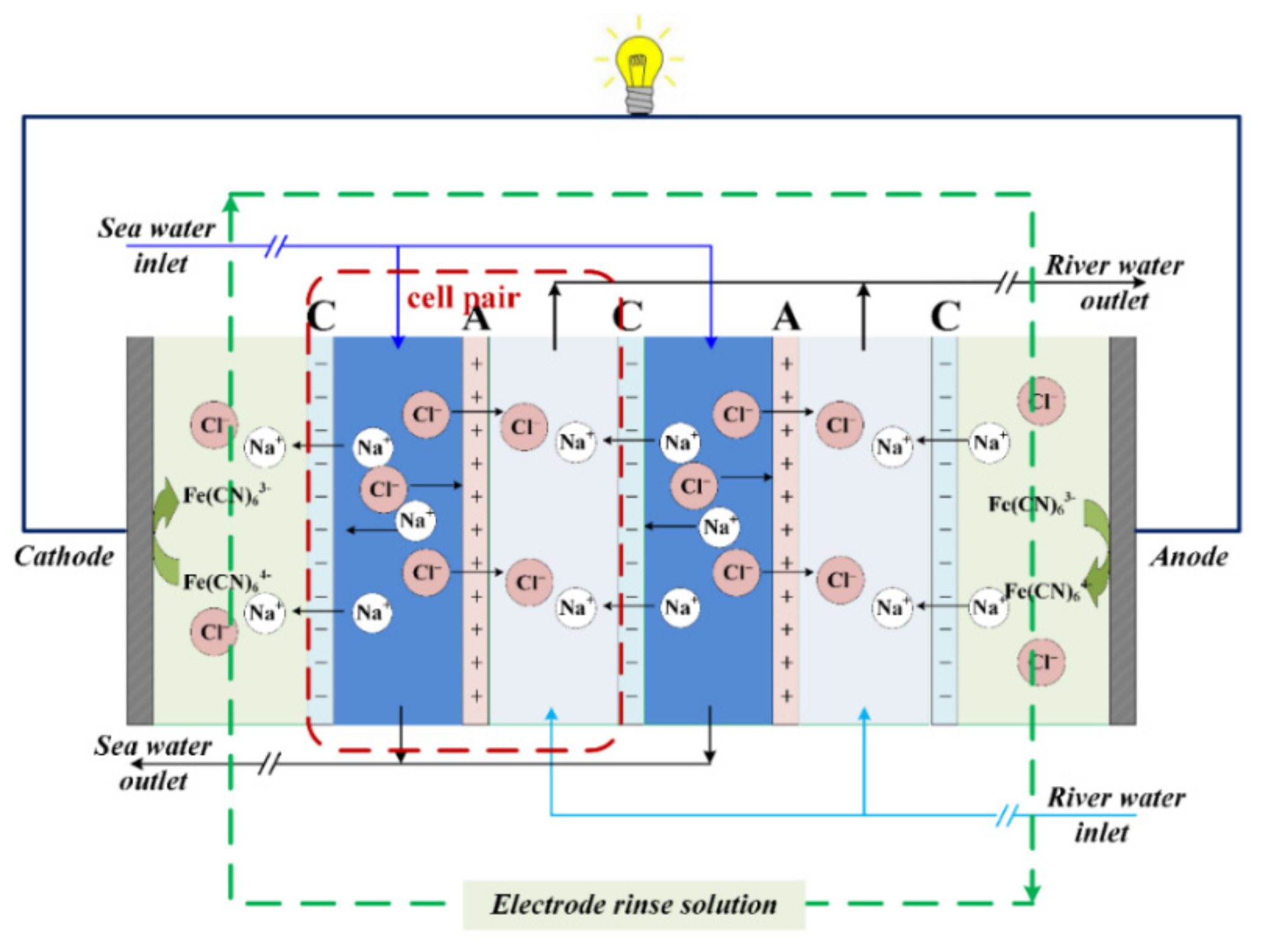
:1. Introduction
2. Results and Discussion
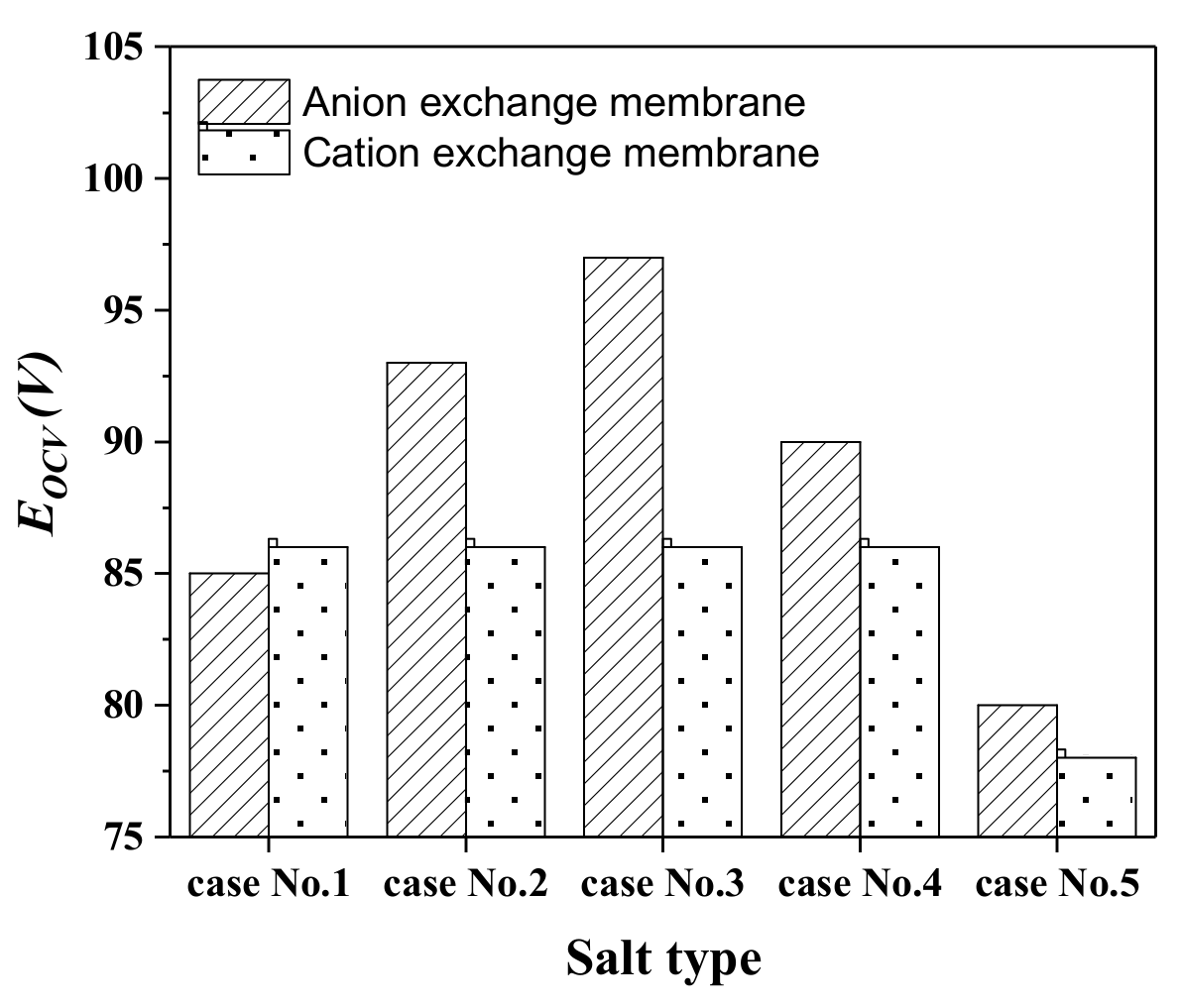
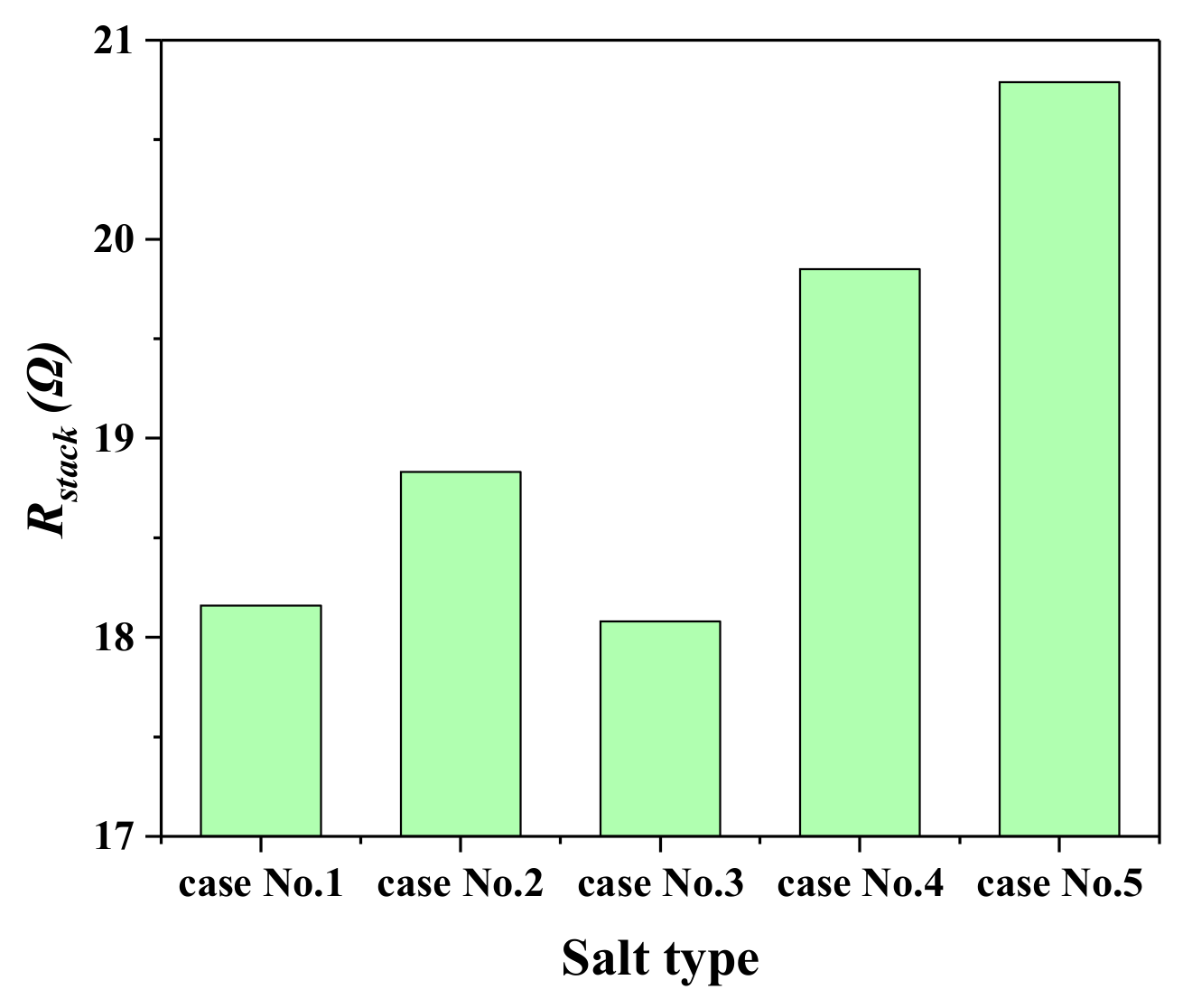
2.1. The Influence of Ion Species on Trans-Membrane Voltage
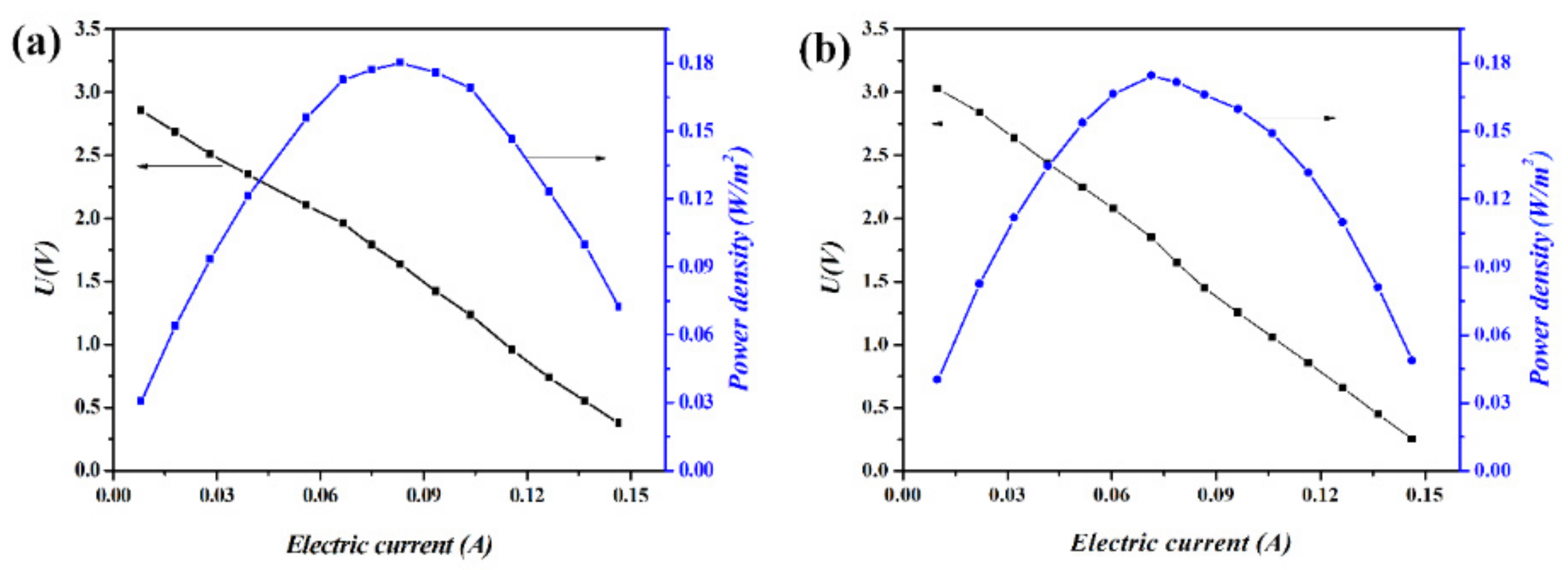
2.2. Polarization Curves of RED
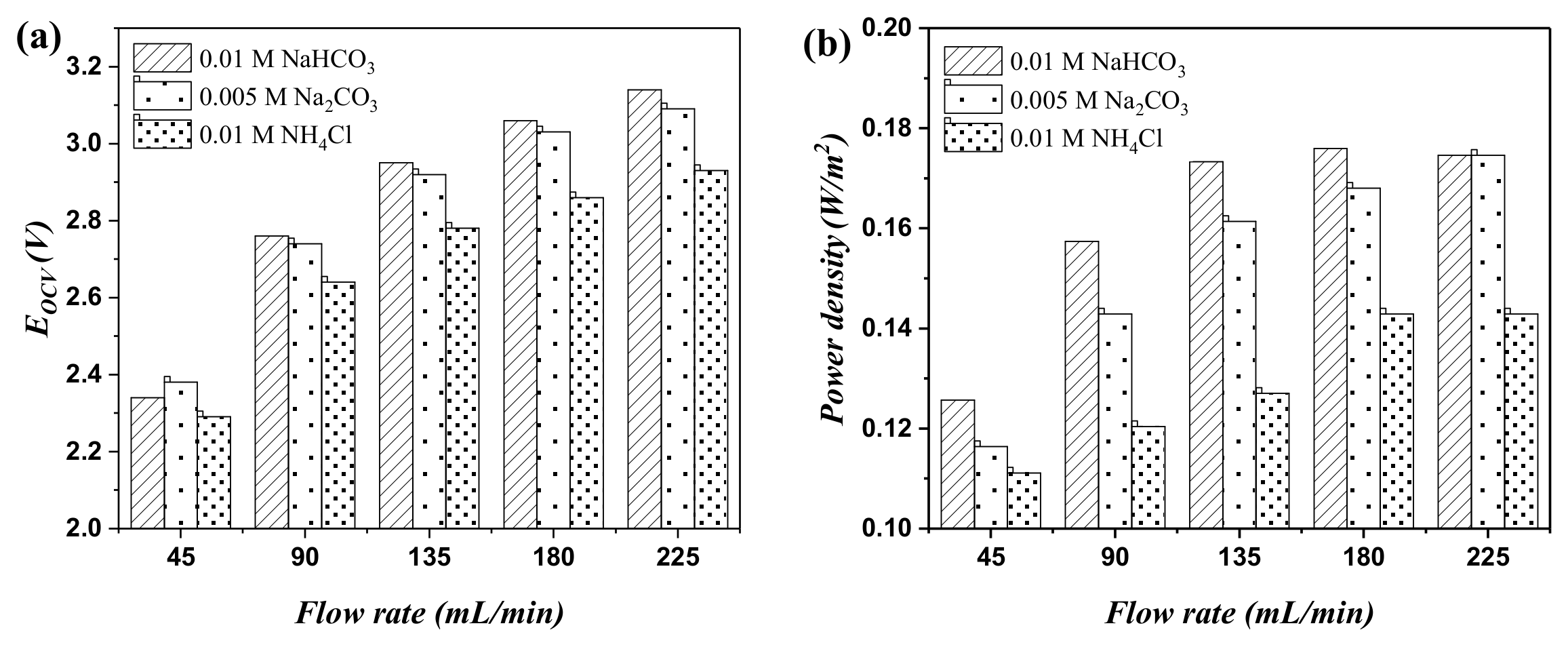
2.3. The Discharging Property of RED
3. Materials and Methods
3.1. Materials
3.2. Transmembrane Voltage Test
3.3. RED Experimental Setup
3.4. RED Performance Tests
3.5. Determination of the RED System
4. Theory
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A | the effective area of a single membrane (m2) |
| ac | the activity of the concentrated salt solution (mol·L−1) |
| ad | the activity of the diluted salt solution (mol·L−1) |
| AEM | anion exchange membrane |
| b | compartment width (cm) |
| Cc | the concentration of HC (mol·L−1) |
| Cd | the concentration of LC (mol·L−1) |
| CEM | cation exchange membrane |
| dc | the thickness of HCC (cm) |
| dd | the thickness of LCC (cm) |
| F | Faraday constant (96485 C·mol−1) |
| HCC | high salt concentration compartment |
| I | current (A) |
| LCC | low salt concentration compartment |
| N | number of cell pairs for RED |
| OCV | open circuit voltage (V) |
| P | output power (W) |
| Pgross | maximum gross power density (W·m−2) |
| Pmax | maximum output power (W) |
| Pnet | net power density (W·m−2) |
| Ppump | power density consumed on pumping (W·m−2) |
| Q | volumetric flow rate (mL·min−1) |
| Qc | the volumetric flow rate of HC (L·s−1) |
| Qd | volumetric flow rate of LC (L s−1) |
| R | gas constant (8.314 J·mol−1·K−1) |
| Ra | the area resistance of anion exchange membrane (Ω·m2) |
| RBL | boundary layer resistance (Ω) |
| Rc | the area resistance of cation exchange membrane (Ω·m2) |
| Rel | the resistance of electrodes (Ω) |
| Rload | load resistance (Ω) |
| Rohmic | ohmic resistance (Ω) |
| Rstack | stack resistance (Ω) |
| R△C | concentration difference resistance (Ω) |
| RED | reverse electrodialysis |
| SGP | salinity gradient power |
| T | absolute temperature (K) |
| U | voltage output |
| V | line flow velocity (cm·min−1) |
| z | electrochemical valence |
| αAEM | permselectivity of the anion exchange membrane |
| αCEM | permselectivity of the cation exchange membrane |
| δ | spacer thickness (cm) |
| εsp | spacer porosity |
| κc | the specific conductivity of the concentrated solution (mS·cm−1) |
| κd | the specific conductivity of the diluted solution (mS·cm−1) |
| ∆pc | the pressure drops along HC (KPa) |
| ∆pd | the pressure drops along LC (KPa) |
References
- Ortiz-Imedio, R.; Gomez-Coma, L.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Comparative performance of Salinity Gradient Power-Reverse Electrodialysis under different operating conditions. Desalination 2019, 457, 8–21. [Google Scholar] [CrossRef]
- Post, J.W.; Hamelers, H.V.M.; Buisman, C.J.N. Energy recovery from controlled mixing salt and fresh water with a reverse electrodialysis system. Environ. Sci. Technol. 2008, 42, 5785–5790. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.B.; Yang, Z.J.; Wang, Y.M.; Jiang, C.X.; Ge, L.; Bakangura, E.; Xu, T.W. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Długołęcki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Membr. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Bajracharya, S.; Sales, B.B.; Saakes, M.; Hamelers, B.; Nijmeijer, K. Clean energy generation using capacitive electrodes in reverse electrodialysis. Energ. Environ. Sci. 2013, 6, 643–651. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Ener.g Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Evaluation of suitable electrode systems. J. Appl. Electrochem. 2010, 40, 1461–1474. [Google Scholar] [CrossRef]
- Luo, F.B.; Wang, Y.M.; Jiang, C.X.; Wu, B.; Feng, H.Y.; Xu, T.W. A power free electrodialysis (PFED) for desalination. Desalination 2017, 404, 138–146. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Crespo, J.G.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energ. 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.P.; Glabman, S.; Uzal, N.; Dou, X.M.; Zhang, H.G.; Wei, X.Z.; Chen, Y.S. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Ciofalo, M.; La Cerva, M.; Di Liberto, M.; Gurreri, L.; Cipollina, A.; Micale, G. Optimization of net power density in Reverse Electrodialysis. Energy 2019, 181, 576–588. [Google Scholar] [CrossRef]
- Gao, H.P.; Zhang, B.P.; Tong, X.; Chen, Y.S. Monovalent-anion selective and antifouling polyelectrolytes multilayer anion exchange membrane for reverse electrodialysis. J. Membr. Sci. 2018, 567, 68–75. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Profiled Ion Exchange Membranes: A Comprehensible Review. Int. J. Mol. Sci. 2019, 20, 165. [Google Scholar] [CrossRef]
- Zhu, X.P.; He, W.H.; Logan, B.E. Influence of solution concentration and salt types on the performance of reverse electrodialysis cells. J. Membr. Sci. 2015, 494, 154–160. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Veerman, J.; Saakes, M.; Nijmeijer, K. Influence of multivalent ions on renewable energy generation in reverse electrodialysis. Energ. Environ. Sci. 2014, 7, 1434–1445. [Google Scholar] [CrossRef]
- Kim, T.; Logan, B.E.; Gorski, C.A. A pH-Gradient Flow Cell for Converting Waste CO2 into Electricity. Environ. Sci. Tech. Let. 2017, 4, 49–53. [Google Scholar] [CrossRef]
- Geise, G.M.; Curtis, A.J.; Hatzell, M.C.; Hickner, M.A.; Logan, B.E. Salt Concentration Differences Alter Membrane Resistance in Reverse Electrodialysis Stacks. Environ. Sci. Tech. Let. 2014, 1, 36–39. [Google Scholar] [CrossRef]
- Jiang, C.X.; Wang, Q.Y.; Li, Y.; Wang, Y.M.; Xu, T.W. Water electro-transport with hydrated cations in electrodialysis. Desalination 2015, 365, 204–212. [Google Scholar] [CrossRef]
- Jiang, C.X.; Zhang, D.Y.; Muhammad, A.S.; Hossain, M.M.; Ge, Z.J.; He, Y.B.; Feng, H.Y.; Xu, T.W. Fouling deposition as an effective approach for preparing monovalent selective membranes. J. Membr. Sci. 2019, 580, 327–335. [Google Scholar] [CrossRef]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse Electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Membr. Sci. 2015. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, C.X.; Shehzad, M.A.; Wang, Y.M.; Feng, H.Y.; Yang, Z.J.; Xu, T.W. Water-Dissociation-Assisted Electrolysis for Hydrogen Production in a Salinity Power Cell. ACS Sustain. Chem. Eng. 2019, 7, 13023–13030. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.V.M.; Biesheuvel, P.M. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membrane. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Güler, E. Anion Exchange Membrane Design for Reverse Electrodialysis; Universiteit Twente: Enschede, The Netherlands, 2014. [Google Scholar]
- Pawlowski, S.; Sistat, P.; Crespo, J.G.; Velizarov, S. Mass transfer in reverse electrodialysis: Flow entrance effects and diffusion boundary layer thickness. J. Membr. Sci. 2014, 471, 72–83. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Enhanced mixing in the diffusive boundary layer for energy generation in reverse electrodialysis. J. Membr. Sci. 2014, 453, 312–319. [Google Scholar] [CrossRef]


| Salt Type | HCC | LCC |
|---|---|---|
| Case No.1 | 0.5 M NaCl | 0.01 M NaCl |
| Case No.2 | 0.5 M NaCl | 0.01 M NaHCO3 |
| Case No.3 | 0.49 M NaCl + 0.01 M NaHCO3 | 0.01 M NaHCO3 |
| Case No.4 | 0.49 M NaCl +0.005 M Na2CO3 | 0.005 M Na2CO3 |
| Case No.5 | 0.49 M NaCl + 0.01 M NH4Cl | 0.01 M NH4Cl |
| Salt Type | HCC | LCC |
|---|---|---|
| Case No.1 | 0.5 M NaCl | 0.01 M NaCl |
| Case No.2 | 0.495 M NaCl + 0.005 M NaHCO3 | 0.005 M NaHCO3 |
| Case No.3 | 0.49 M NaCl + 0.01 M NaHCO3 | 0.01 M NaHCO3 |
| Case No.4 | 0.48 M NaCl +0.02 M NaHCO3 | 0.02 M NaHCO3 |
| Case No.5 | 0.99 M NaCl + 0.01 M NaHCO3 | 0.01 M NaHCO3 |
| Properties | CJ-MC-3 | CJ-MA-2 |
|---|---|---|
| Thickness/mm | 0.220 | 0.185 |
| Ion exchange capacity/mmol/g | 1.50 | 1.25 |
| Water Uptake/% | 35 | 32 |
| Resistance/Ω·cm2 | 3.0 | 1.5 |
| Transfer number/% | 98 | 99 |
| Break stress/MPa | >3.5 | >3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, F.; Wang, Y.; Sha, M.; Wei, Y. Correlations of Ion Composition and Power Efficiency in a Reverse Electrodialysis Heat Engine. Int. J. Mol. Sci. 2019, 20, 5860. https://doi.org/10.3390/ijms20235860
Luo F, Wang Y, Sha M, Wei Y. Correlations of Ion Composition and Power Efficiency in a Reverse Electrodialysis Heat Engine. International Journal of Molecular Sciences. 2019; 20(23):5860. https://doi.org/10.3390/ijms20235860
Chicago/Turabian StyleLuo, Fabao, Yang Wang, Maolin Sha, and Yanxin Wei. 2019. "Correlations of Ion Composition and Power Efficiency in a Reverse Electrodialysis Heat Engine" International Journal of Molecular Sciences 20, no. 23: 5860. https://doi.org/10.3390/ijms20235860
APA StyleLuo, F., Wang, Y., Sha, M., & Wei, Y. (2019). Correlations of Ion Composition and Power Efficiency in a Reverse Electrodialysis Heat Engine. International Journal of Molecular Sciences, 20(23), 5860. https://doi.org/10.3390/ijms20235860




