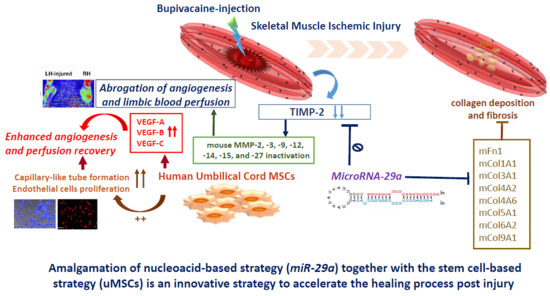
MicroRNA-29a Exhibited Pro-Angiogenic and Anti-Fibrotic Features to Intensify Human Umbilical Cord Mesenchymal Stem Cells—Renovated Perfusion Recovery and Preventing against Fibrosis from Skeletal Muscle Ischemic Injury
Abstract
1. Introduction
2. Results
2.1. Suppression of MicroRNA-29a in Bupivacaine (BPVC)-Injured Gastrocnemius Muscles from C57BL/6 Mice in Comparison with Non-Injured Sham Control Gastrocnemius Muscles
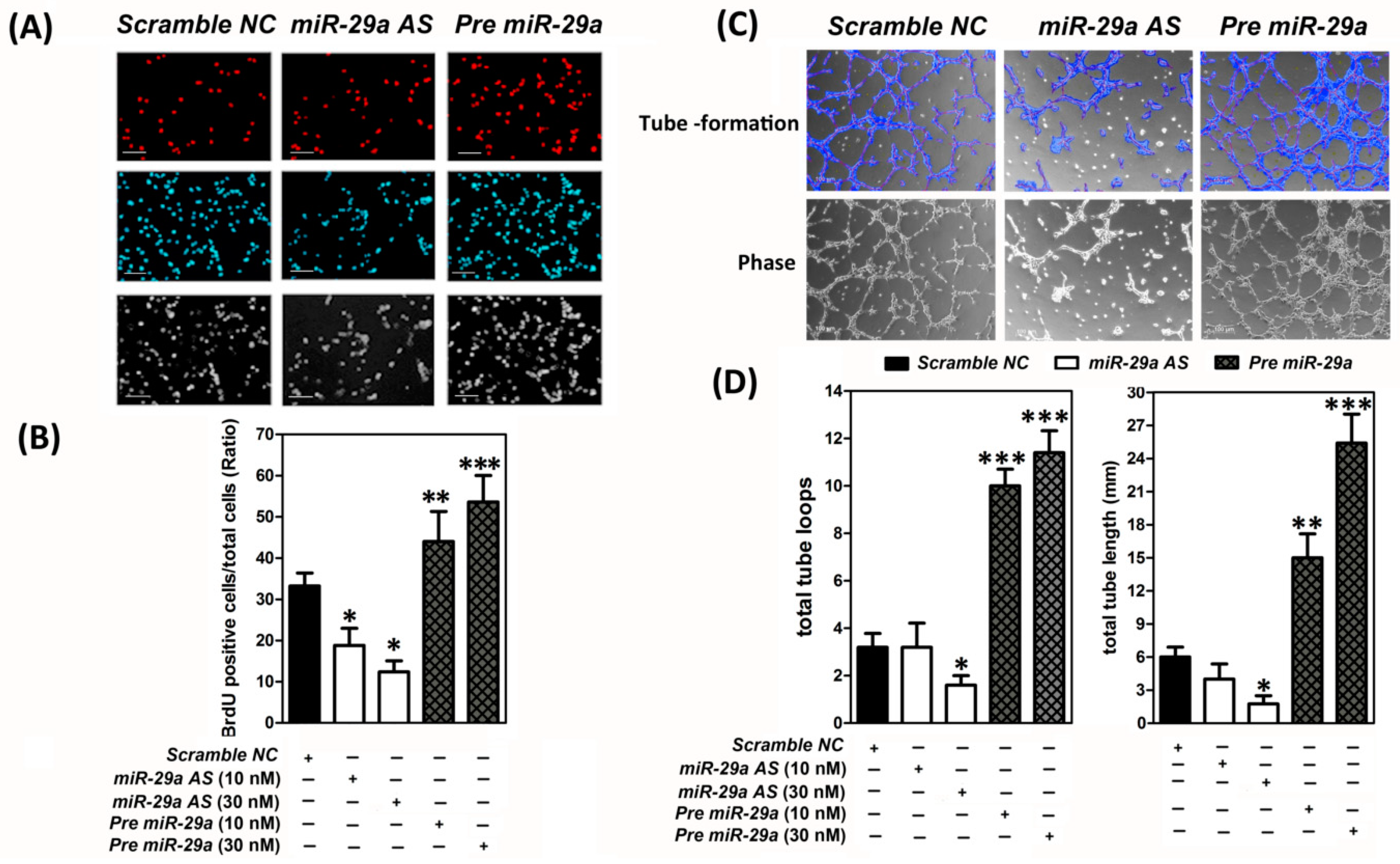
2.2. MicroRNA-29 was a Pro-Angigenesis MicroRNA to Augment the Human Umbilical Vein Endothelial Cells (HUVECs) Proliferation and Capillary-Like Tube Formation In Vitro
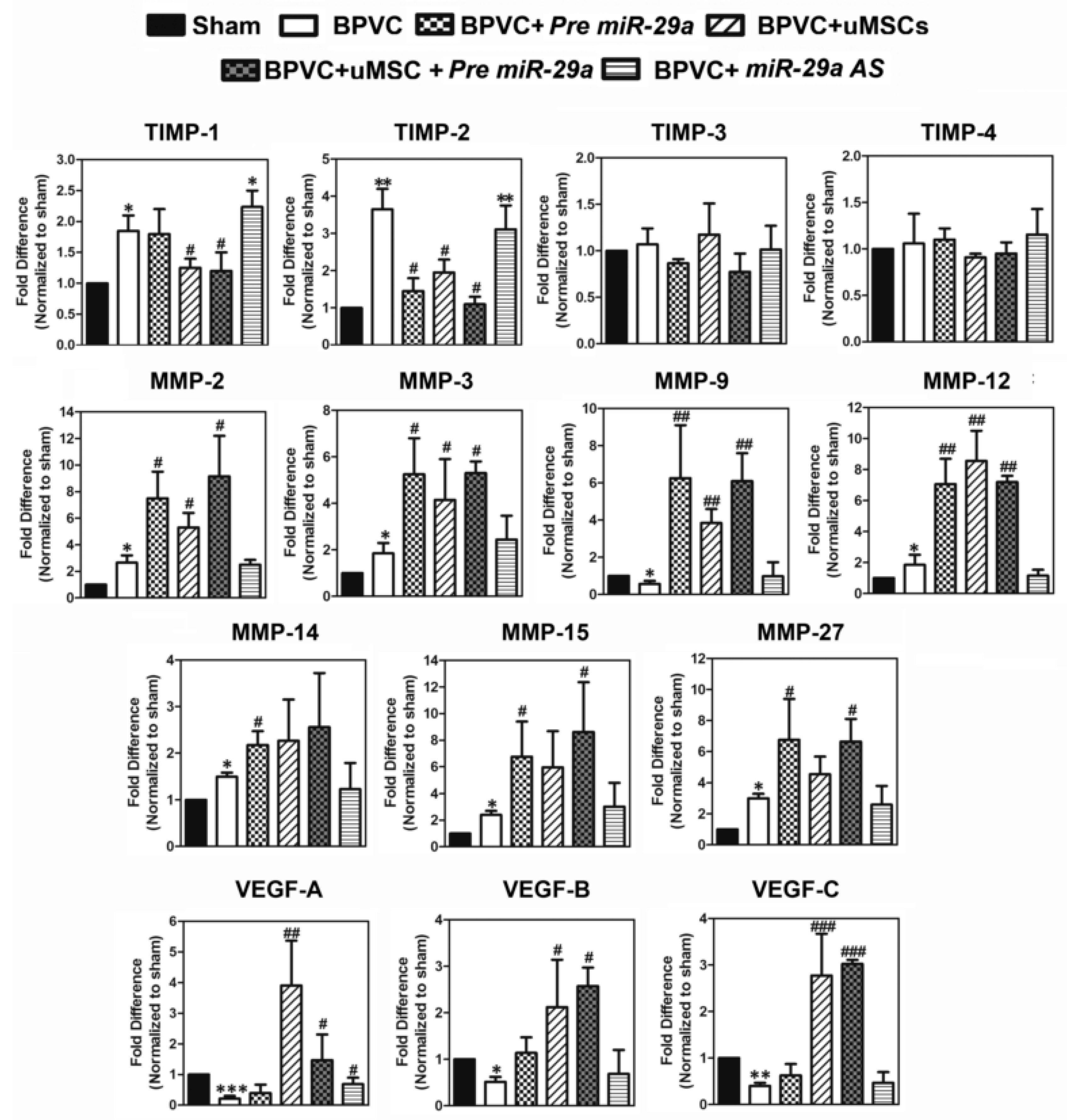
2.3. Exogenous Administration of miR-29a or Transplantation of The Umbilical Cord Wharton’s Jelly (uMSCs) Exhibited the Pro-Angiogensis Properties in Vivo and Successfully Restored the Blood Perfusion of BPVC-Injured Gastrocnemius Muscles of Hind Limbs Measured by Laser Doppler
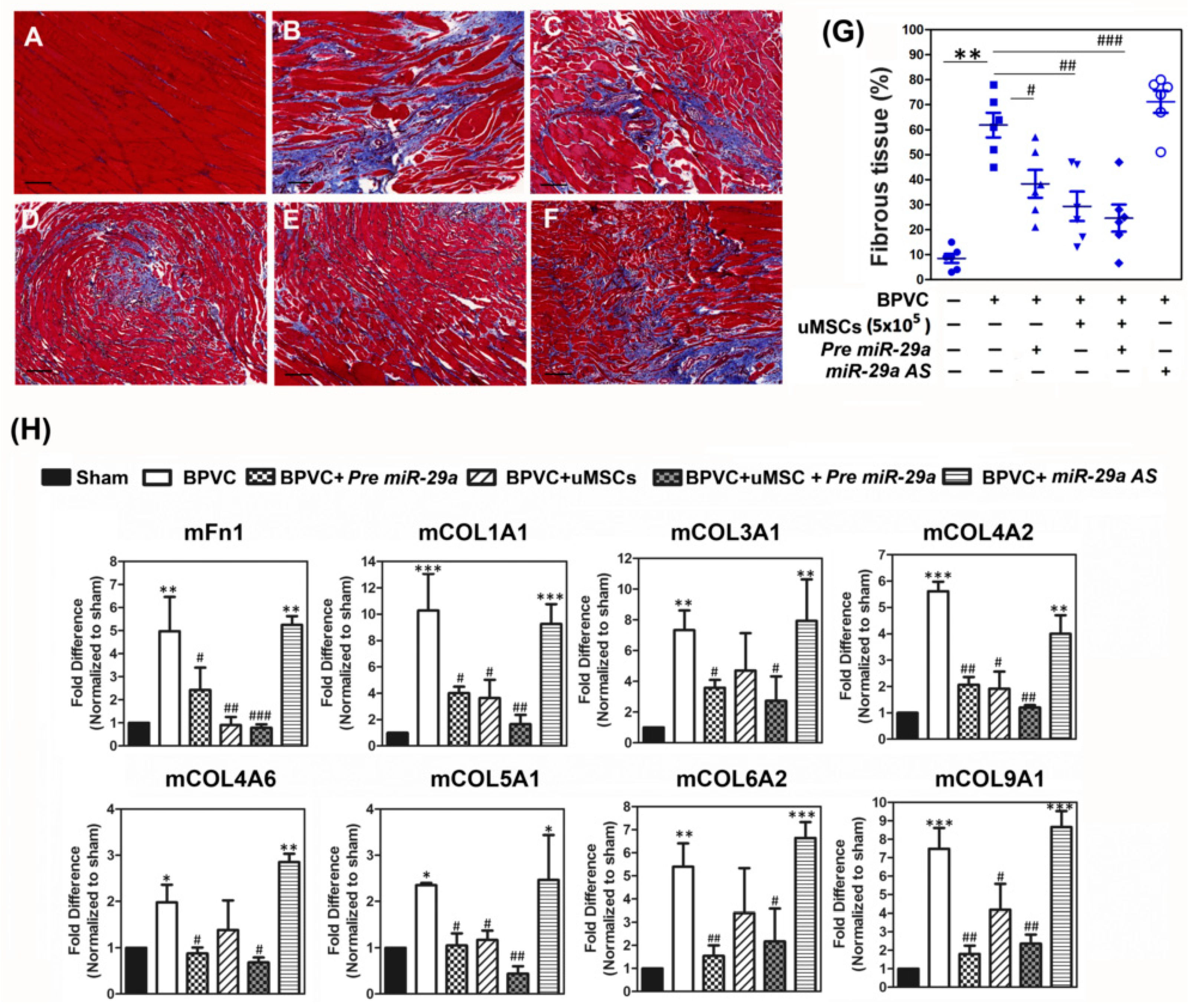
2.4. Forced Expression of miR-29a or Transplantation of the uMSCs Exhibited the Anti-Fibrotic Properties in Vivo and Alleviated BPVC-Induced Gastrocnemius Muscle Fibrosis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation, Cultivation, and Characterization of Human Umbilical Cord Wharton’s Jelly (uMSCs)
4.3. Cultivation of Human Umbilical Cord Vein Endothelial Cells (HUVECs)
4.4. Assessment of MicroRNA Expression by Quantitative RT-PCR
4.5. Transfection of MicroRNA-29a Precursor and Antisense Oligonucleotide into HUVECs
4.6. 5-Bromo-2-Deoxyuridine (BrdU) Cell Proliferation Assay
4.7. In Vitro Capillary-Like Tube Network Formation Assay
4.8. Gastrocnemius Muscles Injury Induced by Bupivacaine Hydrochloride Injection and Administration of MicroRNA-29a or Transplantation of Human uMSCs
4.9. Angeognic Factors Analysis by Quantibody Mouse Angiogenesis Array and Fibrosis-Related Factors Analysis by Quantibody Mouse Extracellular Matrix Array
4.10. Muscle Fibrosis
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Kalimo, H.; Jarvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Ceafalan, L.C.; Manole, E.; Tanase, C.P.; Codrici, E.; Mihai, S.; Gonzalez, A.; Popescu, B.O. Interstitial Outburst of Angiogenic Factors During Skeletal Muscle Regeneration After Acute Mechanical Trauma. Anat. Rec. 2015, 298, 1864–1879. [Google Scholar] [CrossRef]
- Ochoa, O.; Sun, D.; Reyes-Reyna, S.M.; Waite, L.L.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R651–R661. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, D.; Nakamura, A.; Fukushima, K.; Yoshida, K.; Takeda, S.; Ikeda, S. Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum. Mol. Genet. 2011, 20, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Christov, C.; Chretien, F.; Abou-Khalil, R.; Bassez, G.; Vallet, G.; Authier, F.J.; Bassaglia, Y.; Shinin, V.; Tajbakhsh, S.; Chazaud, B.; et al. Muscle satellite cells and endothelial cells: Close neighbors and privileged partners. Mol. Biol. Cell 2007, 18, 1397–1409. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part. B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Phylogenetic and functional relationships between coagulation and the innate immune response. Crit. Care Med. 2000, 28, S77–S80. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Luster, A.D.; Alon, R.; von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef]
- Su, W.H.; Wang, C.J.; Fu, H.C.; Sheng, C.M.; Tsai, C.C.; Cheng, J.H.; Chuang, P.C. Human Umbilical Cord Mesenchymal Stem Cells Extricate Bupivacaine-Impaired Skeletal Muscle Function via Mitigating Neutrophil-Mediated Acute Inflammation and Protecting against Fibrosis. Int. J. Mol. Sci. 2019, 20, 4312. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Q.; Yang, T.; Ding, W.; Zhao, Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 2014, 34, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, A. Endogenous expression of angiogenesis-related factors in response to muscle injury. Mol. Cell Biochem. 2007, 298, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Inflammatory cell response to acute muscle injury. Med. Sci. Sports Exerc. 1995, 27, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Johnson, R.M.; Rathbone, C.R.; Liu, X.; Temm-Grove, C.; Sheehan, S.M.; Hoying, J.B.; Allen, R.E. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am. J. Physiol. Cell Physiol. 2009, 296, C1321–C1328. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Shen, W.; Qiao, C.; Ambrosio, F.; Lavasani, M.; Nozaki, M.; Branca, M.F.; Huard, J. Relationships between transforming growth factor-beta1, myostatin, and decorin: Implications for skeletal muscle fibrosis. J. Biol. Chem. 2007, 282, 25852–25863. [Google Scholar] [CrossRef]
- Darmani, H.; Crossan, J.; McLellan, S.D.; Meek, D.; Adam, C. Expression of nitric oxide synthase and transforming growth factor-beta in crush-injured tendon and synovium. Mediat. Inflamm. 2004, 13, 299–305. [Google Scholar] [CrossRef]
- Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Douglas, K.M.; Koutedakis, Y.; Nevill, A.M.; Panoulas, V.F.; Kita, M.; Kitas, G.D. Blockade of tumour necrosis factor-alpha in rheumatoid arthritis: Effects on components of rheumatoid cachexia. Rheumatology 2007, 46, 1824–1827. [Google Scholar] [CrossRef]
- Rizzi, C.F.; Mauriz, J.L.; Freitas Correa, D.S.; Moreira, A.J.; Zettler, C.G.; Filippin, L.I.; Marroni, N.P.; Gonzalez-Gallego, J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg. Med. 2006, 38, 704–713. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Z.; Han, L.; Zhang, A.-L.; Fu, Y.-C.; Yue, X.; Wang, G.-X.; Jia, Z.-F.; Pu, P.-Y.; Zhang, Q.-Y.; Kang, C.-S. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer 2010, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kinoshita, T.; Nohata, N.; Itesako, T.; Yoshino, H.; Enokida, H.; Nakagawa, M.; Shozu, M.; Seki, N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int. J. Oncol. 2013, 42, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mi, Q.S.; Dong, Z. The regulation and function of microRNAs in kidney diseases. IUBMB Life 2013, 65, 602–614. [Google Scholar] [CrossRef]
- Godwin, J.G.; Ge, X.; Stephan, K.; Jurisch, A.; Tullius, S.G.; Iacomini, J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc. Natl. Acad. Sci. USA 2010, 107, 14339–14344. [Google Scholar] [CrossRef]
- Liu, N.; Williams, A.H.; Maxeiner, J.M.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MicroRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J. Clin. Invest. 2012, 122, 2054–2065. [Google Scholar] [CrossRef]
- Salim, H.; Akbar, N.S.; Zong, D.; Vaculova, A.H.; Lewensohn, R.; Moshfegh, A.; Viktorsson, K.; Zhivotovsky, B. MiRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br. J. Cancer 2012, 107, 1361–1373. [Google Scholar] [CrossRef]
- Alajez, N.M.; Lenarduzzi, M.; Ito, E.; Hui, A.B.; Shi, W.; Bruce, J.; Yue, S.; Huang, S.H.; Xu, W.; Waldron, J.; et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011, 71, 2381–2391. [Google Scholar] [CrossRef]
- Flynt, A.S.; Li, N.; Thatcher, E.J.; Solnica-Krezel, L.; Patton, J.G. Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nat. Genet. 2007, 39, 259–263. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J. Cell Biol. 2006, 174, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chang, P.J.; Ho, C.; Huang, Y.T.; Shih, Y.H.; Wang, C.J.; Lin, C.L. Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/beta-catenin signaling. Sci. Rep. 2016, 6, 30575. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Wang, B.; Beyer, C.; Koh, P.; White, L.; Kantharidis, P.; Gregorevic, P. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J. Biol. Chem. 2011, 286, 13805–13814. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Sun, C.K.; Tsai, T.H.; Chang, L.T.; Leu, S.; Zhen, Y.Y.; Sheu, J.J.; Chua, S.; Yeh, K.H.; Lu, H.I.; et al. Adipose-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffolds promote angiogenesis, preserve heart function, and reduce left ventricular remodeling in rat acute myocardial infarction. Am. J. Transl. Res. 2015, 7, 781–803. [Google Scholar]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef]
- McFarlin, K.; Gao, X.; Liu, Y.B.; Dulchavsky, D.S.; Kwon, D.; Arbab, A.S.; Bansal, M.; Li, Y.; Chopp, M.; Dulchavsky, S.A.; et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006, 14, 471–478. [Google Scholar] [CrossRef]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef]
- Wang, X.H.; Hu, Z.; Klein, J.D.; Zhang, L.; Fang, F.; Mitch, W.E. Decreased miR-29 suppresses myogenesis in CKD. J. Am. Soc. Nephrol. 2011, 22, 2068–2076. [Google Scholar] [CrossRef]
- Guerit, D.; Brondello, J.M.; Chuchana, P.; Philipot, D.; Toupet, K.; Bony, C.; Jorgensen, C.; Noel, D. FOXO3A regulation by miRNA-29a Controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation. Stem Cells Dev. 2014, 23, 1195–1205. [Google Scholar] [CrossRef]
- Kapinas, K.; Kessler, C.; Ricks, T.; Gronowicz, G.; Delany, A.M. MiR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J. Biol. Chem. 2010, 285, 25221–25231. [Google Scholar] [CrossRef]
- Wang, F.S.; Chuang, P.C.; Lin, C.L.; Chen, M.W.; Ke, H.J.; Chang, Y.H.; Chen, Y.S.; Wu, S.L.; Ko, J.Y. MicroRNA-29a protects against glucocorticoid-induced bone loss and fragility in rats by orchestrating bone acquisition and resorption. Arthritis Rheum. 2013, 65, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Dooley, J.; Chung, S.S.; Chandramohan, D.; Cimmino, L.; Mukherjee, S.; Mason, C.E.; de Strooper, B.; Liston, A.; Park, C.Y. MiR-29a maintains mouse hematopoietic stem cell self-renewal by regulating Dnmt3a. Blood 2015, 125, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, I.; Takagi, A.; Ishiura, S.; Nakase, H.; Sugita, H. Pathophysiology of muscle fiber necrosis induced by bupivacaine hydrochloride (Marcaine). Acta Neuropathol. 1983, 60, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.H. Protein synthesis in bupivacaine (marcaine)-treated, regenerating skeletal muscle. Muscle Nerve 1982, 5, 281–290. [Google Scholar] [CrossRef]
- Warren, G.L.; Summan, M.; Gao, X.; Chapman, R.; Hulderman, T.; Simeonova, P.P. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J. Physiol. 2007, 582, 825–841. [Google Scholar] [CrossRef]
- Ji, Q.; Meng, K.; Yu, K.; Huang, S.; Huang, Y.; Min, X.; Zhong, Y.; Wu, B.; Liu, Y.; Nie, S.; et al. Exogenous interleukin 37 ameliorates atherosclerosis via inducing the Treg response in ApoE-deficient mice. Sci. Rep. 2017, 7, 3310. [Google Scholar] [CrossRef]
- Jackson, B.C.; Nebert, D.W.; Vasiliou, V. Update of human and mouse matrix metalloproteinase families. Hum. Genom. 2010, 4, 194–201. [Google Scholar]
- Winkler, T.; von Roth, P.; Matziolis, G.; Schumann, M.R.; Hahn, S.; Strube, P.; Stoltenburg-Didinger, G.; Perka, C.; Duda, G.N.; Tohtz, S.V. Time course of skeletal muscle regeneration after severe trauma. Acta Orthop. 2010, 82, 102–111. [Google Scholar] [CrossRef]
- Sass, F.A.; Fuchs, M.; Pumberger, M.; Geissler, S.; Duda, G.N.; Perka, C.; Schmidt-Bleek, K. Immunology Guides Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 835. [Google Scholar] [CrossRef]
- Limbourg, A.; Korff, T.; Napp, L.C.; Schaper, W.; Drexler, H.; Limbourg, F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009, 4, 1737–1746. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Neth, P.; Nazari-Jahantigh, M.; Schober, A.; Weber, C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc. Res. 2013, 99, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, F.; Bemtgen, X.; Zeiser, R.; Leonhardt, F.; Kreuzaler, S.; Hilgendorf, I.; Smolka, C.; Helbing, T.; Hoefer, I.; Esser, J.S.; et al. MicroRNA-155 Exerts Cell-Specific Antiangiogenic but Proarteriogenic Effects During Adaptive Neovascularization. Circulation 2015, 131, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Ganta, V.C.; Choi, M.H.; Kutateladze, A.; Fox, T.E.; Farber, C.R.; Annex, B.H. A MicroRNA93-Interferon Regulatory Factor-9-Immunoresponsive Gene-1-Itaconic Acid Pathway Modulates M2-Like Macrophage Polarization to Revascularize Ischemic Muscle. Circulation 2017, 135, 2403–2425. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, L.; Zhu, X.; Xu, J.; Jin, R.; Li, G.; Wu, F. MiR-29a modulates the angiogenic properties of human endothelial cells. Biochem. Biophys. Res. Commun. 2013, 434, 143–149. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Ma, Y.; Lan, Y.; Yang, X. Transforming growth factor beta-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J. Biol. Chem. 2013, 288, 10418–10426. [Google Scholar] [CrossRef]
- Nuttall, R.K.; Sampieri, C.L.; Pennington, C.J.; Gill, S.E.; Schultz, G.A.; Edwards, D.R. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004, 563, 129–134. [Google Scholar] [CrossRef]
- Baker, A.H.; Edwards, D.R.; Murphy, G. Metalloproteinase inhibitors: Biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef]
- Chen, T.M.; Chen, Y.H.; Sun, H.S.; Tsai, S.J. Fibroblast growth factors: Potential novel targets for regenerative therapy of osteoarthritis. Chin. J. Physiol. 2019, 62, 2–10. [Google Scholar]
- Hornberger, L.K.; Singhroy, S.; Cavalle-Garrido, T.; Tsang, W.; Keeley, F.; Rabinovitch, M. Synthesis of extracellular matrix and adhesion through beta(1) integrins are critical for fetal ventricular myocyte proliferation. Circ. Res. 2000, 87, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Shohara, R.; Yamamoto, A.; Takikawa, S.; Iwase, A.; Hibi, H.; Kikkawa, F.; Ueda, M. Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy 2012, 14, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Lee, H.S.; Naidansaren, P.; Kim, H.K.; Eunju, O.; Cha, J.H.; Ahn, H.Y.; Yang, P.I.; Shin, J.C.; Joe, Y.A. Proangiogenic features of Wharton’s jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int. J. Biochem. Cell Biol. 2012, 45, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Lilyanna, S.; Martinez, E.C.; Vu, T.D.; Ling, L.H.; Gan, S.U.; Tan, A.L.; Phan, T.T.; Kofidis, T. Cord lining-mesenchymal stem cells graft supplemented with an omental flap induces myocardial revascularization and ameliorates cardiac dysfunction in a rat model of chronic ischemic heart failure. Tissue Eng. Part A 2013, 19, 1303–1315. [Google Scholar] [CrossRef]
- Edwards, S.S.; Zavala, G.; Prieto, C.P.; Elliott, M.; Martinez, S.; Egana, J.T.; Bono, M.R.; Palma, V. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton’s jelly in dermal regeneration. Angiogenesis 2014, 17, 851–866. [Google Scholar] [CrossRef]
- Garg, K.; Corona, B.T.; Walters, T.J. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front. Pharmacol. 2015, 6, 87. [Google Scholar] [CrossRef]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef]
- Schueller, F.; Roy, S.; Vucur, M.; Trautwein, C.; Luedde, T.; Roderburg, C. The Role of miRNAs in the Pathophysiology of Liver Diseases and Toxicity. Int. J. Mol. Sci. 2018, 19, 261. [Google Scholar] [CrossRef]
- Ko, J.Y.; Chuang, P.C.; Chen, M.W.; Ke, H.C.; Wu, S.L.; Chang, Y.H.; Chen, Y.S.; Wang, F.S. MicroRNA-29a ameliorates glucocorticoid-induced suppression of osteoblast differentiation by regulating beta-catenin acetylation. Bone 2013, 57, 468–475. [Google Scholar] [CrossRef]
- Ding, Q.; Chang, C.J.; Xie, X.; Xia, W.; Yang, J.Y.; Wang, S.C.; Wang, Y.; Xia, J.; Chen, L.; Cai, C.; et al. APOBEC3G promotes liver metastasis in an orthotopic mouse model of colorectal cancer and predicts human hepatic metastasis. J. Clin. Invest. 2011, 121, 4526–4536. [Google Scholar] [CrossRef]
- Qin, W.; Chung, A.C.; Huang, X.R.; Meng, X.M.; Hui, D.S.; Yu, C.M.; Sung, J.J.; Lan, H.Y. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J. Am. Soc. Nephrol. 2011, 22, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Itami, S.; Kuroda, M.; Yoshizato, K.; Kawada, N.; Murakami, Y. MiR-29a Assists in Preventing the Activation of Human Stellate Cells and Promotes Recovery From Liver Fibrosis in Mice. Mol. Ther. 2016, 24, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Liou, C.W.; Chen, S.D.; Wang, P.W.; Chuang, J.H.; Tiao, M.M.; Hsu, T.Y.; Lin, H.Y.; Lin, T.K. Mitochondrial Transfer from Wharton’s Jelly Mesenchymal Stem Cell to MERRF Cybrid Reduces Oxidative Stress and Improves Mitochondrial Bioenergetics. Oxid. Med. Cell Longev. 2017, 2017, 5691215. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Liou, C.W.; Chen, S.D.; Hsu, T.Y.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; Lin, T.K.; et al. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 2015, 22, 31–44. [Google Scholar] [CrossRef]
- Su, W.H.; Chen, H.I.; Jen, C.J. Differential movements of VE-cadherin and PECAM-1 during transmigration of polymorphonuclear leukocytes through human umbilical vein endothelium. Blood 2002, 100, 3597–3603. [Google Scholar] [CrossRef]
- Millar, N.L.; Gilchrist, D.S.; Akbar, M.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Murrell, G.A.C.; Liew, F.Y.; Kurowska-Stolarska, M.; McInnes, I.B. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun. 2015, 6, 6774. [Google Scholar] [CrossRef]
- Chang, J.Y.; Kessler, H.P. Masson trichrome stain helps differentiate myofibroma from smooth muscle lesions in the head and neck region. J. Formos. Med. Assoc. 2008, 107, 767–773. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, S.; Yu, F.; Li, H.; Guo, C.; Fan, X. Ghrelin Attenuates Liver Fibrosis through Regulation of TGF-beta1 Expression and Autophagy. Int. J. Mol. Sci. 2015, 16, 21911–21930. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-H.; Wang, C.-J.; Hung, Y.-Y.; Lu, C.-W.; Ou, C.-Y.; Tseng, S.-H.; Tsai, C.-C.; Kao, Y.-T.; Chuang, P.-C. MicroRNA-29a Exhibited Pro-Angiogenic and Anti-Fibrotic Features to Intensify Human Umbilical Cord Mesenchymal Stem Cells—Renovated Perfusion Recovery and Preventing against Fibrosis from Skeletal Muscle Ischemic Injury. Int. J. Mol. Sci. 2019, 20, 5859. https://doi.org/10.3390/ijms20235859
Su W-H, Wang C-J, Hung Y-Y, Lu C-W, Ou C-Y, Tseng S-H, Tsai C-C, Kao Y-T, Chuang P-C. MicroRNA-29a Exhibited Pro-Angiogenic and Anti-Fibrotic Features to Intensify Human Umbilical Cord Mesenchymal Stem Cells—Renovated Perfusion Recovery and Preventing against Fibrosis from Skeletal Muscle Ischemic Injury. International Journal of Molecular Sciences. 2019; 20(23):5859. https://doi.org/10.3390/ijms20235859
Chicago/Turabian StyleSu, Wen-Hong, Ching-Jen Wang, Yi-Yung Hung, Chun-Wun Lu, Chia-Yu Ou, Shun-Hung Tseng, Ching-Chin Tsai, Yun-Ting Kao, and Pei-Chin Chuang. 2019. "MicroRNA-29a Exhibited Pro-Angiogenic and Anti-Fibrotic Features to Intensify Human Umbilical Cord Mesenchymal Stem Cells—Renovated Perfusion Recovery and Preventing against Fibrosis from Skeletal Muscle Ischemic Injury" International Journal of Molecular Sciences 20, no. 23: 5859. https://doi.org/10.3390/ijms20235859
APA StyleSu, W.-H., Wang, C.-J., Hung, Y.-Y., Lu, C.-W., Ou, C.-Y., Tseng, S.-H., Tsai, C.-C., Kao, Y.-T., & Chuang, P.-C. (2019). MicroRNA-29a Exhibited Pro-Angiogenic and Anti-Fibrotic Features to Intensify Human Umbilical Cord Mesenchymal Stem Cells—Renovated Perfusion Recovery and Preventing against Fibrosis from Skeletal Muscle Ischemic Injury. International Journal of Molecular Sciences, 20(23), 5859. https://doi.org/10.3390/ijms20235859