Cytoprotective Preconditioning of Osteoblast-Like Cells with N-Acetyl-L-Cysteine for Bone Regeneration in Cell Therapy
Abstract
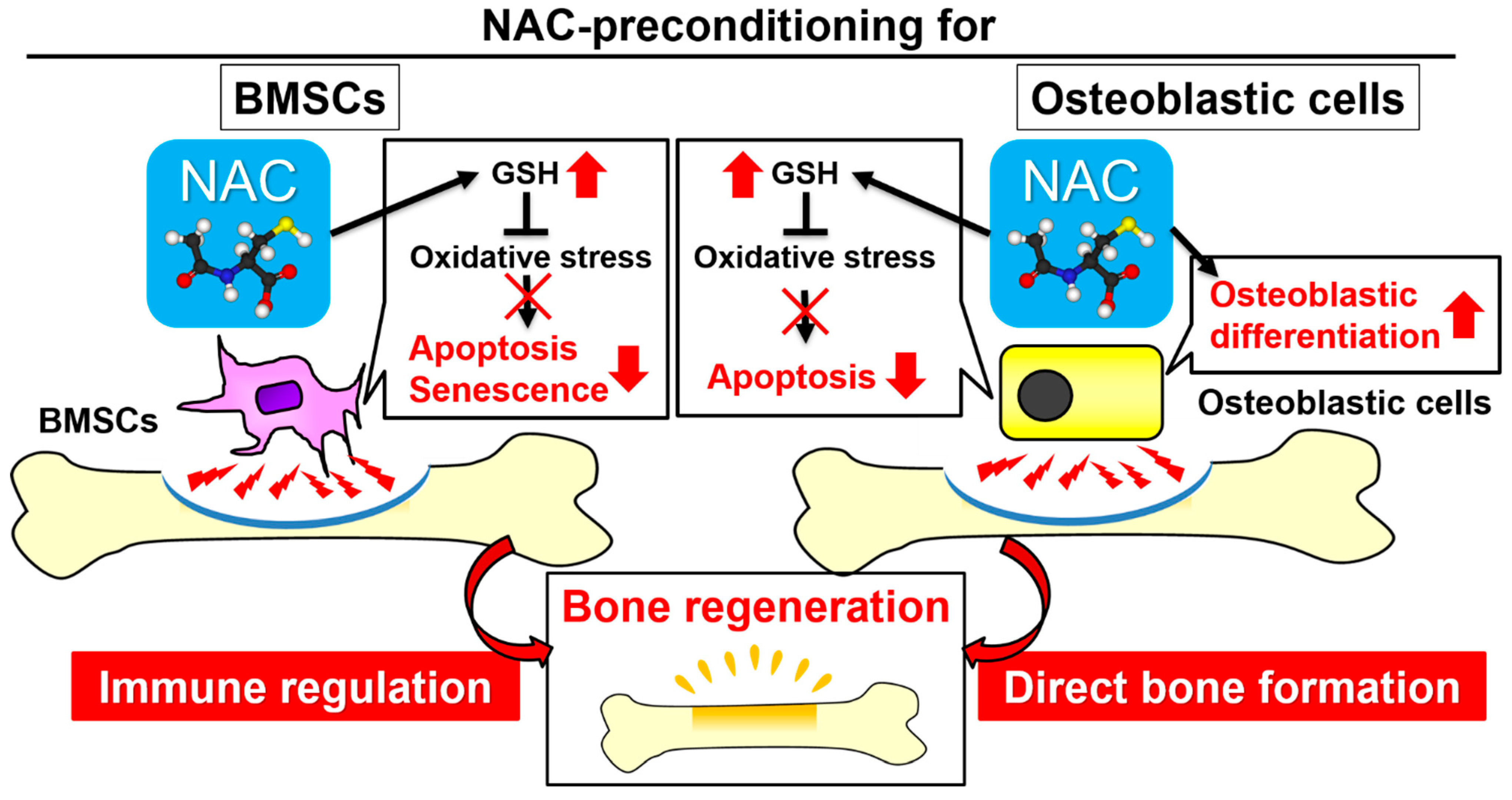
:1. Introduction
2. Results
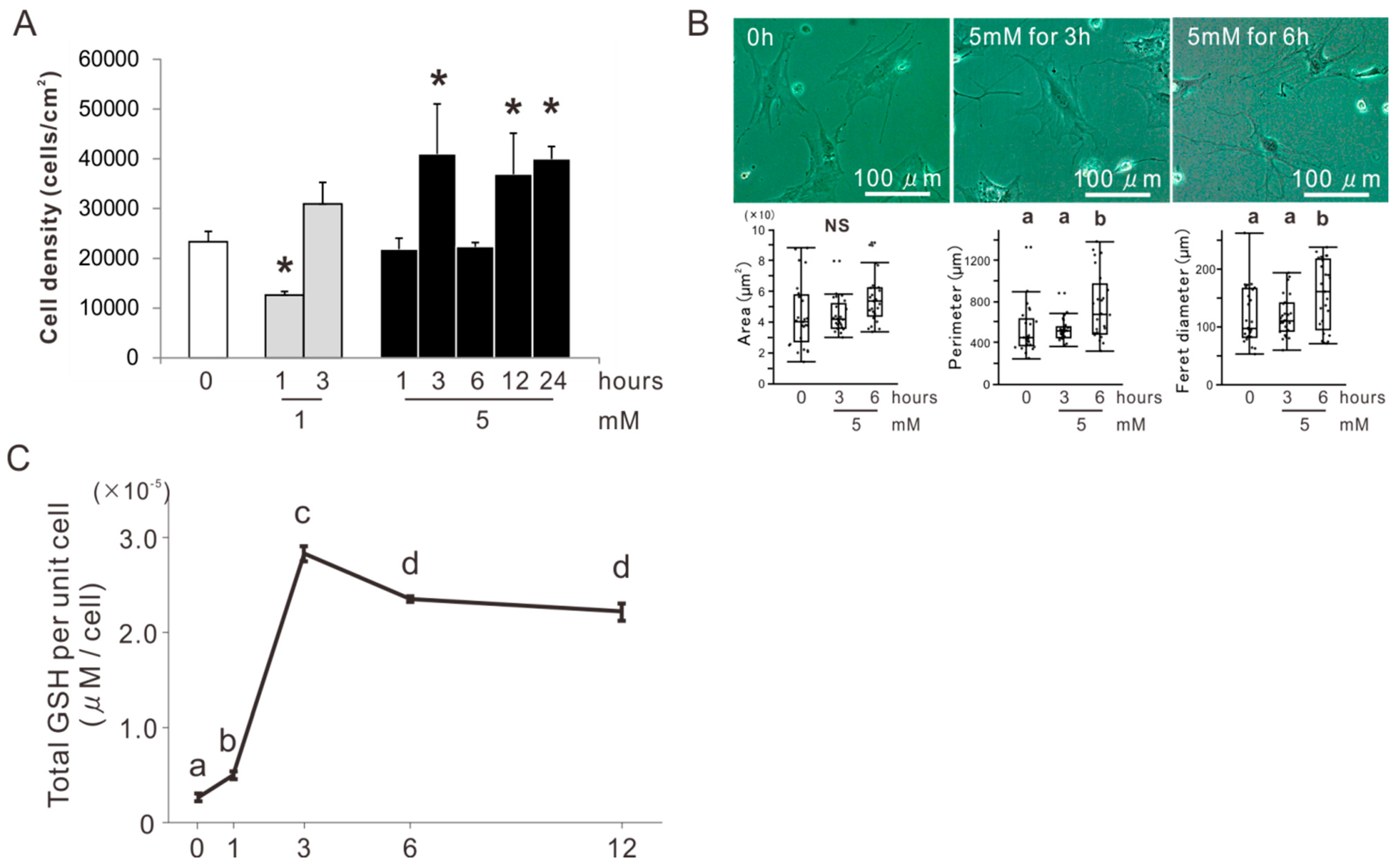
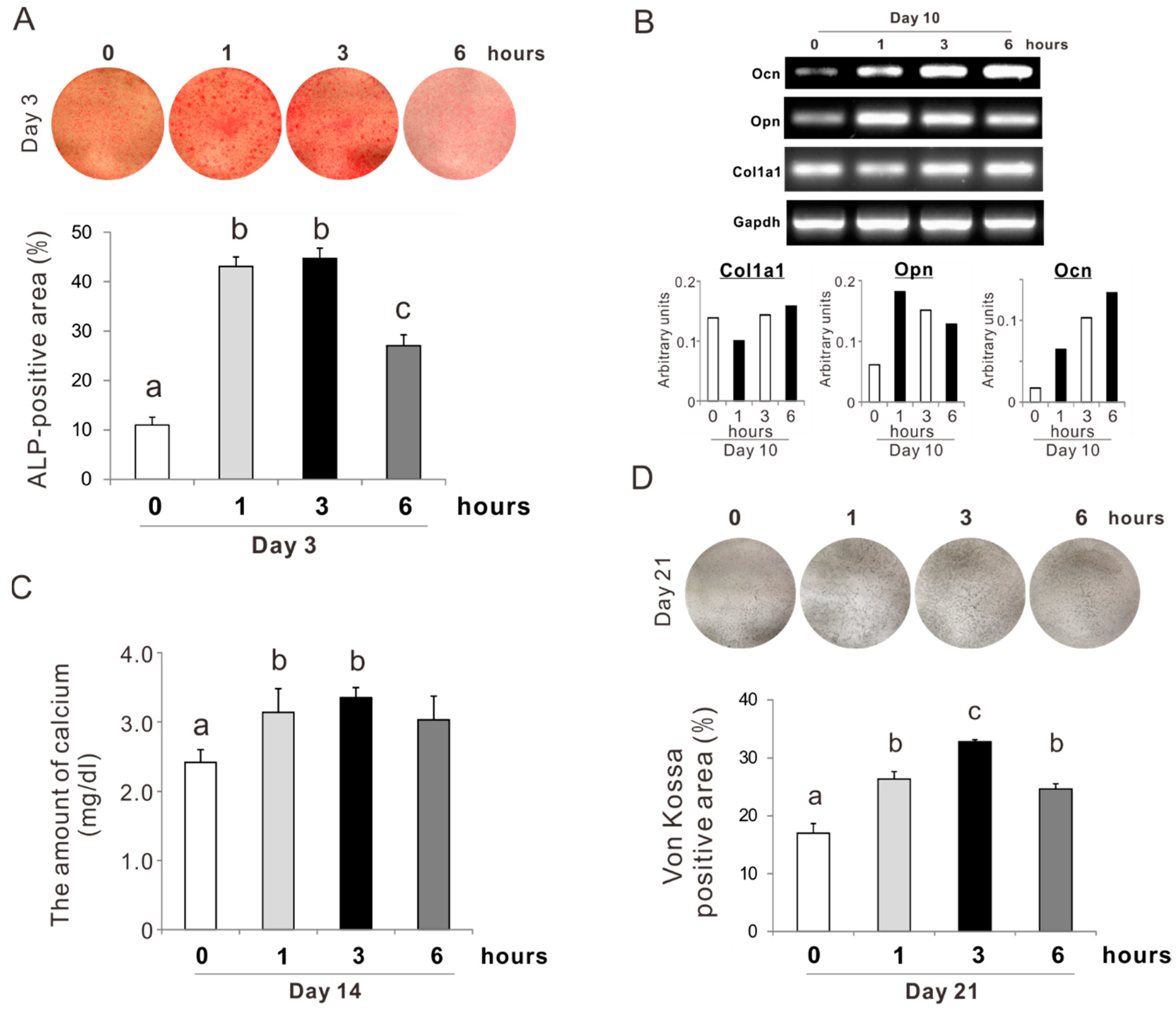
2.1. Determination of the Concentration and Incubation Time of NAC Preconditioning for Osteoblast-Like Cells
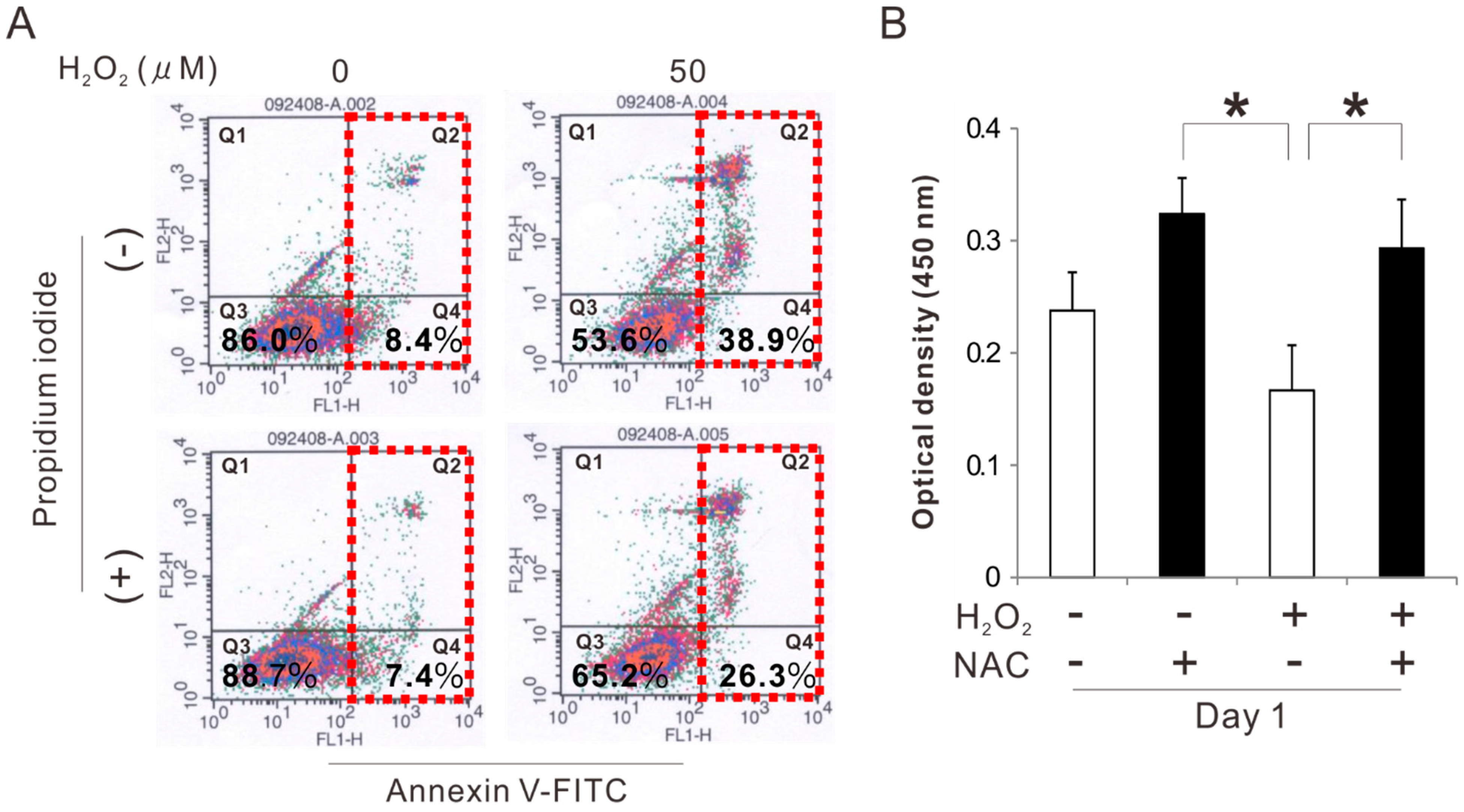
2.2. Effects of Preconditioning Osteoblast-Like Cells with NAC on Cell Viability under Oxidative Stress
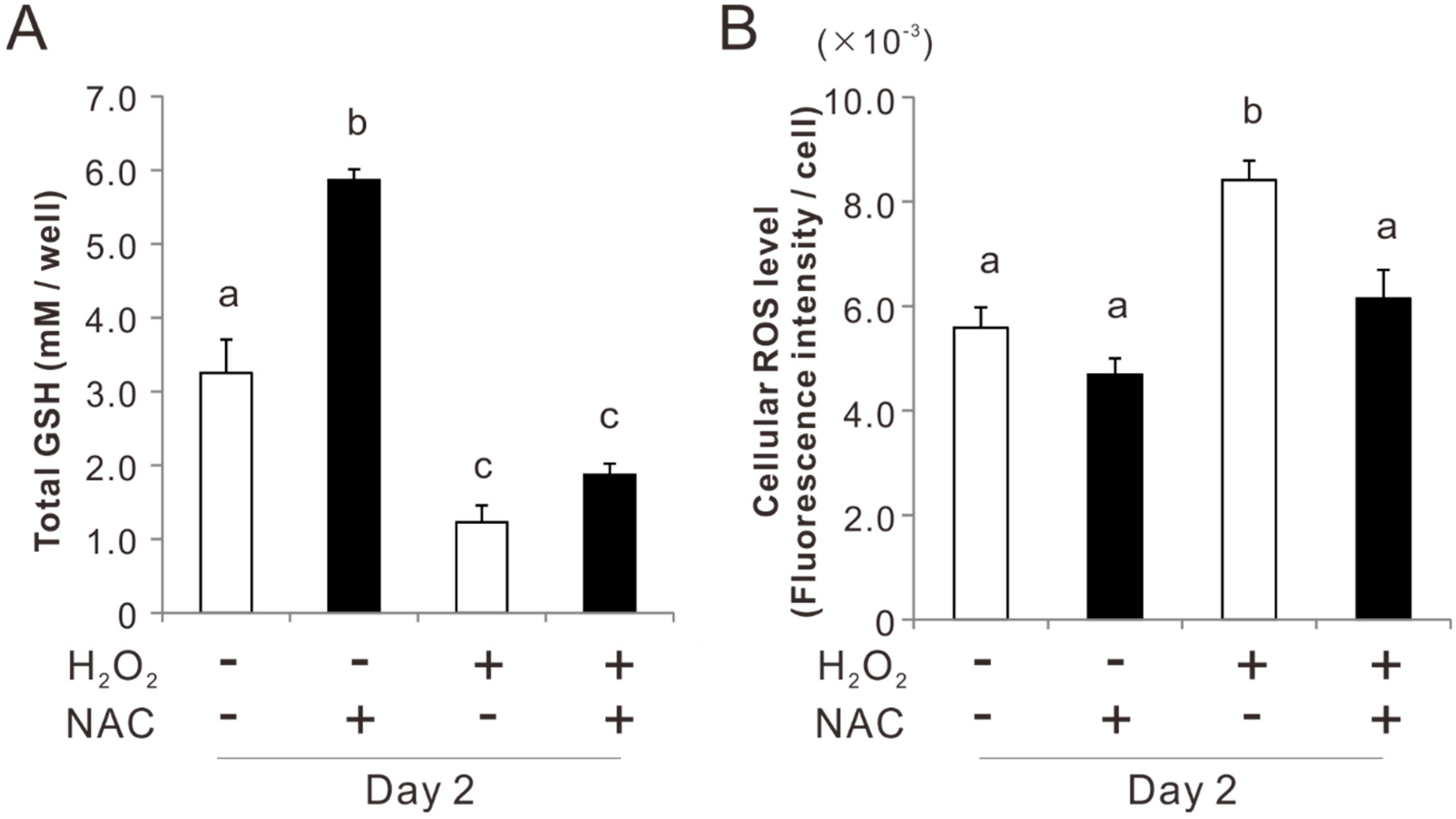
2.3. Effects of Preconditioning Osteoblast-Like Cells with NAC on Cellular Redox Balance under Oxidative Stress
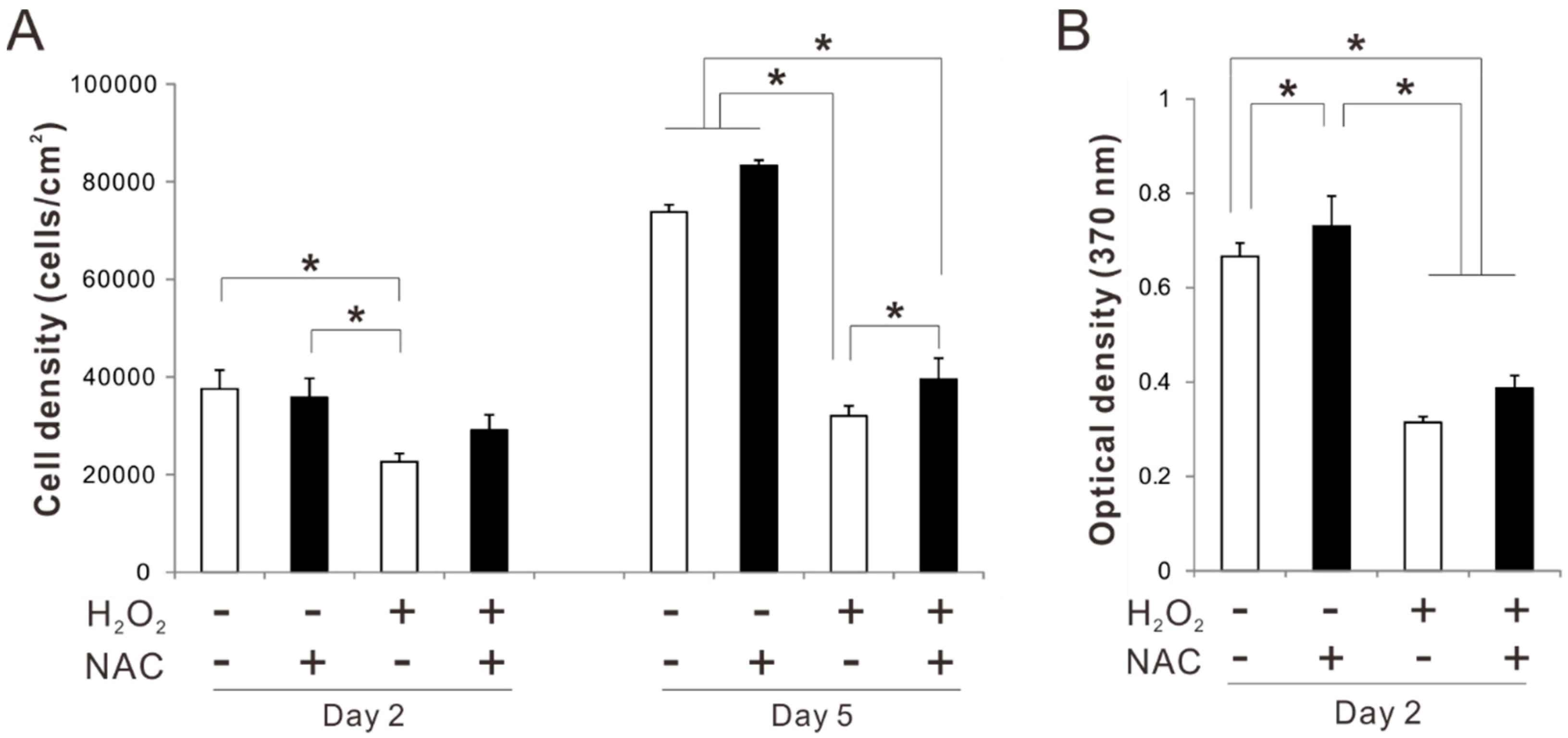
2.4. Effects of Preconditioning Osteoblast-Like Cells with NAC on Proliferation under Oxidative Stress
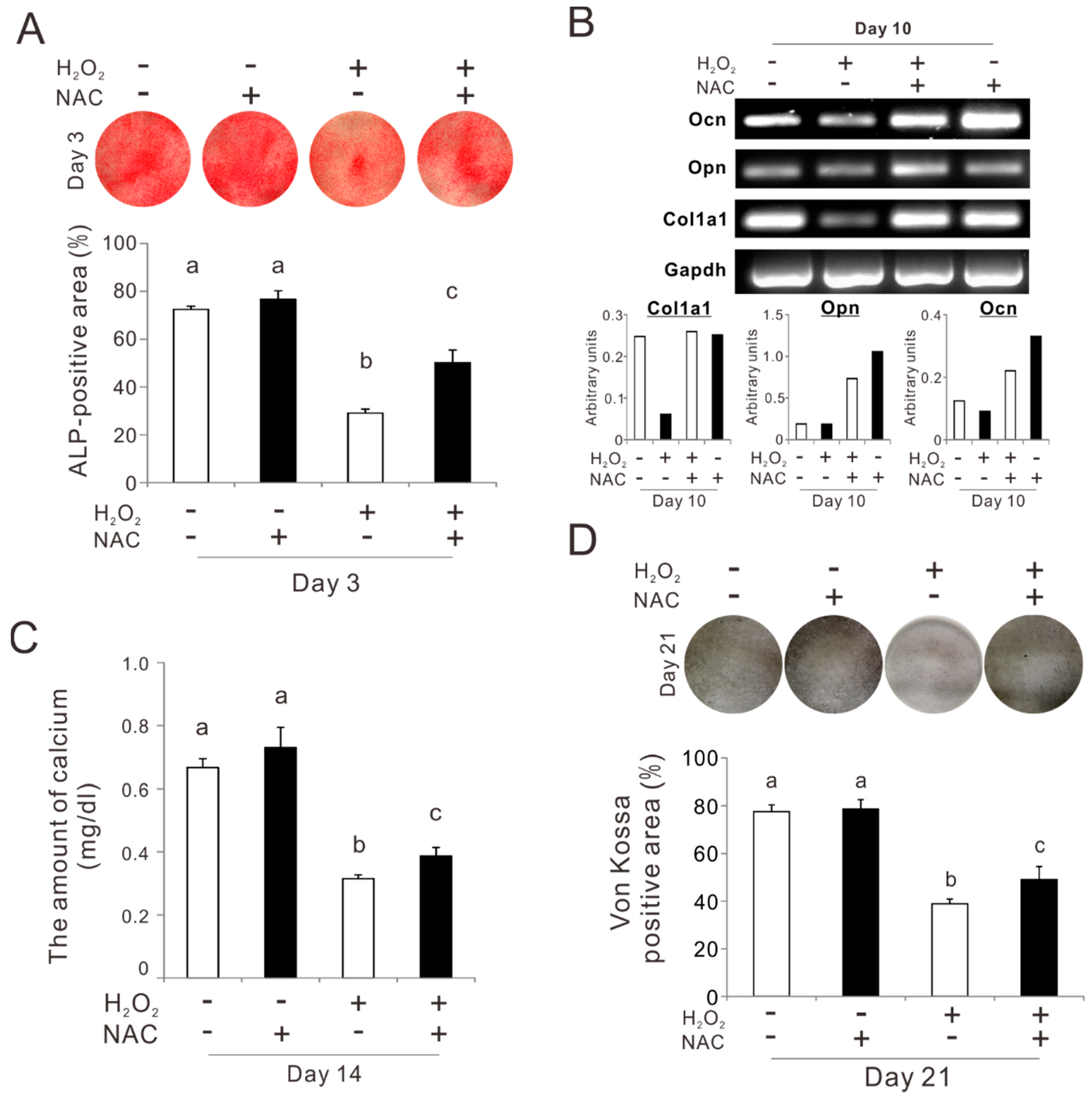
2.5. Effects of Preconditioning Osteoblast-Like Cells with NAC on Osteogenic Differentiation under Oxidative Stress
2.6. Effects of Preconditioning Osteoblast-Like Cells with NAC on Osteogenic Differentiation
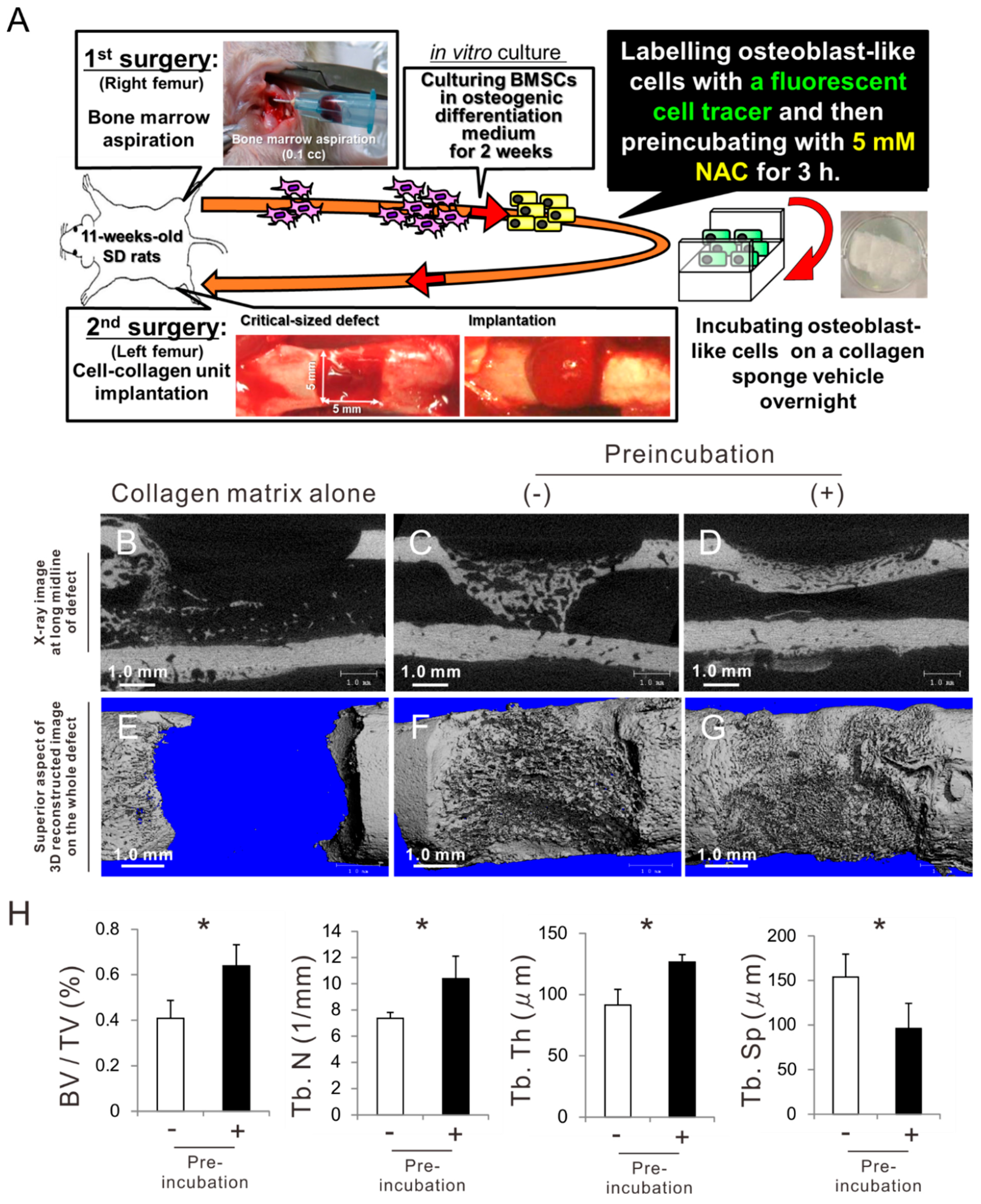
2.7. Effects of Preconditioning Osteoblast-Like Cells with NAC on Bone Regeneration in Autologous Local Cell Transplantation
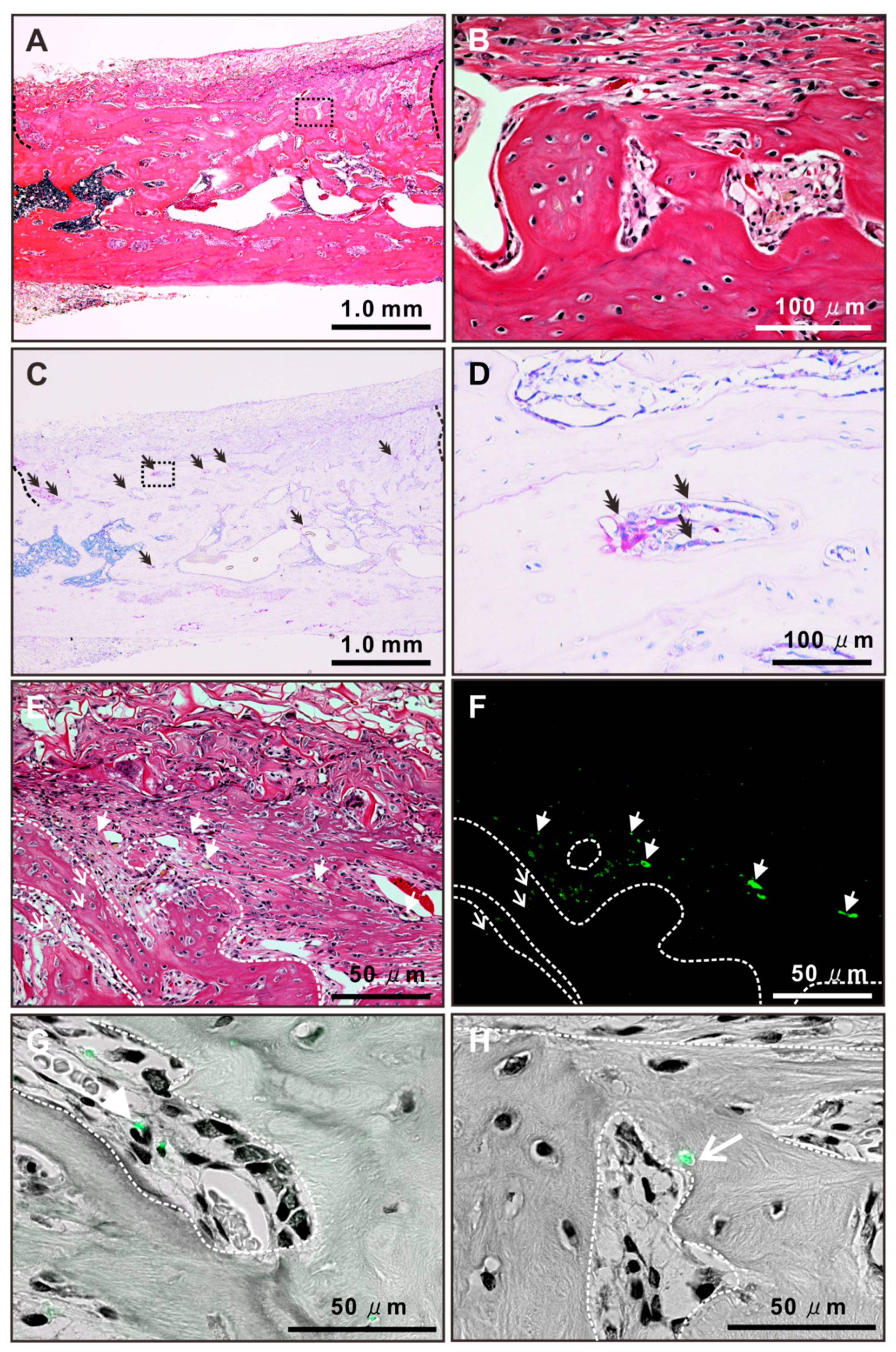
2.8. Histological Features of Newly Formed Bone Tissue after Autologous Local Transplantation of Osteoblast-Like Cells with NAC
3. Discussion
4. Material and Methods
4.1. Reagent Preparation and Application
4.2. Osteoblastic Cell Culture
4.3. Cell Attachment, Cytomorphometry, and Proliferation Assay
4.4. Flow Cytometry for Apoptosis Detection
4.5. GSH Detection Assay
4.6. Intracellular ROS Level
4.7. Gene Expression Analysis
4.8. Alkaline Phosphatase Activity
4.9. Matrix Mineralization Assay
4.10. Autologous Local Transplantation in A Massive Rat Femur Bone Defect
4.11. Micro-Computed Tomography Analysis
4.12. Histological Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boukhechba, F.; Balaguer, T.; Bouvet-Gerbettaz, S.; Michiels, J.F.; Bouler, J.M.; Carle, G.F.; Scimeca, J.C.; Rochet, N. Fate of bone marrow stromal cells in a syngenic model of bone formation. Tissue Eng. Part A 2011, 17, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry--Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Ahn, S.Y.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatric Res. 2018, 83, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Togel, F.; Westenfelder, C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev. Dyn.: Off. Publ. Am. Assoc. Anat. 2007, 236, 3321–3331. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef] [Green Version]
- Amin, H.D.; Brady, M.A.; St-Pierre, J.P.; Stevens, M.M.; Overby, D.R.; Ethier, C.R. Stimulation of chondrogenic differentiation of adult human bone marrow-derived stromal cells by a moderate-strength static magnetic field. Tissue Eng. Part A 2014, 20, 1612–1620. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Satomura, K.; Gotoh, Y.; Kitaoka, E.; Tobiume, S.; Kume, K.; Nagayama, M. Bone formation by transplanted human osteoblasts cultured within collagen sponge with dexamethasone in vitro. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 857–867. [Google Scholar] [CrossRef]
- Olender, E.; Brubaker, S.; Uhrynowska-Tyszkiewicz, I.; Wojtowicz, A.; Kaminski, A. Autologous osteoblast transplantation, an innovative method of bone defect treatment: Role of a tissue and cell bank in the process. Transplant. Proc. 2014, 46, 2867–2872. [Google Scholar] [CrossRef]
- Cahill, R.A.; Jones, O.Y.; Klemperer, M.; Steele, A.; Mueller, T.O.; el-Badri, N.; Chang, Y.; Good, R.A. Replacement of recipient stromal/mesenchymal cells after bone marrow transplantation using bone fragments and cultured osteoblast-like cells. Biol. Blood Marrow Transplant.: J. Am. Soc. Blood Marrow Transplant. 2004, 10, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Shin, Y.W.; Yang, K.H.; Kim, S.B.; Yoo, M.J.; Han, S.K.; Im, S.A.; Won, Y.D.; Sung, Y.B.; Jeon, T.S.; et al. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(Ossron) injection to treat fractures. BMC Musculoskelet. Disord. 2009, 10, 20. [Google Scholar] [CrossRef]
- Mangano, F.G.; Colombo, M.; Veronesi, G.; Caprioglio, A.; Mangano, C. Mesenchymal stem cells in maxillary sinus augmentation: A systematic review with meta-analysis. World J. Stem Cells 2015, 7, 976–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Blanc, K.; Gotherstrom, C.; Ringden, O.; Hassan, M.; McMahon, R.; Horwitz, E.; Anneren, G.; Axelsson, O.; Nunn, J.; Ewald, U.; et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005, 79, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.E.; Gierloff, M.; Hedderich, J.; Acil, Y.; Wiltfang, J.; Terheyden, H. Survival of transplanted rat bone marrow-derived osteogenic stem cells in vivo. Tissue Eng. Part A 2011, 17, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Inayathullah, M.; Lijkwan, M.A.; Zhao, X.; Sun, W.; Park, S.; Hong, W.X.; Parekh, M.B.; Malkovskiy, A.V.; Lau, E.; et al. Prolonged survival of transplanted stem cells after ischaemic injury via the slow release of pro-survival peptides from a collagen matrix. Nat. Biomed. Eng. 2018, 2, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, N.; Hogendoorn, S.; Zurcher, L.; Garavaglia, G.; Yang, S.; Konig, S.; Laumonier, T.; Menetrey, J. Autologous transplantation of porcine myogenic precursor cells in skeletal muscle. Neuromuscul. Disord. Nmd 2005, 15, 237–244. [Google Scholar] [CrossRef]
- Pieri, F.; Lucarelli, E.; Corinaldesi, G.; Aldini, N.N.; Fini, M.; Parrilli, A.; Dozza, B.; Donati, D.; Marchetti, C. Dose-dependent effect of adipose-derived adult stem cells on vertical bone regeneration in rabbit calvarium. Biomaterials 2010, 31, 3527–3535. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef]
- Okabe, Y.T.; Kondo, T.; Mishima, K.; Hayase, Y.; Kato, K.; Mizuno, M.; Ishiguro, N.; Kitoh, H. Biodistribution of locally or systemically transplantedosteoblast-like cells. Bone Jt. Res. 2014, 3, 76–81. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? CellsTissuesOrgans 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef]
- Chen, C.T.; Shih, Y.R.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Pasini, A.; Rigo, A.; Scupoli, M.T.; Tecchio, C.; Malpeli, G.; Scarpa, A.; Dazzi, F.; Pizzolo, G.; Vinante, F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: Inducing cell expansion and reversibly preventing multilineage differentiation. Blood 2005, 106, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef] [PubMed]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci.: Cmls 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cao, Y.; Wang, Y.; Li, W.; Liu, X.; Lv, Y.; Li, X.; Mi, J. Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics 2017, 7, 1036–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftos, J.E.; Whillier, S.; Chapman, B.E.; Kuchel, P.W. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int. J. Biochem. Cell Biol. 2007, 39, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Whillier, S.; Raftos, J.E.; Chapman, B.; Kuchel, P.W. Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep.: Commun. Free Radic. Res. 2009, 14, 115–124. [Google Scholar] [CrossRef]
- Yamada, M.; Ishihara, K.; Ogawa, T.; Sakurai, K. The inhibition of infection by wound pathogens on scaffold in tissue-forming process using N-acetyl cysteine. Biomaterials 2011, 32, 8474–8485. [Google Scholar] [CrossRef] [Green Version]
- Tsukimura, N.; Yamada, M.; Aita, H.; Hori, N.; Yoshino, F.; Chang-Il Lee, M.; Kimoto, K.; Jewett, A.; Ogawa, T. N-acetyl cysteine (NAC)-mediated detoxification and functionalization of poly(methyl methacrylate) bone cement. Biomaterials 2009, 30, 3378–3389. [Google Scholar] [CrossRef]
- Yamada, M.; Ueno, T.; Minamikawa, H.; Sato, N.; Iwasa, F.; Hori, N.; Ogawa, T. N-acetyl cysteine alleviates cytotoxicity of bone substitute. J. Dent. Res. 2010, 89, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tsukimura, N.; Ikeda, T.; Sugita, Y.; Att, W.; Kojima, N.; Kubo, K.; Ueno, T.; Sakurai, K.; Ogawa, T. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials 2013, 34, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Lee, S.H.; Kwak, H.B.; Lee, Z.H.; Seo, S.B.; Woo, K.M.; Ryoo, H.M.; Kim, G.S.; Baek, J.H. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J. Cell. Biochem. 2008, 103, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Yamada, M.; Niibe, K.; Zhang, M.; Kondo, T.; Ishibashi, M.; Egusa, H. Preconditioning of bone marrow-derived mesenchymal stem cells with N-acetyl-L-cysteine enhances bone regeneration via reinforced resistance to oxidative stress. Biomaterials 2018, 185, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kojima, N.; Paranjpe, A.; Att, W.; Aita, H.; Jewett, A.; Ogawa, T. N-acetyl cysteine (NAC)-assisted detoxification of PMMA resin. J. Dent. Res. 2008, 87, 372–377. [Google Scholar] [CrossRef]
- Yamada, M.; Minamikawa, H.; Ueno, T.; Sakurai, K.; Ogawa, T. N-acetyl cysteine improves affinity of beta-tricalcium phosphate granules for cultured osteoblast-like cells. J. Biomater. Appl. 2012, 27, 27–36. [Google Scholar] [CrossRef]
- Yamada, M.; Kubo, K.; Ueno, T.; Iwasa, F.; Att, W.; Hori, N.; Ogawa, T. Alleviation of commercial collagen sponge- and membrane-induced apoptosis and dysfunction in cultured osteoblasts by an amino acid derivative. Int. J. Oral Maxillofac. Implant. 2010, 25, 939–946. [Google Scholar]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef]
- Peter, S.J.; Liang, C.R.; Kim, D.J.; Widmer, M.S.; Mikos, A.G. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J. Cell. Biochem. 1998, 71, 55–62. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Mabuchi, Y.; Morikawa, S.; Harada, S.; Niibe, K.; Suzuki, S.; Renault-Mihara, F.; Houlihan, D.D.; Akazawa, C.; Okano, H.; Matsuzaki, Y. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Rep. 2013, 1, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Singgih, A.; Kapoor, A.; Alarcon, C.M.; Baylink, D.J.; Mohan, S. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. J. Biol. Chem. 2007, 282, 22052–22061. [Google Scholar] [CrossRef] [PubMed]
- Sant, K.E.; Hansen, J.M.; Williams, L.M.; Tran, N.L.; Goldstone, J.V.; Stegeman, J.J.; Hahn, M.E.; Timme-Laragy, A. The role of Nrf1 and Nrf2 in the regulation of glutathione and redox dynamics in the developing zebrafish embryo. Redox Biol. 2017, 13, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hinoi, E.; Fujimori, S.; Wang, L.; Hojo, H.; Uno, K.; Yoneda, Y. Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J. Biol. Chem. 2006, 281, 18015–18024. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Dehnade, F.; Zafarullah, M. Thiol antioxidant, N-acetylcysteine, activates extracellular signal-regulated kinase signaling pathway in articular chondrocytes. Biochem. Biophys. Res. Commun. 2000, 275, 789–794. [Google Scholar] [CrossRef]
- Chan, E.D.; Riches, D.W.; White, C.W. Redox paradox: Effect of N-acetylcysteine and serum on oxidation reduction-sensitive mitogen-activated protein kinase signaling pathways. Am. J. Respir. Cell Mol. Biol. 2001, 24, 627–632. [Google Scholar] [CrossRef]
- Ezerina, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459 e4. [Google Scholar] [CrossRef]
- Imai, T.; Ii, H.; Yaegaki, K.; Murata, T.; Sato, T.; Kamoda, T. Oral malodorous compound inhibits osteoblast proliferation. J. Periodontol. 2009, 80, 2028–2034. [Google Scholar] [CrossRef]
- Maniatopoulos, C.; Sodek, J.; Melcher, A.H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988, 254, 317–330. [Google Scholar] [CrossRef]
- Almazrooa, S.A.; Noonan, V.; Woo, S.B. Resorbable collagen membranes: Histopathologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 236–240. [Google Scholar] [CrossRef]
- Fricain, J.C.; Schlaubitz, S.; Le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A nano-hydroxyapatite--pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, M.; Watanabe, J.; Ueno, T.; Ogawa, T.; Egusa, H. Cytoprotective Preconditioning of Osteoblast-Like Cells with N-Acetyl-L-Cysteine for Bone Regeneration in Cell Therapy. Int. J. Mol. Sci. 2019, 20, 5199. https://doi.org/10.3390/ijms20205199
Yamada M, Watanabe J, Ueno T, Ogawa T, Egusa H. Cytoprotective Preconditioning of Osteoblast-Like Cells with N-Acetyl-L-Cysteine for Bone Regeneration in Cell Therapy. International Journal of Molecular Sciences. 2019; 20(20):5199. https://doi.org/10.3390/ijms20205199
Chicago/Turabian StyleYamada, Masahiro, Jun Watanabe, Takeshi Ueno, Takahiro Ogawa, and Hiroshi Egusa. 2019. "Cytoprotective Preconditioning of Osteoblast-Like Cells with N-Acetyl-L-Cysteine for Bone Regeneration in Cell Therapy" International Journal of Molecular Sciences 20, no. 20: 5199. https://doi.org/10.3390/ijms20205199





