Exposure to the RXR Agonist SR11237 in Early Life Causes Disturbed Skeletal Morphogenesis in a Rat Model
Abstract
1. Introduction
2. Results
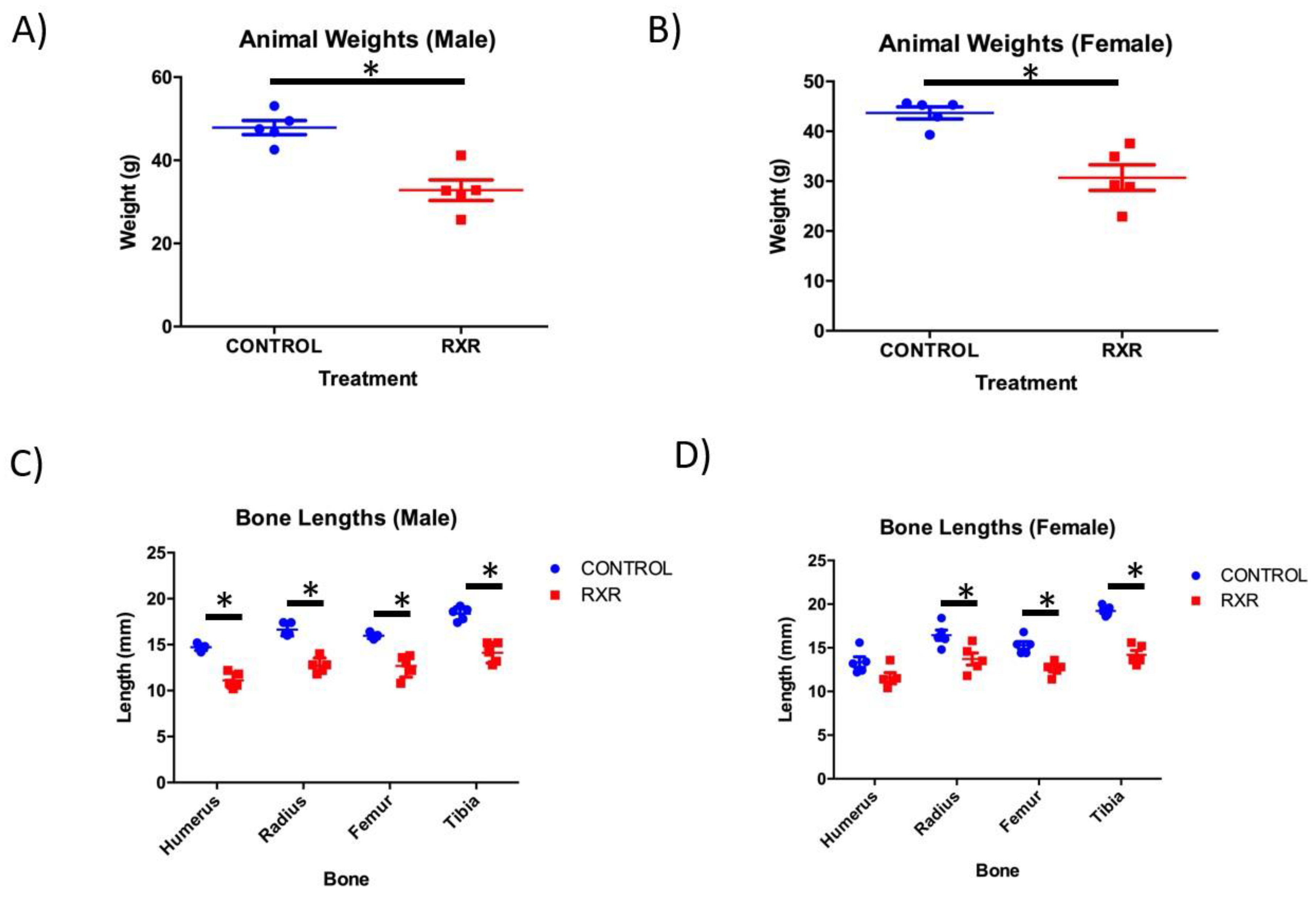
2.1. RXR Activation Results in Reduced Body Weight and Bone Length
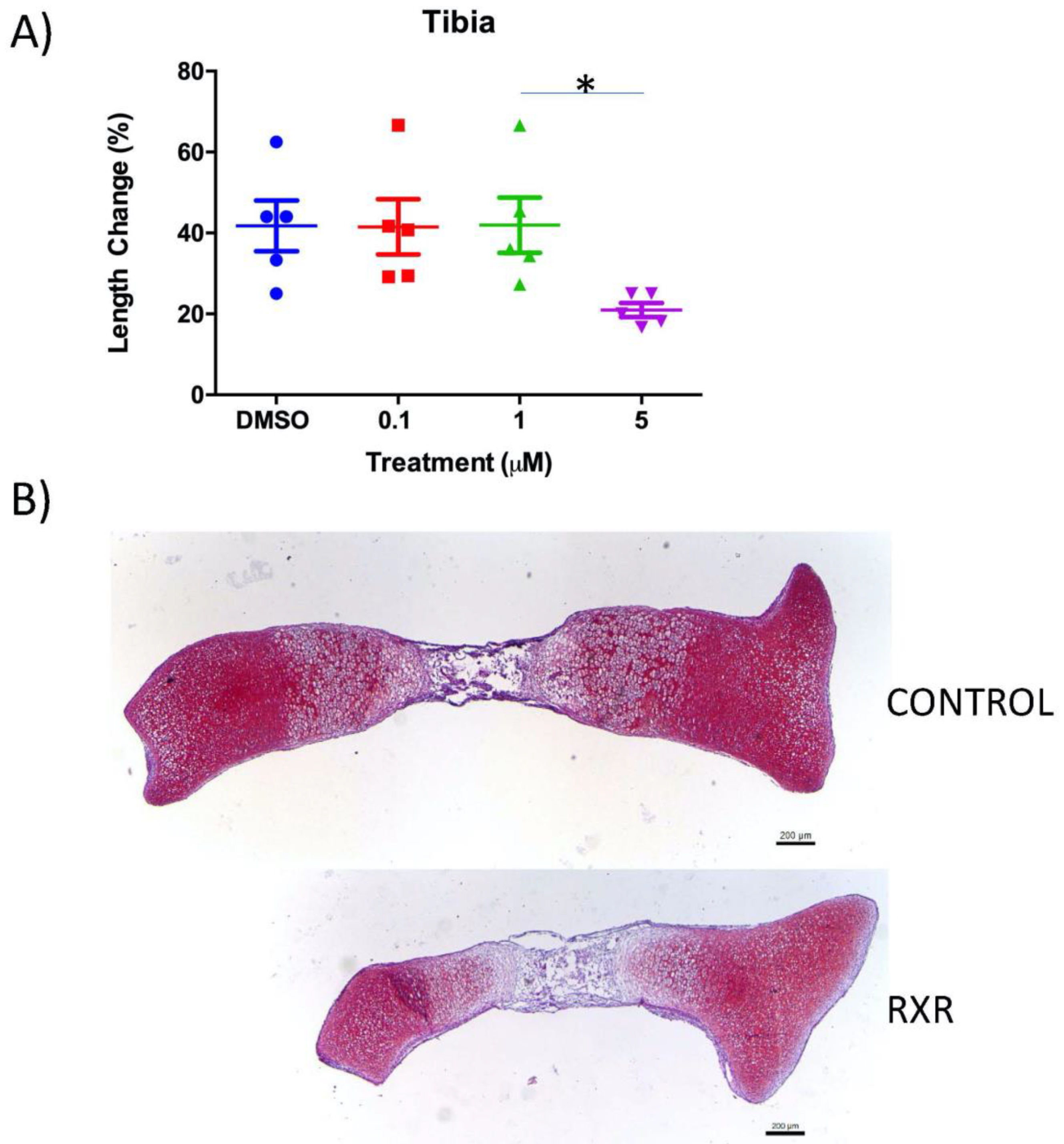
2.2. The RXR Agonist SR11237 Decreases Total Length Change in Cultured Mouse Tibiae
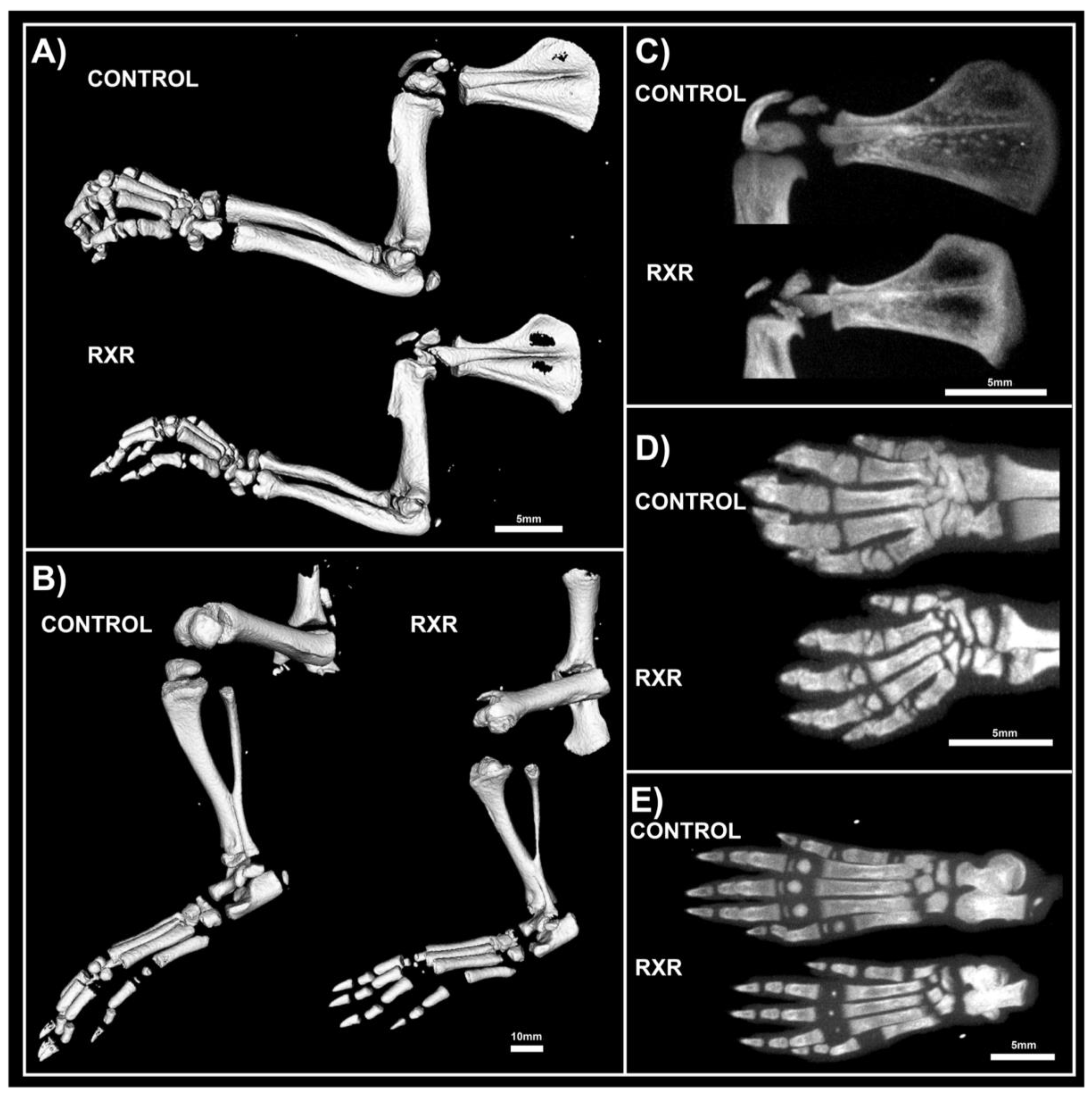
2.3. MicroCT Analyses Shos Abnormal Bone Morphology in RXR Agonist-Treated Rats
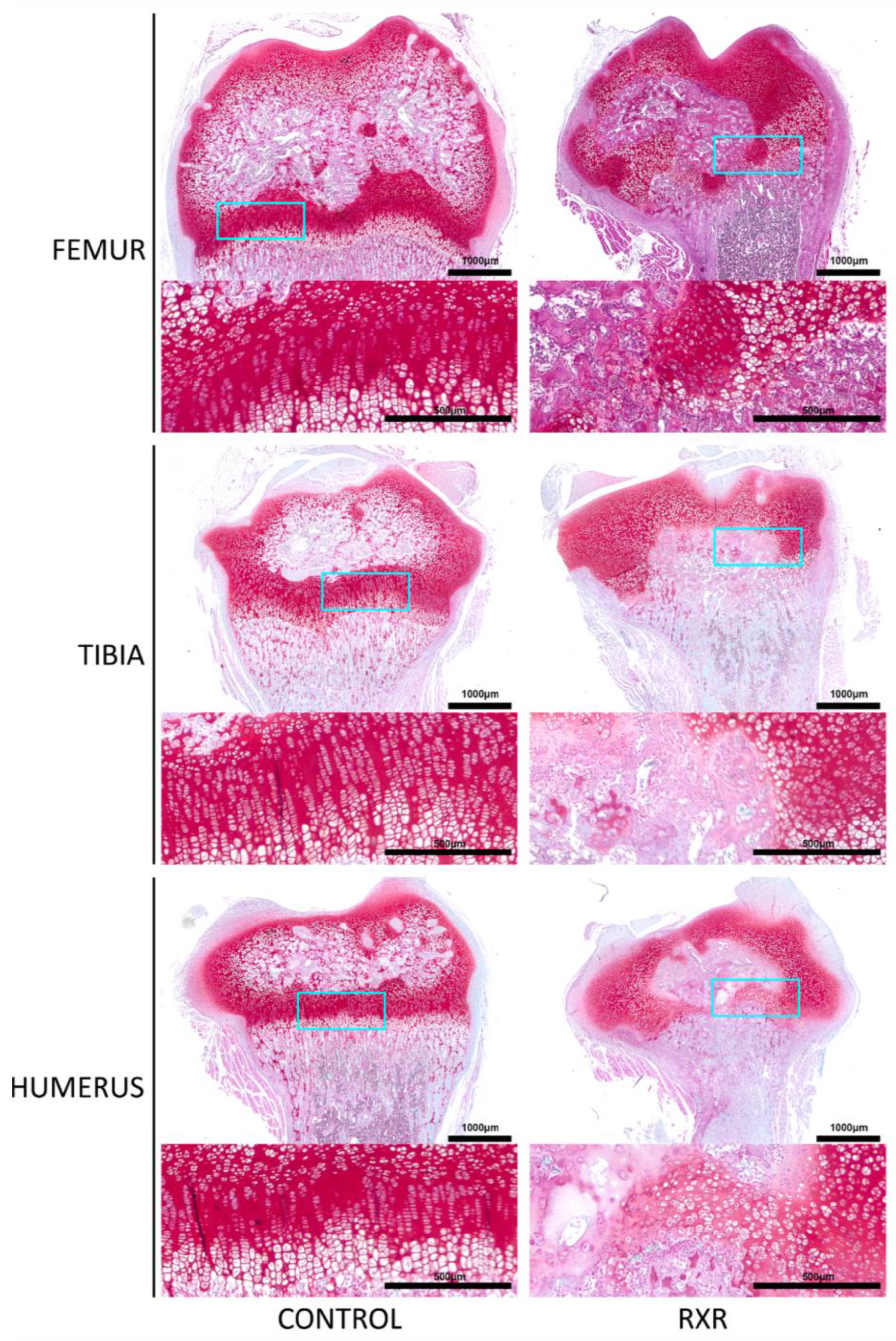
2.4. Disrupted Growth Plate Morphology in P16 Male Rat Long Bones
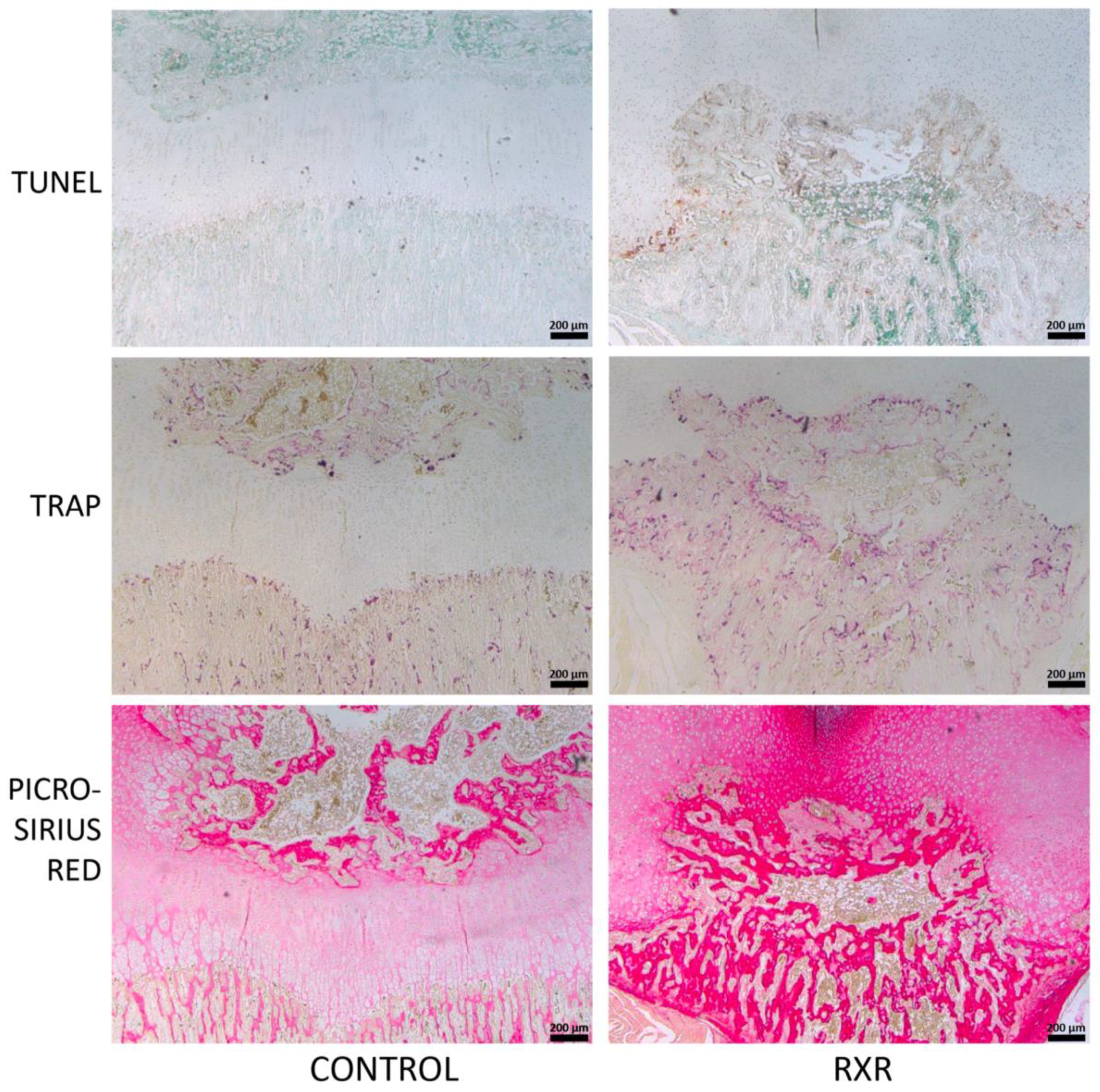
2.5. Immunohistochemistry and Histological Staining of P16 Rat Tibial Sections
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. SR11237 Injections
4.3. Tibia Organ Cultures
4.4. Micro-Computed Tomography (MicroCT)
4.5. Histology and Immunohistochemistry
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EO | Endochondral ossification |
| RXR | Retinoid X receptor |
| SOX9 | Sex-determining region-box 9 |
| RUNX2 | Runt-related transcription factor 2 |
| MEF2C | Myocyte enhancer factor-2c |
| PPAR | Peroxisome proliferator-activated receptor |
| FXR | Farnesoid X receptor |
| LXR | Liver X receptor |
| RAR | Retinoic acid receptor |
| VDR | Vitamin D receptor |
| TR | Thyroid receptor |
| IP | Intraperitoneal |
| DMSO | Dimethyl sulfoxide |
| GLP | Glucagon-like peptide |
| MEM | Minimum essential medium eagle |
| TRAP | Tartrate-resistant acid phosphatase |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick-end labelling |
| PCNA | Proliferating cell nuclear antigen |
| RANKL | Receptor activator of nuclear factor of kappa-B ligand |
References
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Abad, V.; Meyers, J.L.; Weise, M.; Gafni, R.I.; Barnes, K.M.; Nilsson, O.; Bacher, J.D.; Baron, J. The role of the resting zone in growth plate chondrogenesis. Endocrinology 2002, 143, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L.; Oh, S.; Sung, Y.; Dasari, R.R.; Kirschner, M.W.; Tabin, C.J. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 2013, 495, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Bonnamy, J.P.; Owen, M.J.; Ducy, P.; Karsenty, G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001, 15, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Ueta, C.; Iwamoto, M.; Kanatani, N.; Yoshida, C.; Liu, Y.; Enomoto-Iwamoto, M.; Ohmori, T.; Enomoto, H.; Nakata, K.; Takada, K.; et al. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J. Cell Biol. 2001, 153, 87–100. [Google Scholar] [CrossRef]
- Yoshida, C.A.; Yamamoto, H.; Fujita, T.; Furuichi, T.; Ito, K.; Inoue, K.; Yamana, K.; Zanma, A.; Takada, K.; Ito, Y.; et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004, 18, 952–963. [Google Scholar] [CrossRef]
- Arnold, M.A.; Kim, Y.; Czubryt, M.P.; Phan, D.; McAnally, J.; Qi, X.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 2007, 12, 377–389. [Google Scholar] [CrossRef]
- Pest, M.A.; Beier, F. Developmental biology: Is there such a thing as a cartilage-specific knockout mouse? Nat. Rev. Rheumatol. 2014, 10, 702–704. [Google Scholar] [CrossRef]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S.E. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. 2014, 111, 12097–12102. [Google Scholar] [CrossRef]
- Yeung Tsang, K.; Wa Tsang, S.; Chan, D.; Cheah, K.S.E. The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res. C 2014, 102, 52–73. [Google Scholar] [CrossRef]
- Sun, M.M.-G.; Beier, F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res. C 2014, 102, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Nilsson, O.; Baron, J. Recent research on the growth plate: Recent insights into the regulation of the growth plate. J. Mol. Endocrinol. 2014, 53, T1–T9. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.I.; Xia, Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta 2012, 1821, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.; Bourguet, W.; Gronemeyer, H.; de Lera, A.R. Modulation of RXR function through ligand design. Biochim. Biophys. Acta 2012, 1821, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; Chambon, P.; Eichele, G.; Evans, R.M.; Lazar, M.A.; Leid, M.; De Lera, A.R.; Lotan, R.; Mangelsdorf, D.J.; Gronemeyer, H. International Union of Pharmacology. LXIII. Retinoid X Receptors. Pharmacol. Rev. 2006, 58, 760–772. [Google Scholar] [CrossRef]
- Kojetin, D.J.; Matta-Camacho, E.; Hughes, T.S.; Srinivasan, S.; Nwachukwu, J.C.; Cavett, V.; Nowak, J.; Chalmers, M.J.; Marciano, D.P.; Kamenecka, T.M.; et al. Structural mechanism for signal transduction in RXR nuclear receptor heterodimers. Nat. Commun. 2015, 6, 8013. [Google Scholar] [CrossRef] [PubMed]
- Sucov, H.M.; Izpisúa-Belmonte, J.C.; Gañan, Y.; Evans, R.M. Mouse embryos lacking RXR alpha are resistant to retinoic-acid-induced limb defects. Development 1995, 121, 3997–4003. [Google Scholar]
- Kochhar, D.M. Limb development in mouse embryos. I. Analysis of teratogenic effects of retinoic acid. Teratology 1973, 7, 289–295. [Google Scholar] [CrossRef]
- Benoit, G.; Altucci, L.; Flexor, M.; Ruchaud, S.; Lillehaug, J.; Raffelsberger, W.; Gronemeyer, H.; Lanotte, M. RAR-independent RXR signaling induces t(15;17) leukemia cell maturation. EMBO J. 1999, 18, 7011–7018. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Filipiak, J.; Nikodem, A.; Merwid-Ląd, A.; Pieśniewska, M.; Fereniec-Gołębiewska, L.; Kwiatkowska, J.; Szeląg, A. The influence of bexarotene, a selective agonist of the retinoid receptor X (RXR), and tazarotene, a selective agonist of the retinoid acid receptor (RAR), on bone metabolism in rats. Adv. Med. Sci. 2015, 61, 85–89. [Google Scholar] [CrossRef]
- Menéndez-Gutiérrez, M.P.; Rőszer, T.; Fuentes, L.; Núñez, V.; Escolano, A.; Redondo, J.M.; De Clerck, N.; Metzger, D.; Valledor, A.F.; Ricote, M. Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling. J. Clin. Invest. 2015, 125, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Stockmans, I.; Torrekens, S.; Van Looveren, R.; Maes, C.; Carmeliet, P.; Bouillon, R.; Carmeliet, G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J. Clin. Invest. 2006, 116, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, N.; Yamamoto, Y.; Yoshizawa, T.; Sekine, K.; Uematsu, Y.; Murayama, H.; Nagai, Y.; Krezel, W.; Chambon, P.; Matsumoto, T.; et al. Aberrant growth plate development in VDR/RXR gamma double null mutant mice. Endocrinology 2001, 142, 5332–5341. [Google Scholar] [CrossRef][Green Version]
- Jiménez, M.J.; Balbín, M.; Alvarez, J.; Komori, T.; Bianco, P.; Holmbeck, K.; Birkedal-Hansen, H.; López, J.M.; López-Otín, C. A regulatory cascade involving retinoic acid, Cbfa1, and matrix metalloproteinases is coupled to the development of a process of perichondrial invasion and osteogenic differentiation during bone formation. J. Cell Biol. 2001, 155, 1333–1344. [Google Scholar] [CrossRef]
- Menéndez-Gutiérrez, M.P.; Ricote, M. The multi-faceted role of retinoid X receptor in bone remodeling. Cell. Mol. Life Sci. 2017, 74, 2135–2149. [Google Scholar] [CrossRef]
- Stoffers, D.A.; Desai, B.M.; DeLeon, D.D.; Simmons, R.A. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes 2003, 52, 734–740. [Google Scholar] [CrossRef][Green Version]
- Standeven, A.M.; Escobar, M.; Beard, R.L.; Yuan, Y.D.; Chandraratna, R.A. Mitogenic effect of retinoid X receptor agonists in rat liver. Biochem. Pharmacol. 1997, 54, 517–524. [Google Scholar] [CrossRef]
- Agoston, H.; Khan, S.; James, C.G.; Gillespie, J.R.; Serra, R.; Stanton, L.-A.; Beier, F. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Dev. Biol. 2007, 7, 18. [Google Scholar] [CrossRef]
- Pest, M.A.; Russell, B.A.; Zhang, Y.-W.; Jeong, J.-W.; Beier, F. Disturbed cartilage and joint homeostasis resulting from a loss of mitogen-inducible gene 6 in a mouse model of joint dysfunction. Arthritis Rheumatol. 2014, 66, 2816–2827. [Google Scholar] [CrossRef]
- Gillespie, J.R.; Ulici, V.; Dupuis, H.; Higgs, A.; Dimattia, A.; Patel, S.; Woodgett, J.R.; Beier, F. Deletion of glycogen synthase kinase-3β in cartilage results in up-regulation of glycogen synthase kinase-3α protein expression. Endocrinology 2011, 152, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Solomon, L.A.; Li, J.R.; Bérubé, N.G.; Beier, F. Loss of ATRX in chondrocytes has minimal effects on skeletal development. PLoS ONE 2009, 4, e7106. [Google Scholar] [CrossRef] [PubMed]
- Ulici, V.; Hoenselaar, K.D.; Agoston, H.; McErlain, D.D.; Umoh, J.; Chakrabarti, S.; Holdsworth, D.W.; Beier, F. The role of Akt1 in terminal stages of endochondral bone formation: Angiogenesis and ossification. Bone 2009, 45, 1133–1145. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuis, H.; Pest, M.A.; Hadzic, E.; Vo, T.X.; Hardy, D.B.; Beier, F. Exposure to the RXR Agonist SR11237 in Early Life Causes Disturbed Skeletal Morphogenesis in a Rat Model. Int. J. Mol. Sci. 2019, 20, 5198. https://doi.org/10.3390/ijms20205198
Dupuis H, Pest MA, Hadzic E, Vo TX, Hardy DB, Beier F. Exposure to the RXR Agonist SR11237 in Early Life Causes Disturbed Skeletal Morphogenesis in a Rat Model. International Journal of Molecular Sciences. 2019; 20(20):5198. https://doi.org/10.3390/ijms20205198
Chicago/Turabian StyleDupuis, Holly, Michael Andrew Pest, Ermina Hadzic, Thin Xuan Vo, Daniel B. Hardy, and Frank Beier. 2019. "Exposure to the RXR Agonist SR11237 in Early Life Causes Disturbed Skeletal Morphogenesis in a Rat Model" International Journal of Molecular Sciences 20, no. 20: 5198. https://doi.org/10.3390/ijms20205198
APA StyleDupuis, H., Pest, M. A., Hadzic, E., Vo, T. X., Hardy, D. B., & Beier, F. (2019). Exposure to the RXR Agonist SR11237 in Early Life Causes Disturbed Skeletal Morphogenesis in a Rat Model. International Journal of Molecular Sciences, 20(20), 5198. https://doi.org/10.3390/ijms20205198





