Complementary Immunometabolic Effects of Exercise and PPARβ/δ Agonist in the Context of Diet-Induced Weight Loss in Obese Female Mice
Abstract
:1. Introduction
2. Results
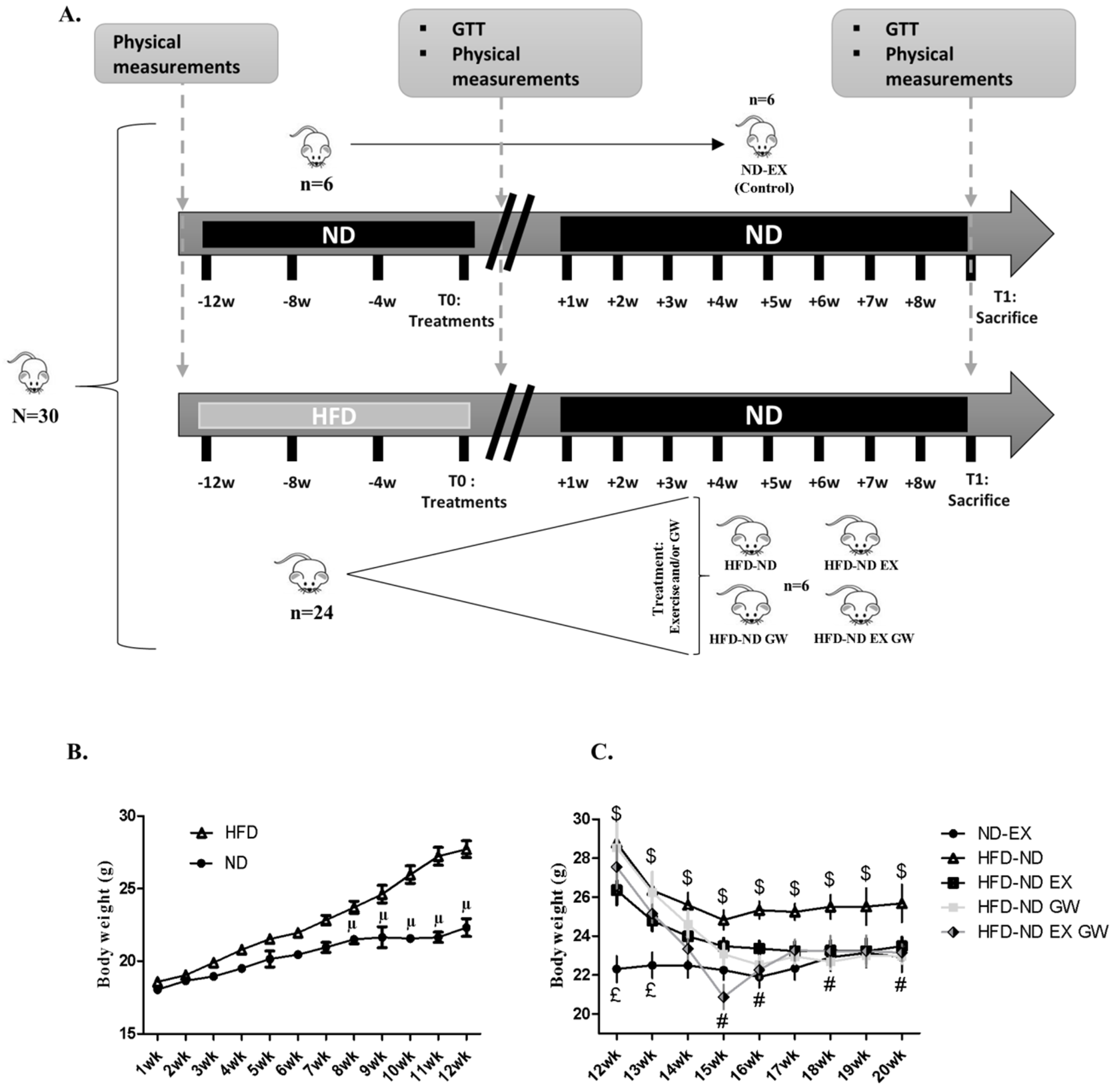
2.1. GW0742 Treatment in Obese Mice Improves Diet-Induced Weight Loss, Visceral Fat Mass Reduction, and Insulin Sensitivity
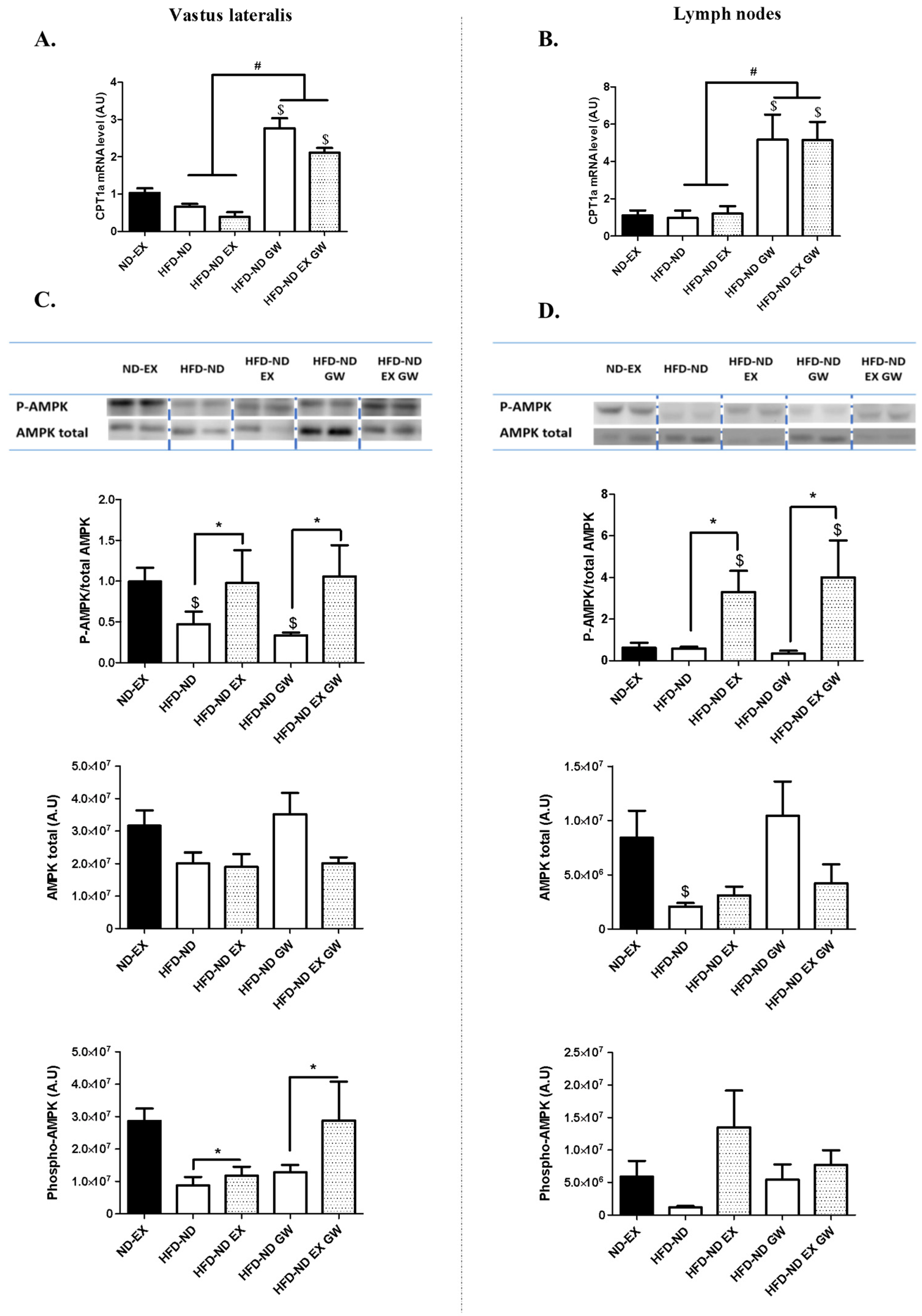
2.2. GW0742 Treatment but not Exercise Increases PPARβ/δ Activity in Both Skeletal Muscle and Lymphoid Tissues, Whereas Exercise but not GW0742 Treatment Improves AMPK Regulation in These Tissues
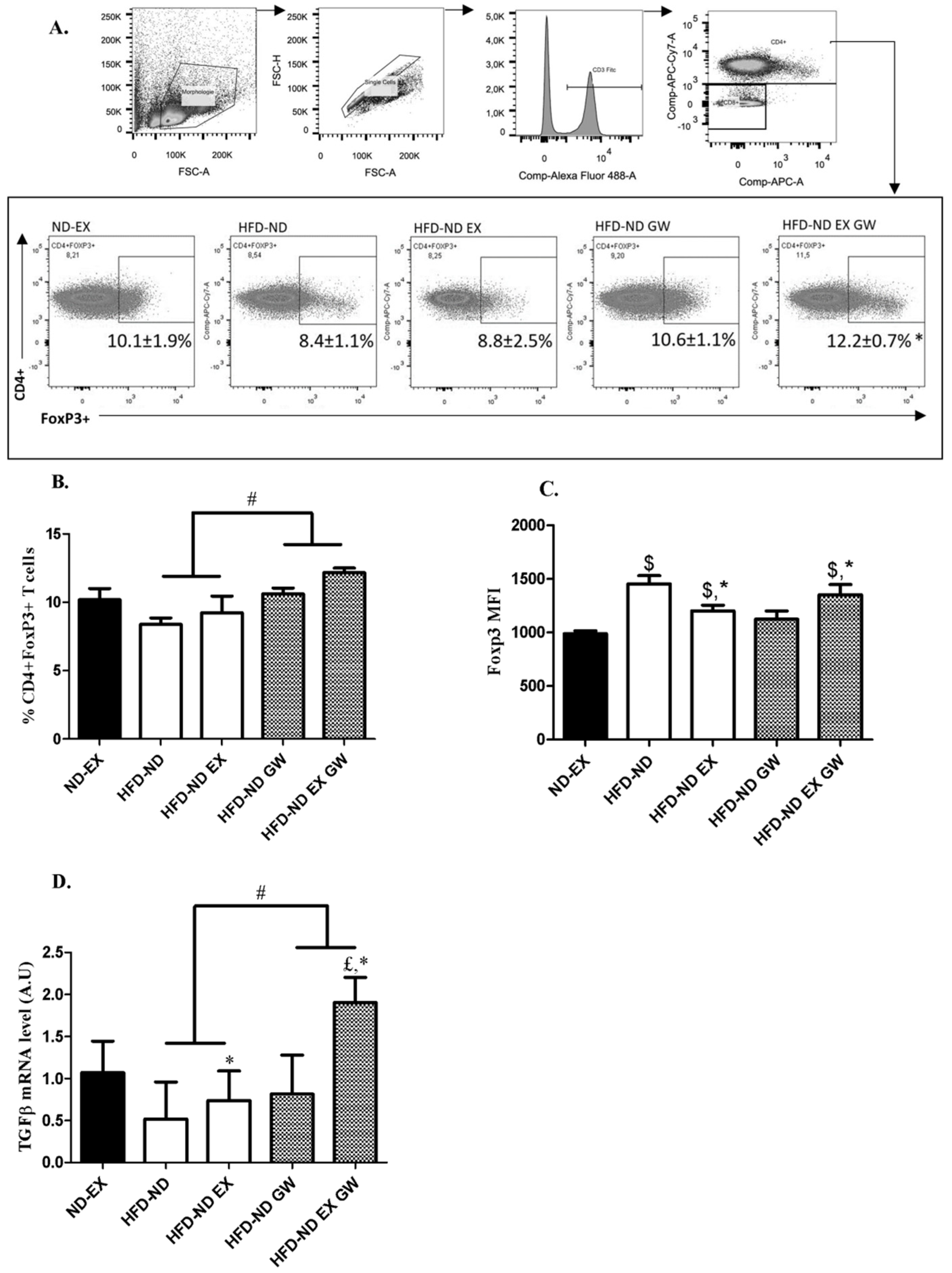
2.3. Prevalence of CD4+Foxp3+ Regulatory T cells increases with GW0742 Treatment in Lymph Nodes
2.4. Exercise Training Reduces Systemic and Skeletal Muscle Inflammation and Impacts Metabolism with Possible Additive Effect of GW0742 Treatment
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Glucose Tolerance Test
4.3. Physical Tests Measurements
4.4. Lymph Node Collection and Cell Preparation
4.5. Flow Cytometry Analysis
4.6. Western Blots Analysis
4.7. RNA Extraction and Quantitative Real-Time PCR
4.8. Quantification of Cytokines with Luminex
4.9. Immunohistological Staining Protocol
4.10. Citrate Synthase Activity
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| BW | Body weight |
| CD3 | Cluster of differentiation 3 |
| CD4 | Cluster of differentiation 4 |
| CPT-1 | Carnitine palmitoyl transferase-1 |
| CS | Citrate synthase |
| DIO | Diet-induced obesity |
| DMSO | Dimethyl sulfoxide |
| EX | Exercise |
| FAO | Fatty acid oxidation |
| FCS | Fœtal calf serum |
| FoxP3 | Forkhead box P3 |
| GTT | Glucose tolerance test |
| HFD | High fat diet |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| IFNγ | Interferon gamma |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IR | Insulin resistance |
| MCP1 | Monocyte chemoattractant protein 1 |
| MFI | Mean fluorescence intensity |
| ND | Normal chow diet |
| PBS | Phosphate-buffered saline |
| PDK4 | Pyruvate dehydrogenase kinase 4 |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| T2DM | Type 2 diabetes mellitus |
| TGF-β | Transforming growth factor beta |
| TLA | Tibialis lateralis anterior |
| Th1 | T helper 1 cells |
| Th2 | T helper 2 cells |
| Tregs | Regulatory T cells |
| Th17 | T helper 17 cells |
References
- Kivimaki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M.; et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120,813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Perrard, X.Y.; Brunner, G.; Lui, H.; Sparks, L.M.; Smith, S.R.; Wang, X.; Shi, Z.Z.; Lewis, D.E.; Wu, H.; et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int. J. Obes. (Lond) 2015, 39, 1607–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, B.H. Mitochondrial deficiency is associated with insulin resistance. Diabetes 2013, 62, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Chin, S.H.; Kahathuduwa, C.N.; Binks, M. Physical activity and obesity: What we know and what we need to know. Obes. Rev. 2016, 17, 1226–1244. [Google Scholar] [CrossRef]
- Cohen, S.; Danzaki, K.; MacIver, N.J. Nutritional effects on T-cell immunometabolism. Eur. J. Immunol. 2017, 47, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Foundations of immunometabolism and implications for metabolic health and disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Iliadis, F.; Angelopoulou, N.; Perrea, D.; Ampatzidis, G.; Liapis, C.D.; Alevizos, M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 837–843. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Handzlik, M.K.; Shaw, A.J.; Dungey, M.; Bishop, N.C.; Gleeson, M. The influence of exercise training status on antigen-stimulated IL-10 production in whole blood culture and numbers of circulating regulatory T cells. Eur. J. Appl. Physiol. 2013, 113, 1839–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, N.; Zhang, H.F.; Wei, Q.; Wang, P.; Guo, W.Y. Expression of CD4+CD25+Foxp3+ regulatory T cells, interleukin 10 and transforming growth factor beta in newly diagnosed type 2 diabetic patients. Exp. Clin. Endocrinol. Diabetes 2018, 126, 96–101. [Google Scholar] [PubMed]
- Han, J.M.; Patterson, S.J.; Speck, M.; Ehses, J.A.; Levings, M.K. Insulin inhibits IL-10-mediated regulatory T cell function: Implications for obesity. J. Immunol. 2014, 192, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Berod, L.; Friedrich, C.; Nandan, A.; Freitag, J.; Hagemann, S.; Harmrolfs, K.; Sandouk, A.; Hesse, C.; Castro, C.N.; Bahre, H.; et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014, 20, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Cluxton, D.; Petrasca, A.; Moran, B.; Fletcher, J.M. Differential regulation of human treg and Th17 cells by fatty acid synthesis and glycolysis. Front. Immunol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef]
- Howie, D.; Cobbold, S.P.; Adams, E.; Ten Bokum, A.; Necula, A.S.; Zhang, W.; Huang, H.; Roberts, D.J.; Thomas, B.; Hester, S.S.; et al. Foxp3 drives oxidative phosphorylation and protection from lipotoxicity. JCI Insight 2017, 2, e89160. [Google Scholar] [CrossRef] [Green Version]
- Norata, G.D.; Caligiuri, G.; Chavakis, T.; Matarese, G.; Netea, M.G.; Nicoletti, A.; O’Neill, L.A.; Marelli–Berg, F.M. The cellular and molecular basis of translational immunometabolism. Immunity 2015, 43, 421–434. [Google Scholar] [CrossRef]
- Neels, J.G.; Grimaldi, P.A. Physiological functions of peroxisome proliferator-activated receptor beta. Physiol. Rev. 2014, 94, 795–858. [Google Scholar] [CrossRef]
- Luquet, S.; Lopez-Soriano, J.; Holst, D.; Fredenrich, A.; Melki, J.; Rassoulzadegan, M.; Grimaldi, P.A. Peroxisome proliferator–activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003, 17, 2299–2301. [Google Scholar] [CrossRef]
- Mothe–Satney, I.; Murdaca, J.; Sibille, B.; Rousseau, A.S.; Squillace, R.; Le Menn, G.; Rekima, A.; Larbret, F.; Pele, J.; Verhasselt, V.; et al. A role for Peroxisome Proliferator-activated receptor beta in T cell development. Sci. Rep. 2016, 6, 34317. [Google Scholar] [CrossRef] [PubMed]
- Kanakasabai, S.; Chearwae, W.; Walline, C.C.; Iams, W.; Adams, S.M.; Bright, J.J. Peroxisome proliferator–activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology 2010, 130, 572–588. [Google Scholar] [CrossRef] [PubMed]
- Mothe–Satney, I.; Piquet, J.; Murdaca, J.; Sibille, B.; Grimaldi, P.A.; Neels, J.G.; Rousseau, A.S. Peroxisome Proliferator Activated Receptor Beta (PPARbeta) activity increases the immune response and shortens the early phases of skeletal muscle regeneration. Biochimie 2017, 136, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Le Menn, G.; Neels, J.G. Regulation of immune cell function by PPARs and the connection with metabolic and neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 1575. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Sainsbury, A.; Stock Conference Working, G. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 2009, 10, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Reue, K. Sex differences in obesity: X chromosome dosage as a risk factor for increased food intake, adiposity and co-morbidities. Physiol. Behav. 2017, 176, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Wood, L.G.; Collins, C.E.; Callister, R. Effectiveness of weight loss interventions--is there a difference between men and women: A systematic review. Obes. Rev. 2015, 16, 171–186. [Google Scholar] [CrossRef]
- Bradley, R.L.; Jeon, J.Y.; Liu, F.F.; Maratos–Flier, E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E586–E594. [Google Scholar] [CrossRef] [Green Version]
- Blagih, J.; Coulombe, F.; Vincent, E.E.; Dupuy, F.; Galicia–Vazquez, G.; Yurchenko, E.; Raissi, T.C.; van der Windt, G.J.; Viollet, B.; Pearce, E.L.; et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 2015, 42, 41–54. [Google Scholar] [CrossRef]
- Newton, R.; Priyadharshini, B.; Turka, L.A. Immunometabolism of regulatory T cells. Nat. Immunol. 2016, 17, 618–625. [Google Scholar] [CrossRef]
- Lendoye, E.; Sibille, B.; Rousseau, A.S.; Murdaca, J.; Grimaldi, P.A.; Lopez, P. PPARbeta activation induces rapid changes of both AMPK subunit expression and AMPK activation in mouse skeletal muscle. Mol. Endocrinol. 2011, 25, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; Hutch, C.R.; Abrishami, S.; Stelmak, D.; Eter, L.; Li, Z.; Chang, E.; Agarwal, D.; Zamarron, B.; Varghese, M.; et al. Inflammatory responses to dietary and surgical weight loss in male and female mice. Biol. Sex. Differ. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.J.; McAuley, E.; Vieira, V.J.; Baynard, T.; Hu, L.; Evans, E.M.; Woods, J.A. Sex differences in the relationship between obesity, C-reactive protein, physical activity, depression, sleep quality and fatigue in older adults. Brain. Behav. Immun. 2009, 23, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.Y.; Ko, H.J.; Lichtman, E.I.; Lee, E.; Lawton, E.; Ong, H.; Yu, K.; Azuma, Y.; Friedline, R.H.; Lee, K.W.; et al. Short-term weight loss attenuates local tissue inflammation and improves insulin sensitivity without affecting adipose inflammation in obese mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E964–E976. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kim, I.Y.; Sung, H.R.; Nam, M.; Kim, Y.J.; Kyung, D.S.; Seong, J.K.; Hwang, G.S. Metabolic dysfunction following weight regain compared to initial weight gain in a high-fat diet-induced obese mouse model. J. Nutr. Biochem. 2019, 69, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Barg, S.; Vallois, D.; Lahiri, S.; Roger, C.; Yessoufou, A.; Pradevand, S.; McDonald, A.; Bonal, C.; Reimann, F.; et al. PPARbeta/delta affects pancreatic beta cell mass and insulin secretion in mice. J. Clin. Investig. 2012, 122, 4105–4117. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.; Ham, S.A.; Lee, W.J.; Hwang, S.I.; Park, J.A.; Hwang, J.S.; Hur, J.; Shin, H.C.; Han, S.G.; Lee, C.H.; et al. Ligand–dependent interaction of PPARdelta with T–Cell protein tyrosine phosphatase 45 enhances insulin signaling. Diabetes 2018, 67, 360–371. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Angelin, A.; Gil–de–Gomez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J., 3rd; Kopinski, P.K.; Wang, L.; et al. Foxp3 reprograms T cell metabolism to function in low–glucose, high–lactate environments. Cell Metab. 2017, 25, 1282–1293. [Google Scholar] [CrossRef]
- Gerriets, V.A.; Kishton, R.J.; Nichols, A.G.; Macintyre, A.N.; Inoue, M.; Ilkayeva, O.; Winter, P.S.; Liu, X.; Priyadharshini, B.; Slawinska, M.E.; et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Investig. 2015, 125, 194–207. [Google Scholar] [CrossRef]
- Lobo, T.F.; Borges, C.M.; Mattar, R.; Gomes, C.P.; de Angelo, A.G.S.; Pendeloski, K.P.T.; Daher, S. Impaired Treg and NK cells profile in overweight women with gestational diabetes mellitus. Am. J. Reprod. Immunol. 2018, 79, e12810. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Lim, J.; Kulkarni, A.B.; Perruche, S.; Chen, W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 2008, 9, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshini, B.; Loschi, M.; Newton, R.H.; Zhang, J.W.; Finn, K.K.; Gerriets, V.A.; Huynh, A.; Rathmell, J.C.; Blazar, B.R.; Turka, L.A. Cutting edge: TGF-beta and phosphatidylinositol 3-Kinase signals modulate distinct metabolism of regulatory T cell subsets. J. Immunol. 2018, 201, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fu, J.; Zhou, Y. Metabolism controls the balance of Th17/T–regulatory cells. Front. Immunol. 2017, 8, 1632. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Turner, N.; Bruce, C.R.; Beale, S.M.; Hoehn, K.L.; So, T.; Rolph, M.S.; Cooney, G.J. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007, 56, 2085–2092. [Google Scholar] [CrossRef]
- De Wilde, J.; Mohren, R.; van den Berg, S.; Boekschoten, M.; Dijk, K.W.; de Groot, P.; Muller, M.; Mariman, E.; Smit, E. Short–term high fat–feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol. Genom. 2008, 32, 360–369. [Google Scholar] [CrossRef]
- Hancock, C.R.; Han, D.H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, E.J.; Camera, D.M.; Jenkins, T.A.; Kosari, S.; Lee, J.S.; Hawley, J.A.; Stepto, N.K. Skeletal muscle respiratory capacity is enhanced in rats consuming an obesogenic Western diet. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1541–E1549. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.R. Cellular Energy Sensing and Metabolism–Implications for Treating Diabetes: The 2017 Outstanding Scientific Achievement Award Lecture. Diabetes 2018, 67, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, M.; Shimabukuro–Vornhagen, A.; Franke, A.; Theurich, S.; Wahl, P.; Hallek, M.; Schmidt, A.; Schinkothe, T.; Mester, J.; von Bergwelt–Baildon, M.; et al. Physical exercise modulates the homeostasis of human regulatory T cells. J. Allergy. Clin. Immunol. 2016, 137, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.S.; Sibille, B.; Murdaca, J.; Mothe–Satney, I.; Grimaldi, P.A.; Neels, J.G. alpha–Lipoic acid up–regulates expression of peroxisome proliferator-activated receptor beta in skeletal muscle: Involvement of the JNK signaling pathway. FASEB J. 2016, 30, 1287–1299. [Google Scholar] [CrossRef] [PubMed]


| ND-EX Control | HFD-ND | HFD-ND EX | HFD-ND GW | HFD-ND EX GW | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| ΔBW (T1−T0) (g) | 1.68 | 1.19 | −2.33 | 1.08 | −2.28 | 1.42 | −6.2 # | 2.86 | −4.4 # | 1.98 | |
| Adipose tissues (mg/g) | Subcutaneous | 17.29 | 3.73 | 21.98 $ | 6.74 | 14.61 * | 2.43 | 14.33 * | 2.86 | 14.67 * | 4.21 |
| Visceral | 12.55 | 3.36 | 21.12 $ | 7.16 | 11.41 * | 3.33 | 11.77 * | 1.10 | 10.93 * | 3.08 | |
| Brown | 3.50 | 1.17 | 3.54 | 0.63 | 2.98 | 0.27 | 3.32 | 0.42 | 2.85 | 0.44 | |
| Skeletal muscles (mg/g) | TLA | 1.87 | 0.74 | 1.45 | 0.18 | 1.65 | 0.08 | 1.59 | 0.06 | 1.56 | 0.11 |
| Vastus lateralis | 6.88 | 0.48 | 6.40 | 0.37 | 7.03 | 0.24 | 6.83 | 0.64 | 7.27 * | 0.41 | |
| Soleus | 0.28 | 0.02 | 0.26 | 0.02 | 0.33 | 0.05 | 0.33 | 0.15 | 0.27 | 0.05 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Garf, S.; Murdaca, J.; Mothe-Satney, I.; Sibille, B.; Le Menn, G.; Chinetti, G.; Neels, J.G.; Rousseau, A.-S. Complementary Immunometabolic Effects of Exercise and PPARβ/δ Agonist in the Context of Diet-Induced Weight Loss in Obese Female Mice. Int. J. Mol. Sci. 2019, 20, 5182. https://doi.org/10.3390/ijms20205182
Le Garf S, Murdaca J, Mothe-Satney I, Sibille B, Le Menn G, Chinetti G, Neels JG, Rousseau A-S. Complementary Immunometabolic Effects of Exercise and PPARβ/δ Agonist in the Context of Diet-Induced Weight Loss in Obese Female Mice. International Journal of Molecular Sciences. 2019; 20(20):5182. https://doi.org/10.3390/ijms20205182
Chicago/Turabian StyleLe Garf, Sébastien, Joseph Murdaca, Isabelle Mothe-Satney, Brigitte Sibille, Gwenaëlle Le Menn, Giulia Chinetti, Jaap G. Neels, and Anne-Sophie Rousseau. 2019. "Complementary Immunometabolic Effects of Exercise and PPARβ/δ Agonist in the Context of Diet-Induced Weight Loss in Obese Female Mice" International Journal of Molecular Sciences 20, no. 20: 5182. https://doi.org/10.3390/ijms20205182






