The Beige Adipocyte as a Therapy for Metabolic Diseases
Abstract
:1. Introduction
2. The Origin of Adipocytes
3. Adipose-Derived Stem Cells (ADMSC)
4. Beige Thermogenesis
5. Epigenetics Modifications in Adipose Cells
5.1. Epigenetic Regulation by DNA Methylation
5.2. Epigenetics and Mechanisms of Chromatin Modification
5.3. Epigenetics Changes by Non-Coding RNA
6. Induction of Beige Adipocyte by Cold
7. Influence of Physical Exercise
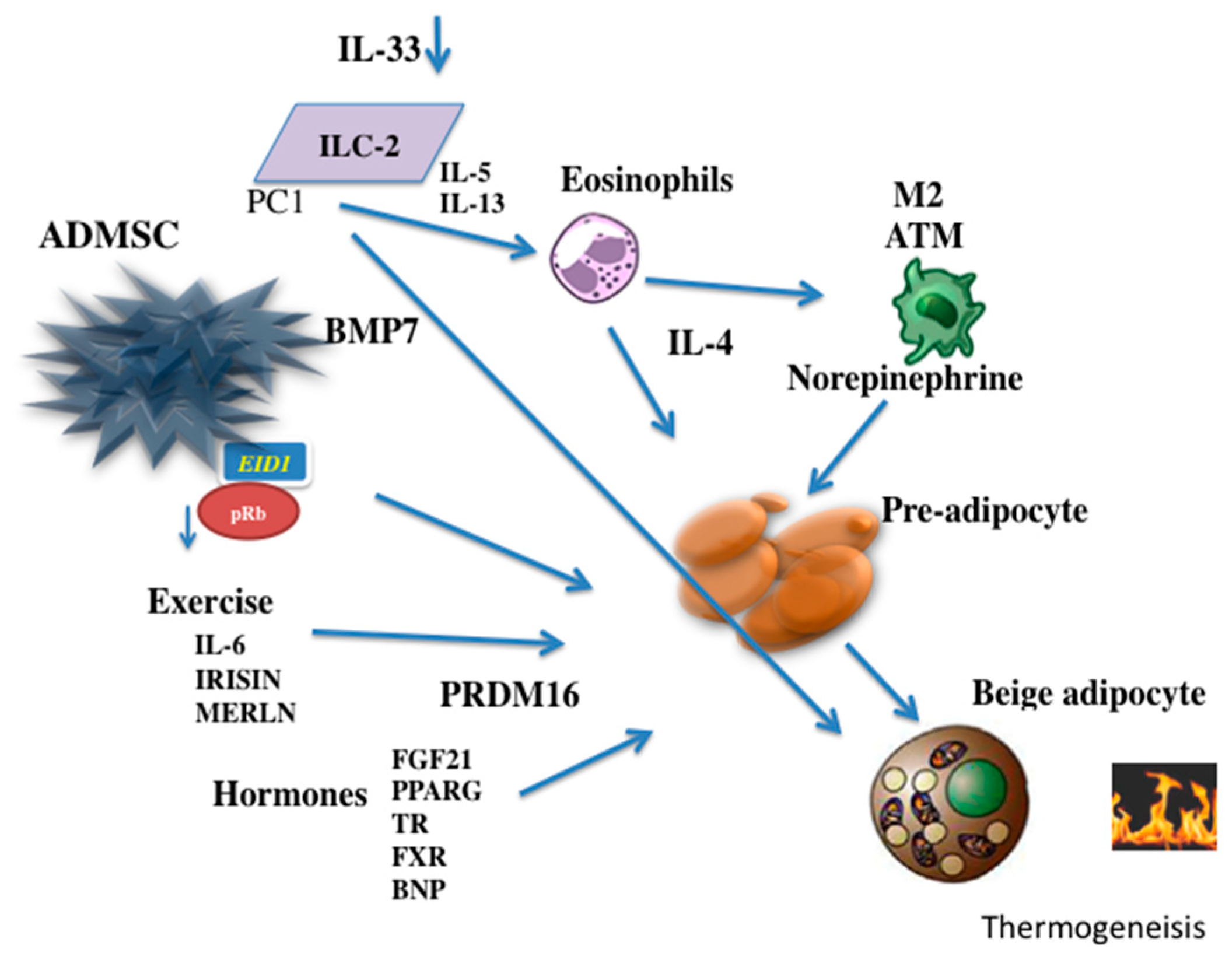
8. Role of Interleukins
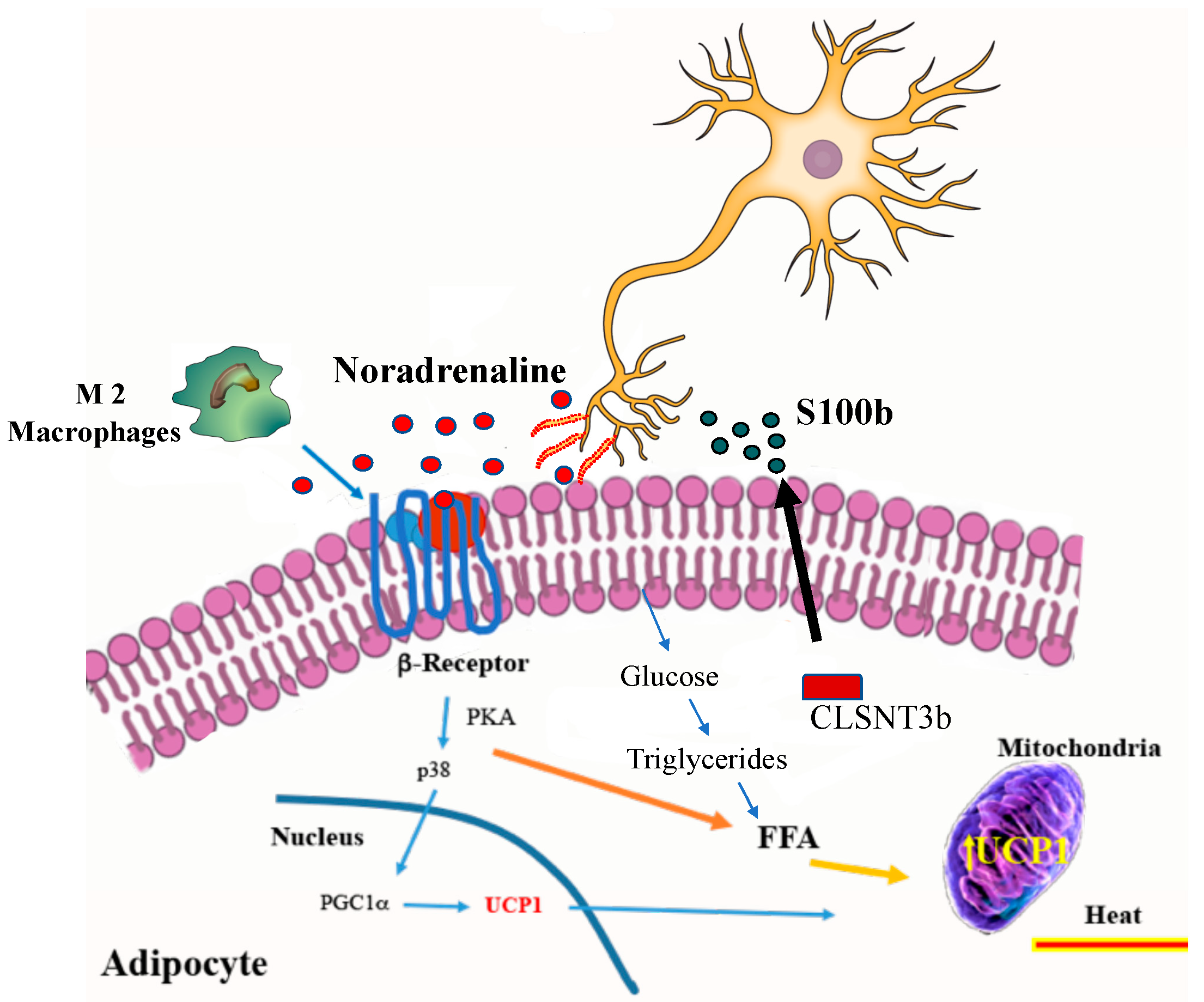
9. Endocrine Factors and Metabolites in Beige Adipocytes
10. Potential Therapeutic Use in Humans
Funding
Acknowledgments
Conflicts of Interest
References
- Rawshani, A.; Rawshani, A.; Franzen, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Wilke, T.; Mueller, S.; Groth, A.; Fuchs, A.; Seitz, L.; Kienhofer, J.; Maywald, U.; Lundershausen, R.; Wehling, M. Treatment-dependent and treatment-independent risk factors associated with the risk of diabetes-related events: A retrospective analysis based on 229,042 patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Riboli, E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Kanat, M.; DeFronzo, R.A.; Abdul-Ghani, M.A. Treatment of prediabetes. World J. Diabetes 2015, 6, 1207–1222. [Google Scholar] [CrossRef]
- Bailey, C.J. The current drug treatment landscape for diabetes and perspectives for the future. Clin. Pharmacol. Ther. 2015, 98, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, D.S.; Sandesara, P.B.; Shapiro, M.D. The intersection of diabetes and cardiovascular disease-a focus on new therapies. Front. Cardiovasc. Med. 2018, 5, 160. [Google Scholar] [CrossRef]
- Van Dijk, S.J.; Molloy, P.L.; Varinli, H.; Morrison, J.L.; Muhlhausler, B.S. Epigenetics and human obesity. Int. J. Obes. 2015, 39, 85–97. [Google Scholar] [CrossRef]
- Srivastava, G.; Apovian, C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef]
- Slyper, A.H. A paradigm shift for the prevention and treatment of individual and global obesity. Diabetes Metab. Syndr. Obes. 2018, 11, 855–861. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Kelton, C.M.; Guo, J.J.; Bian, B.; Heaton, P.C. Treatment of obesity: Pharmacotherapy trends in the United States from 1999 to 2010. Obesity 2015, 23, 1721–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D.; Endocrine, S. Pharmacological management of obesity: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, A.E.; Nolen-Doerr, E.; Farr, O.M.; Mantzoros, C.S. Geoffrey harris prize 2018: Novel pathways regulating neuroendocrine function, energy homeostasis and metabolism in humans. Eur. J. Endocrinol. 2019, 180, R59–R71. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Lazar, M.A. Brown fat in humans: Turning up the heat on obesity. Diabetes 2009, 58, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Spiegelman, B.M. Brown and beige fat: Molecular parts of a thermogenic machine. Diabetes 2015, 64, 2346–2351. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Ye, L.; Wu, J.; Cohen, P.; Kazak, L.; Khandekar, M.J.; Jedrychowski, M.P.; Zeng, X.; Gygi, S.P.; Spiegelman, B.M. Fat cells directly sense temperature to activate thermogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 12480–12485. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and beige fat: Physiological roles beyond heat generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Odegaard, J.I.; Mukundan, L.; Qiu, Y.; Molofsky, A.B.; Nussbaum, J.C.; Yun, K.; Locksley, R.M.; Chawla, A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015, 160, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Keipert, S.; Jastroch, M. Brite/beige fat and UCP1 - is it thermogenesis? Biochim. Biophys. Acta 2014, 1837, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Dempersmier, J.; Sul, H.S. Shades of brown: A model for thermogenic fat. Front. Endocrinol. 2015, 6, 71. [Google Scholar] [CrossRef]
- Lee, P.; Werner, C.D.; Kebebew, E.; Celi, F.S. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int. J. Obes. 2014, 38, 170–176. [Google Scholar] [CrossRef]
- Rahman, S.; Lu, Y.; Czernik, P.J.; Rosen, C.J.; Enerback, S.; Lecka-Czernik, B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 2013, 154, 2687–2701. [Google Scholar] [CrossRef]
- Holmes, D. Epigenetics: On-off switch for obesity. Nat. Rev. Endocrinol. 2016. [Google Scholar]
- Kajimura, S. Engineering fat cell fate to fight obesity and metabolic diseases. Keio. J. Med. 2015, 64, 65. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell. Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Bandiera, R.; Serrels, A.; Martinez-Estrada, O.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; McHaffie, S.; et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell. Biol. 2014, 16, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billon, N.; Dani, C. Developmental origins of the adipocyte lineage: New insights from genetics and genomics studies. Stem Cell Rev. 2012, 8, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Tao, C.; Jiang, L.; Shao, M.; Ye, R.; Zhu, Y.; Gordillo, R.; Ali, A.; Lian, Y.; Holland, W.L.; et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat. Cell. Biol. 2015, 17, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Kady, J.; Nam, M.; Rojas-Rodriguez, R.; Berkenwald, A.; Kim, J.H.; Noh, H.L.; Kim, J.K.; Cooper, M.P.; Fitzgibbons, T.; et al. Human “brite/beige” adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Altshuler-Keylin, S.; Shinoda, K.; Hasegawa, Y.; Ikeda, K.; Hong, H.; Kang, Q.; Yang, Y.; Perera, R.M.; Debnath, J.; Kajimura, S. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell. Metab. 2016, 24, 402–419. [Google Scholar] [CrossRef]
- Roh, H.C.; Tsai, L.T.Y.; Shao, M.; Tenen, D.; Shen, Y.; Kumari, M.; Lyubetskaya, A.; Jacobs, C.; Dawes, B.; Gupta, R.K.; et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell. Metab. 2018, 27, 1121–1137. [Google Scholar] [CrossRef]
- Bonet, M.L.; Oliver, P.; Palou, A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta 2013, 1831, 969–985. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Zhang, P.; Liu, W.; Kuang, S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J. Lipid Res. 2013, 54, 2214–2224. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Gurmaches, J.; Guertin, D.A. Adipocyte lineages: Tracing back the origins of fat. Biochim. Biophys. Acta 2014, 1842, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shan, T.; Yang, X.; Liang, S.; Zhang, P.; Liu, Y.; Liu, X.; Kuang, S. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J. Cell. Sci. 2013, 126, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gurmaches, J.; Hung, C.M.; Sparks, C.A.; Tang, Y.; Li, H.; Guertin, D.A. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell. Metab. 2012, 16, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Petkova, A.P.; Mottillo, E.P.; Granneman, J.G. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell. Metab. 2012, 15, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Z.; Svensson, K.J.; Tsai, L.; Zeng, X.; Roh, H.C.; Kong, X.; Rao, R.R.; Lou, J.; Lokurkar, I.; Baur, W.; et al. A smooth muscle-like origin for beige adipocytes. Cell. Metab. 2014, 19, 810–820. [Google Scholar] [CrossRef]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De Matteis, R.; Cinti, S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1244–E1253. [Google Scholar] [CrossRef] [Green Version]
- Rosenwald, M.; Perdikari, A.; Rulicke, T.; Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell. Biol. 2013, 15, 659–667. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, X.; Yi, F.; Liu, G.H.; Izpisua Belmonte, J.C. Regenerative medicine: Transdifferentiation in vivo. Cell. Res. 2014, 24, 141–142. [Google Scholar] [CrossRef]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef]
- Seale, P. Transcriptional regulatory circuits controlling brown fat development and activation. Diabetes 2015, 64, 2369–2375. [Google Scholar] [CrossRef]
- Wu, J.; Jun, H.; McDermott, J.R. Formation and activation of thermogenic fat. Trends Genet. 2015, 31, 232–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartesaghi, S.; Hallen, S.; Huang, L.; Svensson, P.A.; Momo, R.A.; Wallin, S.; Carlsson, E.K.; Forslow, A.; Seale, P.; Peng, X.R. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol. Endocrinol. 2015, 29, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Huang, H.; Tang, Q. Brown and beige fat: The metabolic function, induction, and therapeutic potential. Front. Med. 2015, 9, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White fat progenitor cells reside in the adipose vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Lazar, M.A. Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc. Natl. Acad. Sci. USA 1994, 91, 1786–1790. [Google Scholar] [CrossRef]
- Jiang, Y.; Berry, D.C.; Tang, W.; Graff, J.M. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014, 9, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Jiang, Y.; Graff, J.M. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat. Commun. 2016, 7, 10184. [Google Scholar] [CrossRef]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.; Both, S.K.; Odorfer, K.I.; Koevoet, W.; Kops, N.; O’Brien, F.J.; Baatenburg de Jong, R.J.; Verhaar, J.A.; Cuijpers, V.; Jansen, J.; et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet. Disord. 2011, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B.; Lindberg, O. Microcalorimetry of isolated mammalian cells. Nature 1977, 267, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. The hunt for the molecular mechanism of brown fat thermogenesis. Biochimie 2017, 134, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell. Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays. Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Fromme, T.; Schweizer, S.; Schottl, T.; Klingenspor, M. Taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured primary brown and brite/beige adipocytes. EMBO Rep. 2014, 15, 1069–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Kazak, L.; Jedrychowski, M.P.; Lu, G.Z.; Erickson, B.K.; Szpyt, J.; Pierce, K.A.; Laznik-Bogoslavski, D.; Vetrivelan, R.; Clish, C.B.; et al. Corrigendum: Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 2016, 536, 360. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enerback, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rossmeisl, M.; McClaine, J.; Riachi, M.; Harper, M.E.; Kozak, L.P. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J. Clin. Investig. 2003, 111, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Rahbani, J.F.; Samborska, B.; Lu, G.Z.; Jedrychowski, M.P.; Lajoie, M.; Zhang, S.; Ramsay, L.C.; Dou, F.Y.; Tenen, D.; et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab. 2019, 1, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, A.M.; Kazak, L.; Chouchani, E.T.; Bogaczynska, M.G.; Paranjpe, I.; Wainwright, G.L.; Betourne, A.; Kajimura, S.; Spiegelman, B.M.; Kirichok, Y. Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell. Metab. 2017, 25, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Lu, G.Z.; Jedrychowski, M.P.; Bare, C.J.; Mina, A.I.; Kumari, M.; Zhang, S.; Vuckovic, I.; Laznik-Bogoslavski, D.; et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell. Metab. 2017, 26, 660–671. [Google Scholar] [CrossRef]
- Gerngross, C.; Schretter, J.; Klingenspor, M.; Schwaiger, M.; Fromme, T. Active brown fat during (18)F-FDG PET/CT imaging defines a patient group with characteristic traits and an increased probability of brown fat redetection. J. Nucl. Med. 2017, 58, 1104–1110. [Google Scholar] [CrossRef]
- Bowers, R.R.; Kim, J.W.; Otto, T.C.; Lane, M.D. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc. Natl. Acad. Sci. USA 2006, 103, 13022–13027. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.S.; Wu, R.; Yang, X.; Kou, S.; MacDougald, O.A.; Yu, L.; Shi, H.; Xue, B. Inhibiting DNA methylation switches adipogenesis to osteoblastogenesis by activating Wnt10a. Sci. Rep. 2016, 6, 25283. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Park, J.H.; Weigel, C.; Liesenfeld, D.B.; Weichenhan, D.; Plass, C.; Seo, D.G.; Lindroth, A.M.; Park, Y.J. TET-mediated hydroxymethylcytosine at the Ppargamma locus is required for initiation of adipogenic differentiation. Int. J. Obes. 2017, 41, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Chia, S.Y.; Jin, S.; Han, W.; Ding, C.; Sun, L. Dynamic DNA methylation landscape defines brown and white cell specificity during adipogenesis. Mol. Metab. 2016, 5, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Procino, A.; Cillo, C. The HOX genes network in metabolic diseases. Cell. Biol. Int. 2013, 37, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Gnad, T.; Scheibler, S.; von Kugelgen, I.; Scheele, C.; Kilic, A.; Glode, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Scheele, C.; Larsen, T.J.; Nielsen, S. Novel nuances of human brown fat. Adipocyte 2014, 3, 54–57. [Google Scholar] [CrossRef]
- Shore, A.; Karamitri, A.; Kemp, P.; Speakman, J.R.; Lomax, M.A. Role of Ucp1 enhancer methylation and chromatin remodelling in the control of Ucp1 expression in murine adipose tissue. Diabetologia 2010, 53, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Patti, M.E. Gene expression in humans with diabetes and prediabetes: What have we learned about diabetes pathophysiology? Curr. Opin. Clin. Nutr. Metab. Care. 2004, 7, 383–390. [Google Scholar] [CrossRef]
- Sun, W.; Dong, H.; Becker, A.S.; Dapito, D.H.; Modica, S.; Grandl, G.; Opitz, L.; Efthymiou, V.; Straub, L.G.; Sarker, G.; et al. Cold-induced epigenetic programming of the sperm enhances brown adipose tissue activity in the offspring. Nat. Med. 2018, 24, 1372–1383. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Wang, B.; Pan, H.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 2016, 594, 4453–4466. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/alpha-Ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell. Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Huang, L.; Zhu, L.J.; Zou, T.; Ou, J.; Zhou, W.; Wang, Y.X. Jmjd3-mediated H3K27me3 dynamics orchestrate brown fat development and regulate white fat plasticity. Dev. Cell. 2015, 35, 568–583. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Shinoda, K.; Ohyama, K.; Sharp, L.Z.; Kajimura, S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 2013, 504, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, Y.; Fujiwara, Y.; Takahashi, H.; Matsumura, Y.; Sawada, T.; Jiang, S.; Nakaki, R.; Uchida, A.; Nagao, N.; Naito, M.; et al. Histone demethylase JMJD1A coordinates acute and chronic adaptation to cold stress via thermogenic phospho-switch. Nat. Commun. 2018, 9, 1566. [Google Scholar] [CrossRef]
- Duteil, D.; Tosic, M.; Willmann, D.; Georgiadi, A.; Kanouni, T.; Schule, R. Lsd1 prevents age-programed loss of beige adipocytes. Proc. Natl. Acad. Sci. USA 2017, 114, 5265–5270. [Google Scholar] [CrossRef] [Green Version]
- Tanimura, K.; Suzuki, T.; Vargas, D.; Shibata, H.; Inagaki, T. Epigenetic regulation of beige adipocyte fate by histone methylation. Endocr. J. 2019, 66, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Cardamone, M.D.; Tanasa, B.; Chan, M.; Cederquist, C.T.; Andricovich, J.; Rosenfeld, M.G.; Perissi, V. GPS2/KDM4A pioneering activity regulates promoter-specific recruitment of PPARgamma. Cell. Rep. 2014, 8, 163–176. [Google Scholar] [CrossRef]
- Lizcano, F.; Romero, C.; Vargas, D. Regulation of adipogenesis by nuclear receptor PPARgamma is modulated by the histone demethylase JMJD2C. Genet. Mol. Biol. 2011, 34, 19–24. [Google Scholar]
- Gray, S.G.; Iglesias, A.H.; Lizcano, F.; Villanueva, R.; Camelo, S.; Jingu, H.; Teh, B.T.; Koibuchi, N.; Chin, W.W.; Kokkotou, E.; et al. Functional characterization of JMJD2A, a histone deacetylase- and retinoblastoma-binding protein. J. Biol. Chem. 2005, 280, 28507–28518. [Google Scholar] [CrossRef]
- Abe, Y.; Rozqie, R.; Matsumura, Y.; Kawamura, T.; Nakaki, R.; Tsurutani, Y.; Tanimura-Inagaki, K.; Shiono, A.; Magoori, K.; Nakamura, K.; et al. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nat. Commun. 2015, 6, 7052. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, T.; Tachibana, M.; Magoori, K.; Kudo, H.; Tanaka, T.; Okamura, M.; Naito, M.; Kodama, T.; Shinkai, Y.; Sakai, J. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes Cells 2009, 14, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Okada, Y.; Kallin, E.M.; Zhang, Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 2009, 458, 757–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier-Daire, V.; Molinari, F.; Rio, M.; Raoul, O.; de Blois, M.C.; Romana, S.; Vekemans, M.; Munnich, A.; Colleaux, L. Cryptic terminal deletion of chromosome 9q34: A novel cause of syndromic obesity in childhood? J. Med. Genet. 2003, 40, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Nagano, G.; Ohno, H.; Oki, K.; Kobuke, K.; Shiwa, T.; Yoneda, M.; Kohno, N. Activation of classical brown adipocytes in the adult human perirenal depot is highly correlated with PRDM16-EHMT1 complex expression. PLoS ONE 2015, 10, e0122584. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, R.; Cui, X.; Zha, L.; Yu, L.; Shi, H.; Xue, B. Histone deacetylase 1 (HDAC1) negatively regulates thermogenic program in brown adipocytes via coordinated regulation of H3K27 deacetylation and methylation. J. Biol. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Li, F.; Wu, R.; Artinian, L.; Rehder, V.; Yu, L.; Liang, H.; Xue, B.; Shi, H. The histone demethylase UTX promotes brown adipocyte thermogenic program via coordinated regulation of H3K27 demethylation and acetylation. J. Biol. Chem. 2015, 290, 25151–25163. [Google Scholar] [CrossRef]
- Kang, S.C.; Kim, S.K.; Chai, J.C.; Kim, S.H.; Won, K.J.; Lee, Y.S.; Jung, K.H.; Chai, Y.G. Transcriptomic profiling and H3K27me3 distribution reveal both demethylase-dependent and independent regulation of developmental gene transcription in cell differentiation. PLoS ONE 2015, 10, e0135276. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.; Xu, F.; Moran, E. Cooperative activation of tissue-specific genes by pRB and E2F1. Cancer Res. 2013, 73, 2150–2158. [Google Scholar] [CrossRef]
- Calo, E.; Quintero-Estades, J.A.; Danielian, P.S.; Nedelcu, S.; Berman, S.D.; Lees, J.A. Rb regulates fate choice and lineage commitment in vivo. Nature 2010, 466, 1110–1114. [Google Scholar] [CrossRef] [Green Version]
- Auffret, J.; Viengchareun, S.; Carre, N.; Denis, R.G.; Magnan, C.; Marie, P.Y.; Muscat, A.; Feve, B.; Lombes, M.; Binart, N. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J. 2012, 26, 3728–3737. [Google Scholar] [CrossRef]
- Vargas, D.; Shimokawa, N.; Kaneko, R.; Rosales, W.; Parra, A.; Castellanos, A.; Koibuchi, N.; Lizcano, F. Regulation of human subcutaneous adipocyte differentiation by EID1. J. Mol. Endocrinol. 2016, 56, 113–122. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.; Porras, D.P.; Perry, C.G.; Seale, P.; Scime, A. p107 is a crucial regulator for determining the adipocyte lineage fate choices of stem cells. Stem Cells 2014, 32, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bi, P.; Shan, T.; Yang, X.; Yin, H.; Wang, Y.X.; Liu, N.; Rudnicki, M.A.; Kuang, S. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013, 9, e1003626. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, H.; Mori, M.A.; Alexander, R.; Yuan, B.; Hattangadi, S.M.; Liu, Q.; Kahn, C.R.; Lodish, H.F. Mir193b-365 is essential for brown fat differentiation. Nat. Cell. Biol. 2011, 13, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, M.; Ahmed, K.; Esau, C.C.; Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell. Biol. 2012, 14, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Vallespinos-Serrano, M.; Trabulo, S.M.; Fernandez-Marcos, P.J.; Firment, A.N.; Vazquez, B.N.; Vieira, C.R.; Mulero, F.; Camara, J.A.; Cronin, U.P.; et al. MiR-93 controls adiposity via inhibition of Sirt7 and Tbx3. Cell. Rep. 2015, 12, 1594–1605. [Google Scholar] [CrossRef]
- Mori, M.; Nakagami, H.; Rodriguez-Araujo, G.; Nimura, K.; Kaneda, Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012, 10, e1001314. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mao, C.; Quattrochi, B.; Friedline, R.H.; Zhu, L.J.; Jung, D.Y.; Kim, J.K.; Lewis, B.; Wang, Y.X. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat. Commun. 2014, 5, 4725. [Google Scholar] [CrossRef]
- Hu, F.; Wang, M.; Xiao, T.; Yin, B.; He, L.; Meng, W.; Dong, M.; Liu, F. miR-30 promotes thermogenesis and the development of beige fat by targeting RIP140. Diabetes 2015, 64, 2056–2068. [Google Scholar] [CrossRef]
- Fu, T.; Seok, S.; Choi, S.; Huang, Z.; Suino-Powell, K.; Xu, H.E.; Kemper, B.; Kemper, J.K. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol. Cell. Biol. 2014, 34, 4130–4142. [Google Scholar] [CrossRef]
- Karbiener, M.; Pisani, D.F.; Frontini, A.; Oberreiter, L.M.; Lang, E.; Vegiopoulos, A.; Mossenbock, K.; Bernhardt, G.A.; Mayr, T.; Hildner, F.; et al. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem Cells 2014, 32, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Gelling, R.W.; Yan, W.; Al-Noori, S.; Pardini, A.; Morton, G.J.; Ogimoto, K.; Schwartz, M.W.; Dempsey, P.J. Deficiency of TNFalpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology 2008, 149, 6053–6064. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dominguez, J.R.; Bai, Z.; Xu, D.; Yuan, B.; Lo, K.A.; Yoon, M.J.; Lim, Y.C.; Knoll, M.; Slavov, N.; Chen, S.; et al. De novo reconstruction of adipose tissue transcriptomes reveals long non-coding RNA regulators of brown adipocyte development. Cell. Metab. 2015, 21, 764–776. [Google Scholar] [CrossRef]
- Mi, L.; Zhao, X.Y.; Li, S.; Yang, G.; Lin, J.D. Conserved function of the long noncoding RNA Blnc1 in brown adipocyte differentiation. Mol. Metab. 2017, 6, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mi, L.; Yu, L.; Yu, Q.; Liu, T.; Wang, G.X.; Zhao, X.Y.; Wu, J.; Lin, J.D. Zbtb7b engages the long noncoding RNA Blnc1 to drive brown and beige fat development and thermogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E7111–E7120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.Y.; Li, S.; DelProposto, J.L.; Liu, T.; Mi, L.; Porsche, C.; Peng, X.; Lumeng, C.N.; Lin, J.D. The long noncoding RNA Blnc1 orchestrates homeostatic adipose tissue remodeling to preserve metabolic health. Mol. Metab. 2018, 14, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Collins, S. Beta-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol. 2011, 2, 102. [Google Scholar] [CrossRef]
- Murano, I.; Barbatelli, G.; Giordano, A.; Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 2009, 214, 171–178. [Google Scholar] [CrossRef]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef]
- Vargas, D.; Rosales, W.; Lizcano, F. Modifications of human subcutaneous ADMSC after PPARgamma activation and cold exposition. Stem Cells Int. 2015, 2015, 196348. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Choi, E.Y.; Liu, X.; Martin, A.; Wang, C.; Xu, X.; During, M.J. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell. Metab. 2011, 14, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Foglesong, G.D.; Huang, W.; Liu, X.; Slater, A.M.; Siu, J.; Yildiz, V.; Salton, S.R.; Cao, L. Role of hypothalamic VGF in energy balance and metabolic adaption to environmental enrichment in mice. Endocrinology 2016, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ye, M.; Resch, J.M.; Jedrychowski, M.P.; Hu, B.; Lowell, B.B.; Ginty, D.D.; Spiegelman, B.M. Innervation of thermogenic adipose tissue via a calsyntenin 3beta-S100b axis. Nature 2019, 569, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Jedrychowski, M.P.; Chen, Y.; Serag, S.; Lavery, G.G.; Gygi, S.P.; Spiegelman, B.M. Lysine-specific demethylase 1 promotes brown adipose tissue thermogenesis via repressing glucocorticoid activation. Genes Dev. 2016, 30, 1822–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riuzzi, F.; Chiappalupi, S.; Arcuri, C.; Giambanco, I.; Sorci, G.; Donato, R. S100 proteins in obesity: Liaisons dangereuses. Cell. Mol. Life Sci. 2019. [Google Scholar] [CrossRef]
- Ringseis, R.; Mooren, F.C.; Keller, J.; Couturier, A.; Wen, G.; Hirche, F.; Stangl, G.I.; Eder, K.; Kruger, K. Regular endurance exercise improves the diminished hepatic carnitine status in mice fed a high-fat diet. Mol. Nutr. Food Res. 2011, 55, S193–S202. [Google Scholar] [CrossRef] [PubMed]
- Ronn, T.; Ling, C. Effect of exercise on DNA methylation and metabolism in human adipose tissue and skeletal muscle. Epigenomics 2013, 5, 603–605. [Google Scholar] [CrossRef]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell. Res. 2012, 22, 551–564. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Bostrom, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. Beta-aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell. Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell. Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Vosselman, M.J.; Hoeks, J.; Brans, B.; Pallubinsky, H.; Nascimento, E.B.; van der Lans, A.A.; Broeders, E.P.; Mottaghy, F.M.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. 2015, 39, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- May, F.J.; Baer, L.A.; Lehnig, A.C.; So, K.; Chen, E.Y.; Gao, F.; Narain, N.R.; Gushchina, L.; Rose, A.; Doseff, A.I.; et al. Lipidomic adaptations in white and brown adipose tissue in response to exercise demonstrate molecular species-specific remodeling. Cell. Rep. 2017, 18, 1558–1572. [Google Scholar] [CrossRef]
- Sutherland, W.H.; Woodhouse, S.P.; Heyworth, M.R. Physical training and adipose tissue fatty acid composition in men. Metabolism 1981, 30, 839–844. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Mougios, V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med. 2004, 34, 1051–1076. [Google Scholar] [CrossRef]
- Sekine, K.; Nagata, N.; Sakamoto, K.; Arai, T.; Shimbo, T.; Shinozaki, M.; Okubo, H.; Watanabe, K.; Imbe, K.; Mikami, S.; et al. Abdominal visceral fat accumulation measured by computed tomography associated with an increased risk of gallstone disease. J. Gastroenterol. Hepatol. 2015, 30, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, P.; Sierra-Johnson, J.; Kallings, L.V.; Cederholm, T.; Kolak, M.; Halldin, M.; Brismar, K.; de Faire, U.; Hellenius, M.L.; Fisher, R.M. Functional changes in adipose tissue in a randomised controlled trial of physical activity. Lipids Health Dis. 2012, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.A.; Wieling, W.; Havekes, L.; Leuven, J.G.; Smit, E.M.; Dunning, A.J. Effect of physical exercise on blood lipids and adipose tissue composition in young healthy men. Atherosclerosis 1984, 53, 83–90. [Google Scholar] [CrossRef]
- Carriere, A.; Jeanson, Y.; Berger-Muller, S.; Andre, M.; Chenouard, V.; Arnaud, E.; Barreau, C.; Walther, R.; Galinier, A.; Wdziekonski, B.; et al. Browning of white adipose cells by intermediate metabolites: An adaptive mechanism to alleviate redox pressure. Diabetes 2014, 63, 3253–3265. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clement, K.; Butler-Browne, G.S.; Lacasa, D. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef] [PubMed]
- Franck, N.; Maris, M.; Nalbandian, S.; Talukdar, S.; Schenk, S.; Hofmann, H.P.; Bullough, D.; Osborn, O. Knock-down of IL-1Ra in obese mice decreases liver inflammation and improves insulin sensitivity. PLoS ONE 2014, 9, e107487. [Google Scholar] [CrossRef] [PubMed]
- Cron, L.; Allen, T.; Febbraio, M.A. The role of gp130 receptor cytokines in the regulation of metabolic homeostasis. J. Exp. Biol. 2016, 219, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Tesz, G.J.; Guilherme, A.; Guntur, K.V.; Hubbard, A.C.; Tang, X.; Chawla, A.; Czech, M.P. Tumor necrosis factor alpha (TNFalpha) stimulates Map4k4 expression through TNFalpha receptor 1 signaling to c-Jun and activating transcription factor 2. J. Biol. Chem. 2007, 282, 19302–19312. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, J.I.; Ganeshan, K.; Chawla, A. Adipose tissue macrophages: Amicus adipem? Cell. Metab. 2013, 18, 767–768. [Google Scholar] [CrossRef]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.E.; Van Dyken, S.J.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J. Metabolism: Type 2 immunity at the origin of beige adipocytes. Nat. Rev. Endocrinol. 2014, 10, 443. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Red Eagle, A.; Odegaard, J.I.; Jouihan, H.; Morel, C.R.; Heredia, J.E.; Mukundan, L.; Wu, D.; Locksley, R.M.; Chawla, A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 22617–22622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.S.; Lin, Y.W.; Burton, F.H.; Wei, L.N. Injecting engineered anti-inflammatory macrophages therapeutically induces white adipose tissue browning and improves diet-induced insulin resistance. Adipocyte 2015, 4, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, P.; Cui, R.; Zhang, M.; Li, H.; Qian, C.; Sheng, C.; Qu, S.; Bu, L. Eosinophils reduce chronic inflammation in adipose tissue by secreting Th2 cytokines and promoting M2 macrophages polarization. Int. J. Endocrinol. 2015, 2015, 565760. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Van Gool, F.; Liang, H.E.; Van Dyken, S.J.; Nussbaum, J.C.; Lee, J.; Bluestone, J.A.; Locksley, R.M. Interleukin-33 and Interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 2015, 43, 161–174. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef]
- Schulz, T.J.; Huang, T.L.; Tran, T.T.; Zhang, H.; Townsend, K.L.; Shadrach, J.L.; Cerletti, M.; McDougall, L.E.; Giorgadze, N.; Tchkonia, T.; et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA 2011, 108, 143–148. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vazquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, B.; Vienberg, S.G.; Smyth, G.; Cheng, C.; Stanford, K.I.; Arumugam, M.; Michael, M.D.; Adams, A.C.; Kharitonenkov, A.; Kahn, C.R. Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Investig. 2014, 124, 515–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegiopoulos, A.; Muller-Decker, K.; Strzoda, D.; Schmitt, I.; Chichelnitskiy, E.; Ostertag, A.; Berriel Diaz, M.; Rozman, J.; Hrabe de Angelis, M.; Nusing, R.M.; et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 2010, 328, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, I.; Babaeikelishomi, R.; Kocanova, S.; Sousa, I.S.; Lerch, S.; Hardt, O.; Wild, S.; Bosio, A.; Bystricky, K.; Herzig, S.; et al. Transcriptional pathways in cPGI2-induced adipocyte progenitor activation for browning. Front. Endocrinol. 2015, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Bordicchia, M.; Liu, D.; Amri, E.Z.; Ailhaud, G.; Dessi-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.J. Beige fat, adaptive thermogenesis, and its regulation by exercise and thyroid hormone. Biology 2019, 8, 57. [Google Scholar] [CrossRef]
- Garcia-Alonso, V.; Lopez-Vicario, C.; Titos, E.; Moran-Salvador, E.; Gonzalez-Periz, A.; Rius, B.; Parrizas, M.; Werz, O.; Arroyo, V.; Claria, J. Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor gamma (PPARgamma) in the conversion of white-to-brown adipocytes. J. Biol. Chem. 2013, 288, 28230–28242. [Google Scholar] [CrossRef]
- Zinn, A.; Felson, S.; Fisher, E.; Schwartzbard, A. Reassessing the cardiovascular risks and benefits of thiazolidinediones. Clin. Cardiol. 2008, 31, 397–403. [Google Scholar] [CrossRef]
- Loft, A.; Forss, I.; Siersbaek, M.S.; Schmidt, S.F.; Larsen, A.S.; Madsen, J.G.; Pisani, D.F.; Nielsen, R.; Aagaard, M.M.; Mathison, A.; et al. Browning of human adipocytes requires KLF11 and reprogramming of PPARgamma superenhancers. Genes Dev. 2015, 29, 7–22. [Google Scholar] [CrossRef]
- DePaoli, A.M.; Higgins, L.S.; Henry, R.R.; Mantzoros, C.; Dunn, F.L.; Group, I.N.T.S. Can a selective PPARgamma modulator improve glycemic control in patients with type 2 diabetes with fewer side effects compared with pioglitazone? Diabetes Care 2014, 37, 1918–1923. [Google Scholar] [CrossRef] [PubMed]
- Pino, E.; Wang, H.; McDonald, M.E.; Qiang, L.; Farmer, S.R. Roles for peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgamma coactivators 1alpha and 1beta in regulating response of white and brown adipocytes to hypoxia. J. Biol. Chem. 2012, 287, 18351–18358. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Tsai, M.L.; Badmaev, V.; Jimenez, M.; Ho, C.T.; Pan, M.H. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J. Agric. Food Chem. 2012, 60, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell. Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Koibuchi, N.; Fukuda, H.; Dangond, F.; Chin, W.W. Cell type-specific roles of histone deacetylase in TR ligand-independent transcriptional repression. Mol. Cell. Endocrinol. 2001, 172, 13–20. [Google Scholar] [CrossRef]
- Yen, P.M.; Chin, W.W. New advances in understanding the molecular mechanisms of thyroid hormone action. Trends Endocrinol. Metab. 1994, 5, 65–72. [Google Scholar] [CrossRef]
- Sadow, P.M.; Chassande, O.; Gauthier, K.; Samarut, J.; Xu, J.; O’Malley, B.W.; Weiss, R.E. Specificity of thyroid hormone receptor subtype and steroid receptor coactivator-1 on thyroid hormone action. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E36–E46. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef]
- Irrcher, I.; Adhihetty, P.J.; Sheehan, T.; Joseph, A.M.; Hood, D.A. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Cell. Physiol. 2003, 284, C1669–C1677. [Google Scholar] [CrossRef]
- Wulf, A.; Harneit, A.; Kroger, M.; Kebenko, M.; Wetzel, M.G.; Weitzel, J.M. T3-mediated expression of PGC-1alpha via a far upstream located thyroid hormone response element. Mol. Cell Endocrinol. 2008, 287, 90–95. [Google Scholar] [CrossRef]
- Warner, A.; Rahman, A.; Solsjo, P.; Gottschling, K.; Davis, B.; Vennstrom, B.; Arner, A.; Mittag, J. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor alpha1. Proc. Natl. Acad. Sci. USA 2013, 110, 16241–16246. [Google Scholar] [CrossRef] [PubMed]
- Dittner, C.; Lindsund, E.; Cannon, B.; Nedergaard, J. At thermoneutrality, acute thyroxine-induced thermogenesis and pyrexia are independent of UCP1. Mol. Metab. 2019, 25, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Kim, A.M.; Somayaji, V.R.; Dong, J.Q.; Rolph, T.P.; Weng, Y.; Chabot, J.R.; Gropp, K.E.; Talukdar, S.; Calle, R.A. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes. Metab. 2017, 19, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Sacripanti, G.; Nguyen, N.M.; Lorenzini, L.; Frascarelli, S.; Saba, A.; Zucchi, R.; Ghelardoni, S. 3,5-Diiodo-l-thyronine increases glucose consumption in cardiomyoblasts without affecting the contractile performance in rat heart. Front. Endocrinol. 2018, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Frascarelli, S.; Ghelardoni, S.; Chiellini, G.; Galli, E.; Ronca, F.; Scanlan, T.S.; Zucchi, R. Cardioprotective effect of 3-iodothyronamine in perfused rat heart subjected to ischemia and reperfusion. Cardiovasc. Drugs Ther. 2011, 25, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006, 86, 435–464. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Cioffi, M.; Goglia, F. Effect of 3,3’-di-iodothyronine and 3,5-di-iodothyronine on rat liver mitochondria. J. Endocrinol. 1993, 136, 59–64. [Google Scholar] [CrossRef]
- Bianco, A.C.; Silva, J.E. Optimal response of key enzymes and uncoupling protein to cold in BAT depends on local T3 generation. Am. J. Physiol. 1987, 253, E255–E263. [Google Scholar] [CrossRef]
- De Jesus, L.A.; Carvalho, S.D.; Ribeiro, M.O.; Schneider, M.; Kim, S.W.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Investig. 2001, 108, 1379–1385. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; Goglia, F. 3,5-Diiodo-L-thyronine and 3,5,3’-triiodo-L-thyronine both improve the cold tolerance of hypothyroid rats, but possibly via different mechanisms. Pflugers Arch. 1998, 436, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Guzman, G. Estrogen deficiency and the origin of obesity during menopause. Biomed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Lynch, L.; Hogan, A.E.; Duquette, D.; Lester, C.; Banks, A.; LeClair, K.; Cohen, D.E.; Ghosh, A.; Lu, B.; Corrigan, M.; et al. iNKT Cells Induce FGF21 for Thermogenesis and Are Required for Maximal Weight Loss in GLP1 Therapy. Cell. Metab. 2016, 24, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchoukalova, Y.D.; Votruba, S.B.; Tchkonia, T.; Giorgadze, N.; Kirkland, J.L.; Jensen, M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl. Acad. Sci. USA 2010, 107, 18226–18231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.W.; Tang, Y.; Li, X.; Liu, Y.; Zhang, Y.Y.; Huang, H.Y.; Xue, R.D.; Yu, H.Y.; Guo, L.; Gao, H.D.; et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E798–E807. [Google Scholar] [CrossRef] [Green Version]
- Svensson, K.J.; Long, J.Z.; Jedrychowski, M.P.; Cohen, P.; Lo, J.C.; Serag, S.; Kir, S.; Shinoda, K.; Tartaglia, J.A.; Rao, R.R.; et al. A secreted Slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell. Metab. 2016, 23, 454–466. [Google Scholar] [CrossRef]
- McQueen, A.E.; Kanamaluru, D.; Yan, K.; Gray, N.E.; Wu, L.; Li, M.L.; Chang, A.; Hasan, A.; Stifler, D. The C-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure. J. Biol. Chem. 2017, 292, 16122–16134. [Google Scholar] [CrossRef] [Green Version]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell. Metab. 2018, 27, 378–392. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chondronikola, M.; Volpi, E.; Borsheim, E.; Porter, C.; Annamalai, P.; Enerback, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Gennemark, P.; O’Mahony, G.; Bartesaghi, S. Unlock the thermogenic potential of adipose tissue: Pharmacological modulation and implications for treatment of diabetes and obesity. Front. Endocrinol. 2015, 6, 174. [Google Scholar] [CrossRef] [PubMed]
| White Adipocyte | Brown Adipocyte | Beige Adipocyte 1 Cold, Noradrenaline, TZD, FGF21, IL-4; IL-6 |
|---|---|---|
| Unilocular adipocyte | Multilocular adipocytes | Unilocular → Multilocular |
| Lipid storage (+++) | Lipid storage (+) | Lipid storage (+++) → Lipid storage (+) |
| Mitochondria (+) | Mitochondria (+++) | Mitochondria (+) → Mitochondria (+++) |
| Fatty acid oxidation (+) | Fatty acid oxidation (+++) | Fatty acid oxidation (+) → Fatty acid oxidation (+++) |
| Respiratory chain (+) | Respiratory chain (+++) | Respiratory chain (+) → Respiratory chain (+++) |
| UCP1 (−) | UCP1 (+++) | UCP1 (−) → UCP1 (+++) |
| PGC-1α (+) | PGC-1α (+++) | PGC-1α (+) → PGC-1α (+++) |
| Markers: Resistin, ASC-1, FAB4 | Markers: Zic1, Lhx8, Eva1, Pdk4, CLSTN3b | Markers: CD137, Tbx1, Cited1, Tmem26, CIDEA, CLSTN3b |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. https://doi.org/10.3390/ijms20205058
Lizcano F. The Beige Adipocyte as a Therapy for Metabolic Diseases. International Journal of Molecular Sciences. 2019; 20(20):5058. https://doi.org/10.3390/ijms20205058
Chicago/Turabian StyleLizcano, Fernando. 2019. "The Beige Adipocyte as a Therapy for Metabolic Diseases" International Journal of Molecular Sciences 20, no. 20: 5058. https://doi.org/10.3390/ijms20205058
APA StyleLizcano, F. (2019). The Beige Adipocyte as a Therapy for Metabolic Diseases. International Journal of Molecular Sciences, 20(20), 5058. https://doi.org/10.3390/ijms20205058





