Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Results
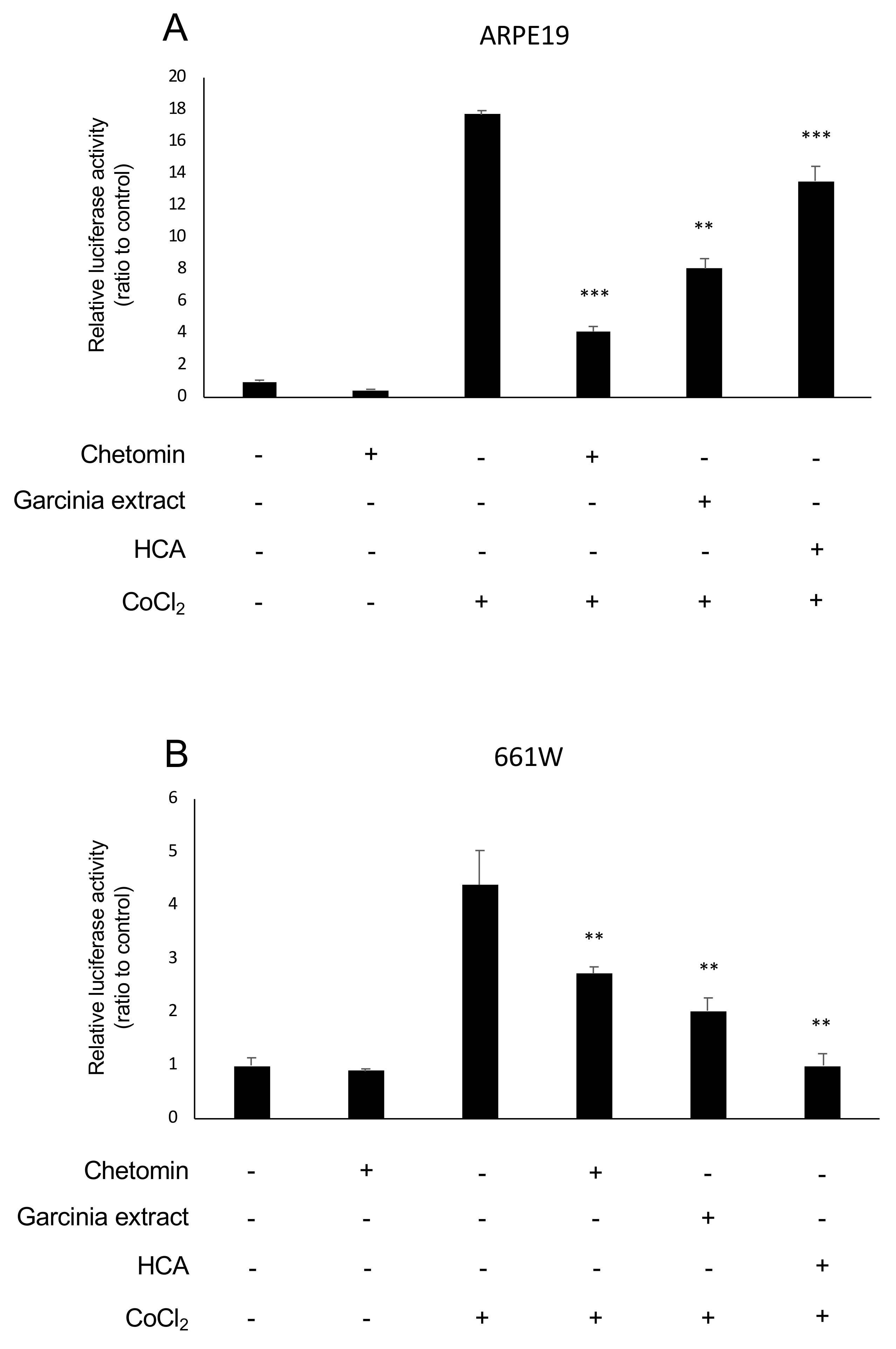
2.1. HIF Activation Suppressed by Garcinia Extract and HCA Administration in Vitro
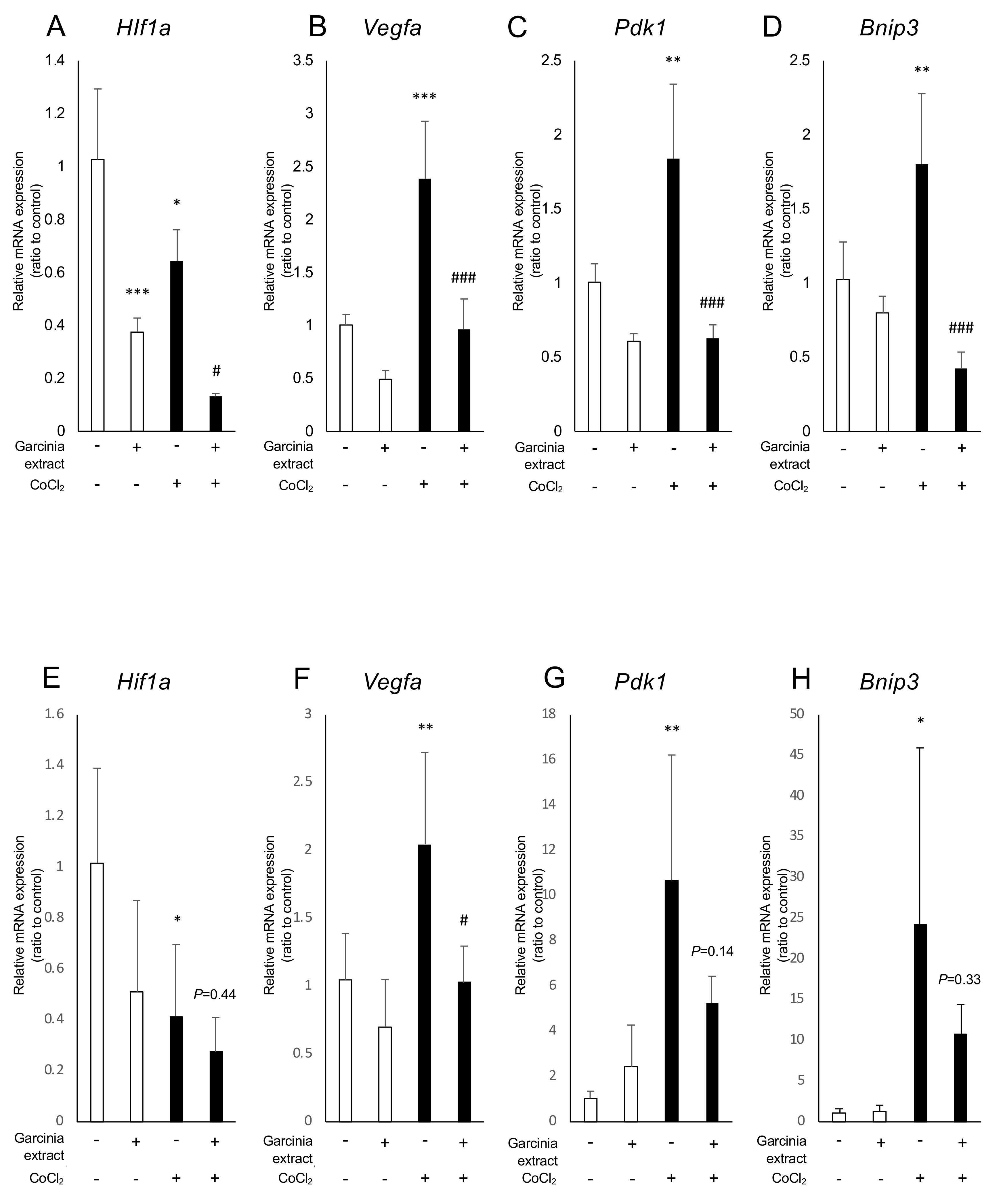
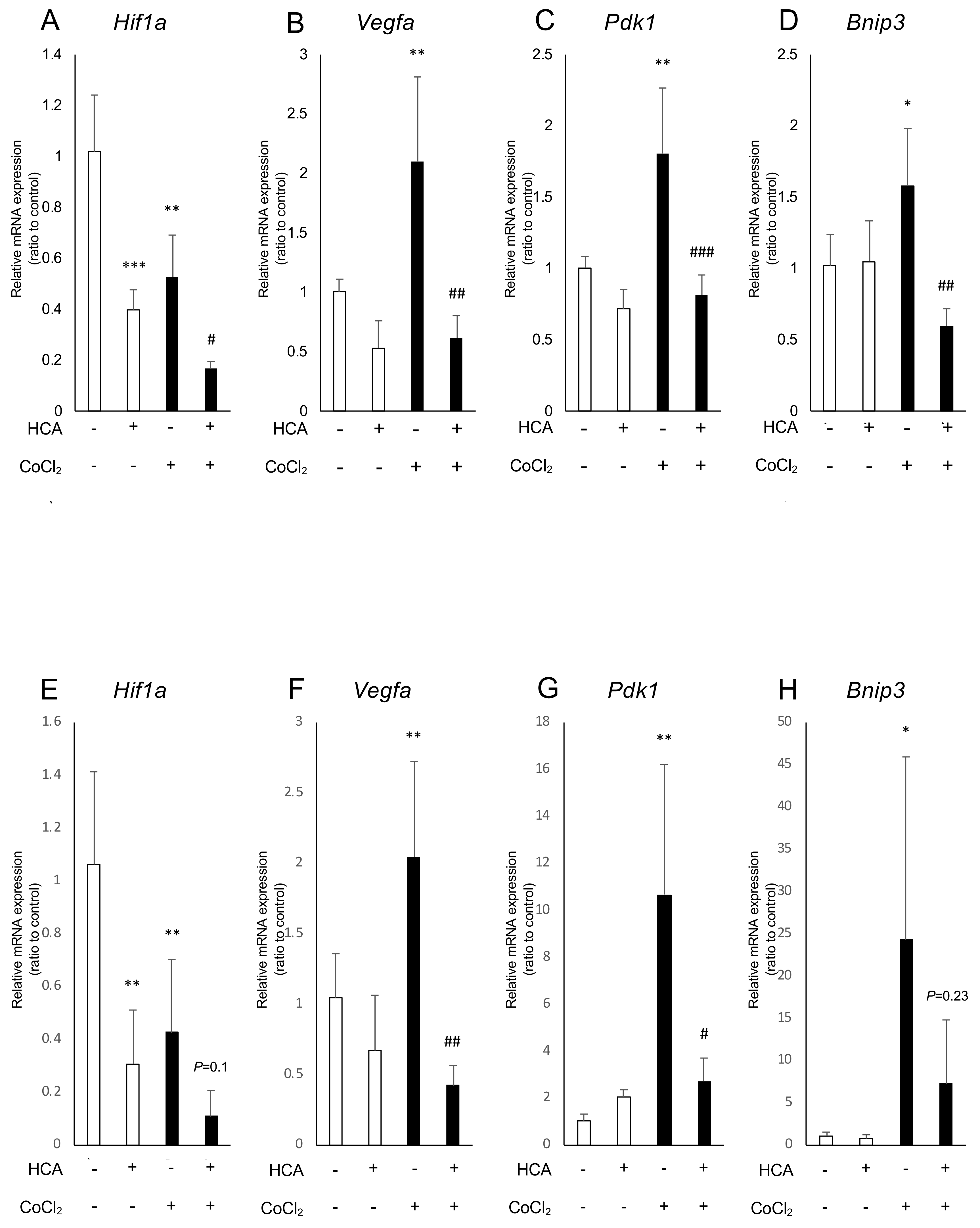
2.2. Administration of Garcinia Extract and HCA Downregulated Hif1a and Downstream Genes
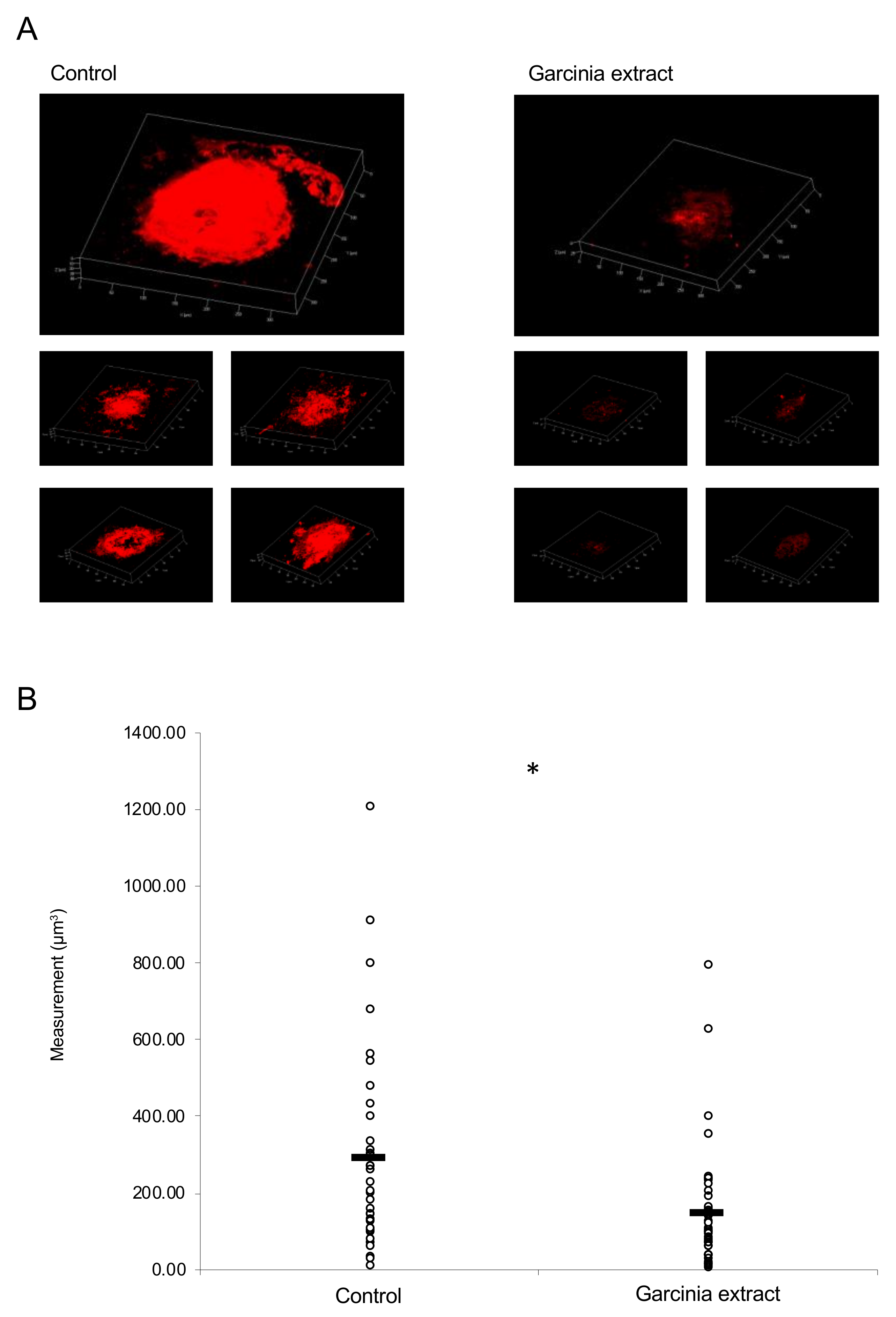
2.3. Administration of Garcinia Extract Suppressed CNV Volume in the Model Mice
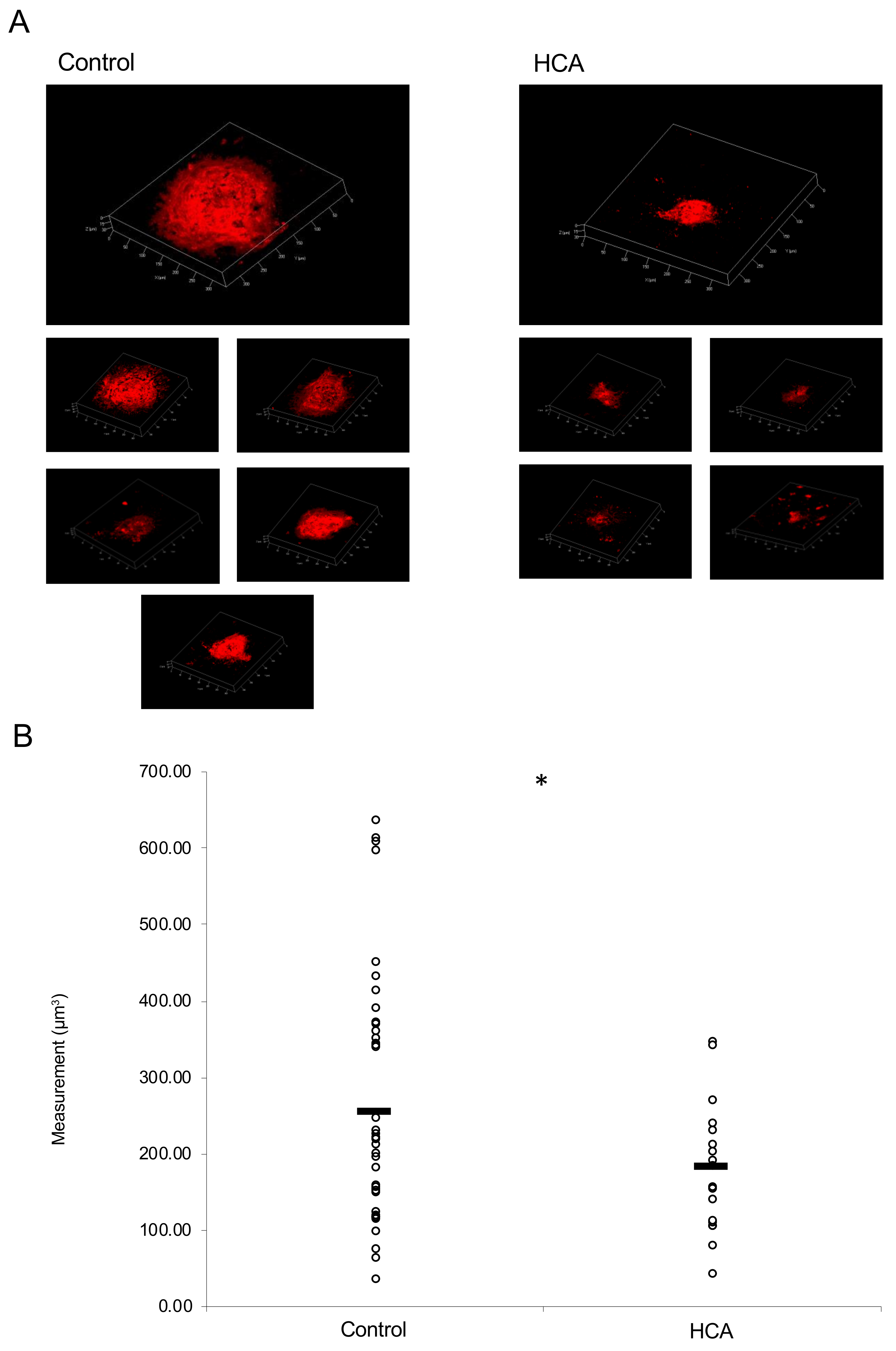
2.4. Administration of HCA Suppressed CNV Volume in the Model Mice
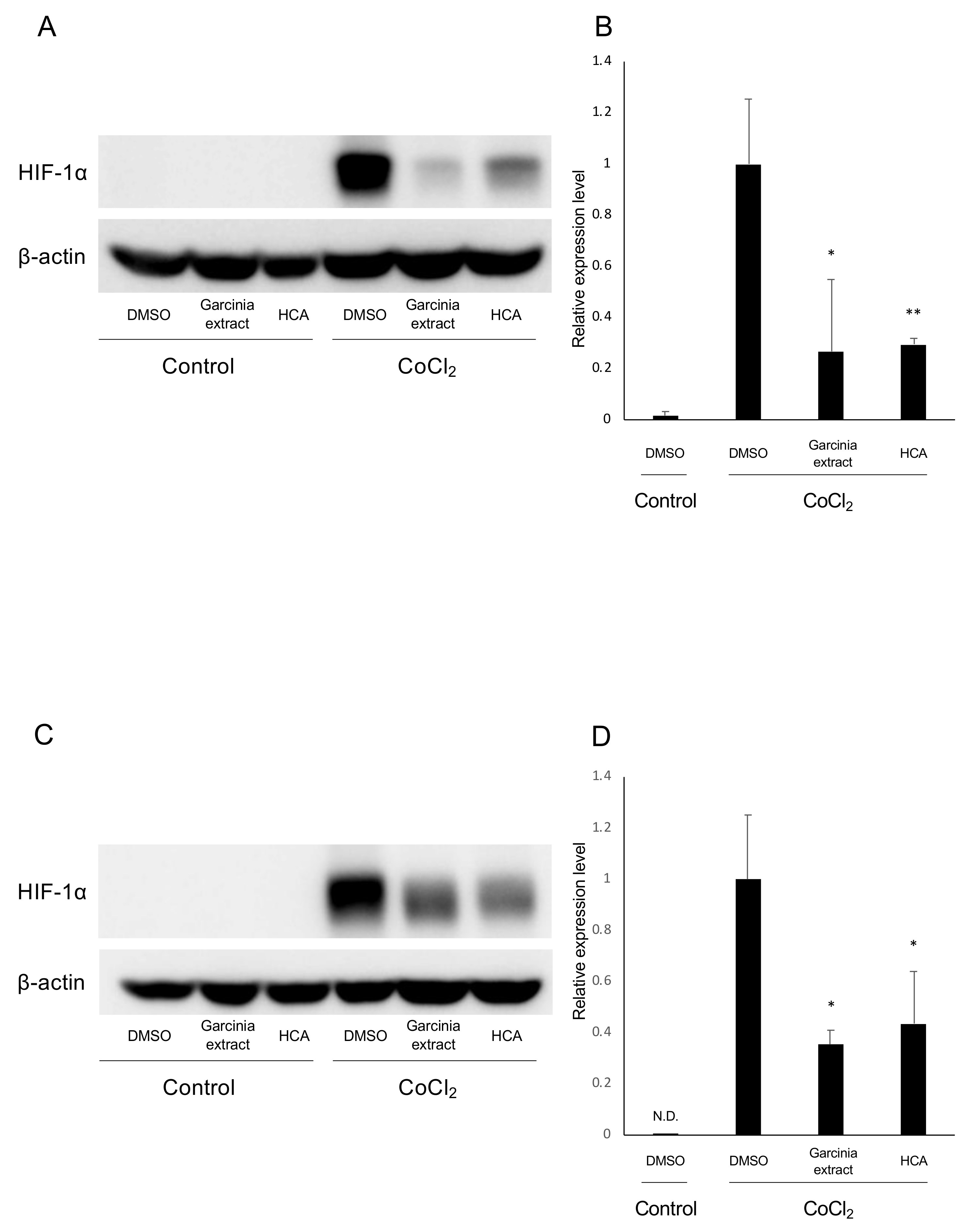
2.5. Administration of HCA Suppressed HIF-1α Expression in Vivo
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Luciferase Assay
4.3. Laser-Induced CNV
4.4. CNV Volume Measurement
4.5. Administration of Garcinia Extract and HCA to Mice
4.6. Real-Time PCR (Polymerase Chain Reaction)
4.7. Western Blot
4.8. Statistics
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| BNIP3 | BCL2 interacting protein 3 |
| CNV | Choroidal neovascularization |
| CoCl2 | Cobalt (II) chloride hexahydrate |
| DMSO | Dimethyl sulfoxide |
| GLUT1 | Glucose transporter 1 |
| HCA | Hydroxycitric acid |
| HIF | Hypoxia-inducible factor |
| NIH | The national institutes of health |
| PCR | Polymerase chain reaction |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| RPE | Retinal pigment epithelium |
| VEGF | Vascular endothelial growth factor |
| VHL | Von Hippel-Lindau protein |
References
- Pascolini, D.; Mariotti, S.P.; Pokharel, G.P.; Pararajasegaram, R.; Etya’ale, D.; Negrel, A.D.; Resnikoff, S. 2002 global update of available data on visual impairment: A compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004, 11, 67–115. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Hyman, L.; Neborsky, R. Risk factors for age-related macular degeneration: An update. Curr. Opin. Ophthalmol. 2002, 13, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- De Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Pierce, E.A.; Foley, E.D.; Takagi, H.; Chen, H.; Riddle, L.; Ferrara, N.; King, G.L.; Smith, L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10457–10461. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.I.; Evans, S.M.; Craft, J.R.; Capozzi, M.E.; McCollum, G.W.; Yang, R.; Marnett, L.J.; Uddin, M.J.; Jayagopal, A.; Penn, J.S. In vivo imaging of retinal hypoxia in a model of oxygen-induced retinopathy. Sci. Rep. 2016, 6, 31011. [Google Scholar] [CrossRef]
- Pieramici, D.J.; Rabena, M.D. Anti-VEGF therapy: Comparison of current and future agents. Eye 2008, 22, 1330–1336. [Google Scholar] [CrossRef]
- Maguire, M.G.; Martin, D.F.; Ying, G.S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L., 3rd; Fine, S.L. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The comparison of age-related macular degeneration treatments trials. Ophthalmology 2016, 123, 1751–1761. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Daniel, E.; Huang, J.; Ying, G.S.; Maguire, M.G.; Toth, C.A.; Jaffe, G.J.; Fine, S.L.; Blodi, B.; Klein, M.L.; et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014, 121, 150–161. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mole, D.R.; Blancher, C.; Copley, R.R.; Pollard, P.J.; Gleadle, J.M.; Ragoussis, J.; Ratcliffe, P.J. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009, 284, 16767–16775. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Westenskow, P.D.; Bravo, S.; Aguilar, E.; Friedlander, M. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Invest. 2012, 122, 4213–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, Y.; Hoshino, Y.; Shoda, C.; Jiang, X.; Tsubota, K.; Kurihara, T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019, 128, 21–31. [Google Scholar] [CrossRef]
- Rapisarda, A.; Uranchimeg, B.; Sordet, O.; Pommier, Y.; Shoemaker, R.H.; Melillo, G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: Mechanism and therapeutic implications. Cancer Res. 2004, 64, 1475–1482. [Google Scholar] [CrossRef]
- Yu, T.; Tang, B.; Sun, X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer therapy. Yonsei. Med. J. 2017, 58, 489–496. [Google Scholar] [CrossRef]
- Hoskins, P.; Eisenhauer, E.; Beare, S.; Roy, M.; Drouin, P.; Stuart, G.; Bryson, P.; Grimshaw, R.; Capstick, V.; Zee, B. Randomized phase II study of two schedules of topotecan in previously treated patients with ovarian cancer: A national cancer institute of canada clinical trials group study. J. Clin. Oncol. 1998, 16, 2233–2237. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.N.; Kim, Y.J.; Park, T. Garcinia cambogia extract ameliorates visceral adiposity in C57BL/6J mice fed on a high-fat diet. Biosci. Biotechnol. Biochem. 2008, 72, 1772–1780. [Google Scholar] [CrossRef]
- Rahtu-Korpela, L.; Karsikas, S.; Horkko, S.; Blanco Sequeiros, R.; Lammentausta, E.; Makela, K.A.; Herzig, K.H.; Walkinshaw, G.; Kivirikko, K.I.; Myllyharju, J.; et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 2014, 63, 3324–3333. [Google Scholar] [CrossRef]
- Kunimi, H.; Miwa, Y.; Inoue, H.; Tsubota, K.; Kurihara, T. A novel HIF inhibitor halofuginone prevents neurodegeneration in a murine model of retinal ischemia-reperfusion. Int. J. Mol. Sci. 2019, 20, 3171. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kurihara, T.; Miyauchi, M.; Ishida, A.; Jiang, X.; Ikeda, S.I.; Torii, H.; Tsubota, K. Oral crocetin administration suppressed refractive shift and axial elongation in a murine model of lens-induced myopia. Sci. Rep. 2019, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, Y.; Zhou, H.; Yang, L.; Yu, S.; Gao, Y.; Huang, Y.; Lu, L.; Liang, X. Tetramethylpyrazine protects CoCl2-induced apoptosis in human umbilical vein endothelial cells by regulating the PHD2/HIF/1alpha-VEGF pathway. Mol. Med. Rep. 2016, 13, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Luo, X.; Liang, J.; Xiao, M.; Sun, X. Antiangiogenic Effects of Doxazosin on Experimental Choroidal Neovascularization in Mice. J. Ocul. Pharm. Ther. 2017, 33, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ke, X.; Wang, W.; Zhang, H.; Ma, N.; Fu, W.; Zhao, M.; Gao, X.; Hao, X.; Zhang, Z. Aloe-emodin suppresses hypoxia-induced retinal angiogenesis via inhibition of HIF-1alpha/VEGF pathway. Int. J. Biol. Sci. 2016, 12, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.; Park, S.W.; Jo, D.H.; Kim, D.; Kim, J.H.; Cho, H.Y.; Kim, J.; Kim, J.H.; Kim, J.S. CRISPR-LbCpf1 prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Nat. Commun. 2018, 9, 1855. [Google Scholar] [CrossRef]
- Yoshida, T.; Zhang, H.; Iwase, T.; Shen, J.; Semenza, G.L.; Campochiaro, P.A. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. Faseb. J. 2010, 24, 1759–1767. [Google Scholar] [CrossRef]
- Jena, B.S.; Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Chemistry and biochemistry of (-)-hydroxycitric acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10–22. [Google Scholar] [CrossRef]
- Stallings, W.C.; Blount, J.F.; Srere, P.A.; Glusker, J.P. Structural studies of hydroxycitrates and their relevance to certain enzymatic mechanisms. Arch. Biochem. Biophys. 1979, 193, 431–448. [Google Scholar] [CrossRef]
- Ohia, S.E.; Opere, C.A.; LeDay, A.M.; Bagchi, M.; Bagchi, D.; Stohs, S.J. Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCA-SX). Mol. Cell Biochem. 2002, 238, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.; Monjok, E.; Kouamou, G.; Ohia, S.E.; Bagchi, D.; Lokhandwala, M.F. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance, and body weight in developing obese Zucker rats. Mol. Cell. Biochem. 2007, 304, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Garis, R.I.; Bramble, J.D.; Bagchi, D.; Bagchi, M.; Rao, C.V.; Satyanarayana, S. Efficacy of a novel calcium/potassium salt of (-)-hydroxycitric acid in weight control. Int. J. Clin. Pharm. Res. 2005, 25, 133–144. [Google Scholar]
- Li, L.; Peng, M.; Ge, C.; Yu, L.; Ma, H. Hydroxycitric acid reduced lipid droplets accumulation via decreasing acetyl-coa supply and accelerating energy metabolism in cultured primary chicken hepatocytes. Cell Physiol. Biochem. 2017, 43, 812–831. [Google Scholar] [CrossRef] [PubMed]
- Nisha, V.M.; Priyanka, A.; Anusree, S.S.; Raghu, K.G. (-)-Hydroxycitric acid attenuates endoplasmic reticulum stress-mediated alterations in 3T3-L1 adipocytes by protecting mitochondria and downregulating inflammatory markers. Free Radic. Res. 2014, 48, 1386–1396. [Google Scholar] [CrossRef]
- Goudarzvand, M.; Afraei, S.; Yaslianifard, S.; Ghiasy, S.; Sadri, G.; Kalvandi, M.; Alinia, T.; Mohebbi, A.; Yazdani, R.; Azarian, S.K.; et al. Hydroxycitric acid ameliorates inflammation and oxidative stress in mouse models of multiple sclerosis. Neural. Regen. Res. 2016, 11, 1610–1616. [Google Scholar] [CrossRef]
- Ono, H.; Tamura, H.; Yamashita, Y.; Tamura, K.; Iwakura, K. In vitro chromosome aberration test and in vivo micronucleus test of Ca-type Garcinia extract. Shokuhin Eiseigaku Zasshi 2006, 47, 80–84. [Google Scholar] [CrossRef]
- Montezuma, S.R.; Dolezal, L.D.; Rageh, A.A.; Mar, K.; Jordan, M.; Ferrington, D.A. Lactoferrin reduces chorioretinal damage in the murine laser model of choroidal neovascularization. Curr. Eye Res. 2015, 40, 946–953. [Google Scholar] [CrossRef]

| Parameter | Specification Result |
|---|---|
| Main Characteristics | |
| Description | Pale brown color with characteristic odor |
| Identification | Compliance with IR (infrared spectroscopy) spectrum |
| Loss on drying | 2.87% w/w |
| Solubility | Slightly soluble in hot water and insoluble in water |
| Particle Information | |
| pH (1% w/v solution) | 9.7 |
| Tapped bulk density | 0.61 g/mL |
| Loose bulk density | 0.36 g/mL |
| Sieve test (mesh size) | 100% passed with 60 mesh |
| Active Ingredients | Results |
| HCA | 56.55% w/w |
| Calcium by titration | 19.70% w/w |
| Contaminants | |
| Heavy Metals | <10 ppm |
| Lead | 1.0 ppm |
| Arsenic | <0.1 ppm |
| Cadmium | <0.01 ppm |
| Mercury | 0.1 ppm |
| Microbiological profile | |
| Total microbial plate count | 600 CFU/g |
| Total yeast and mold count | 10 CFU/g |
| Escherichia coli | Absent |
| Coliforms | Absent |
| Salmonella | Absent |
| Staphylococcus aureus | Absent |
| Pseudomonas aeruginosa | Absent |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 5049. https://doi.org/10.3390/ijms20205049
Ibuki M, Shoda C, Miwa Y, Ishida A, Tsubota K, Kurihara T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2019; 20(20):5049. https://doi.org/10.3390/ijms20205049
Chicago/Turabian StyleIbuki, Mari, Chiho Shoda, Yukihiro Miwa, Ayako Ishida, Kazuo Tsubota, and Toshihide Kurihara. 2019. "Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration" International Journal of Molecular Sciences 20, no. 20: 5049. https://doi.org/10.3390/ijms20205049
APA StyleIbuki, M., Shoda, C., Miwa, Y., Ishida, A., Tsubota, K., & Kurihara, T. (2019). Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. International Journal of Molecular Sciences, 20(20), 5049. https://doi.org/10.3390/ijms20205049







