Disruptions of Autophagy in the Rat Retina with Age During the Development of Age-Related-Macular-Degeneration-like Retinopathy
Abstract
1. Introduction
2. Results
2.1. Differentially Expressed Genes (DEGs) Associated with Autophagy in OXYS Rats
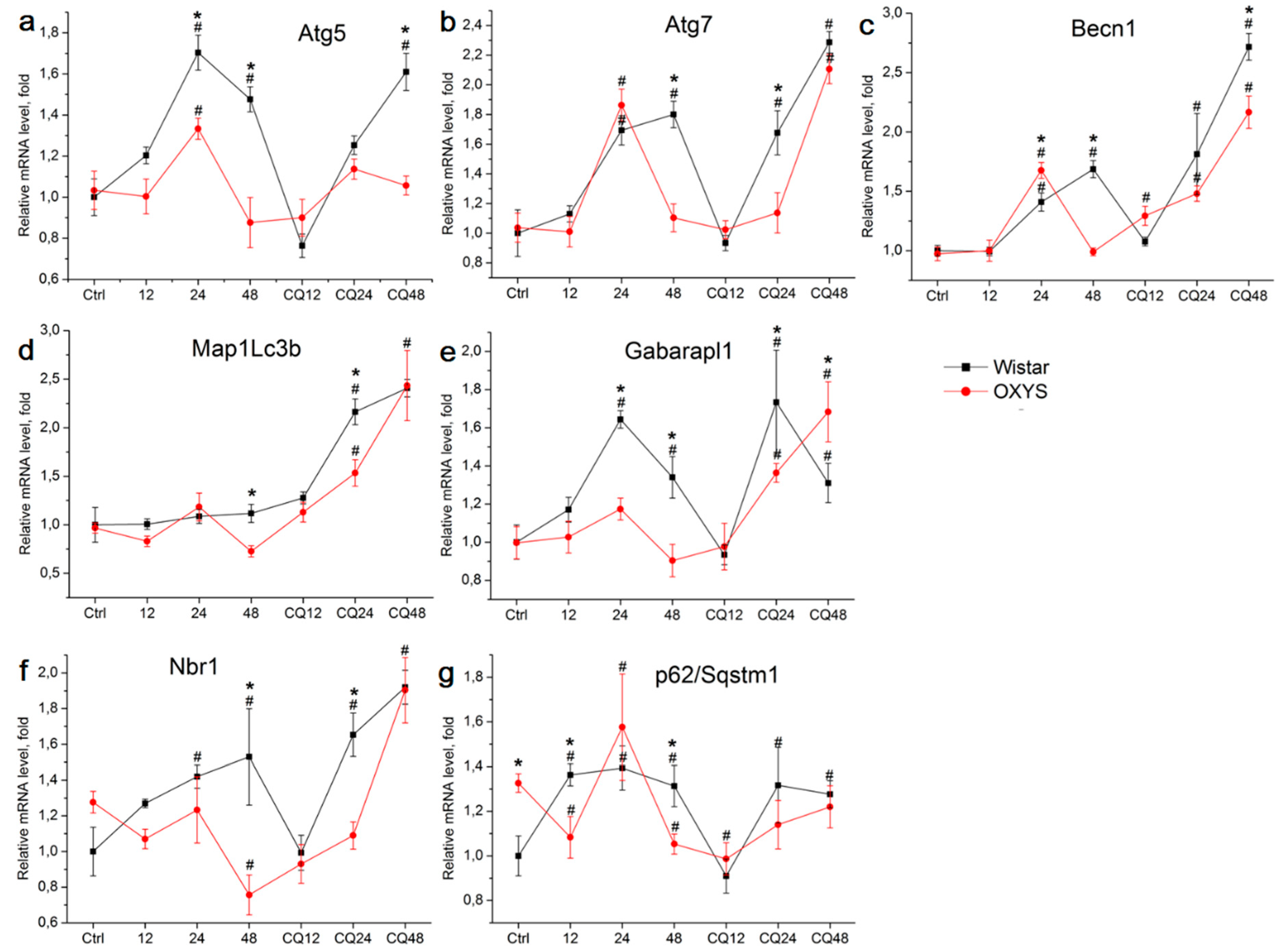
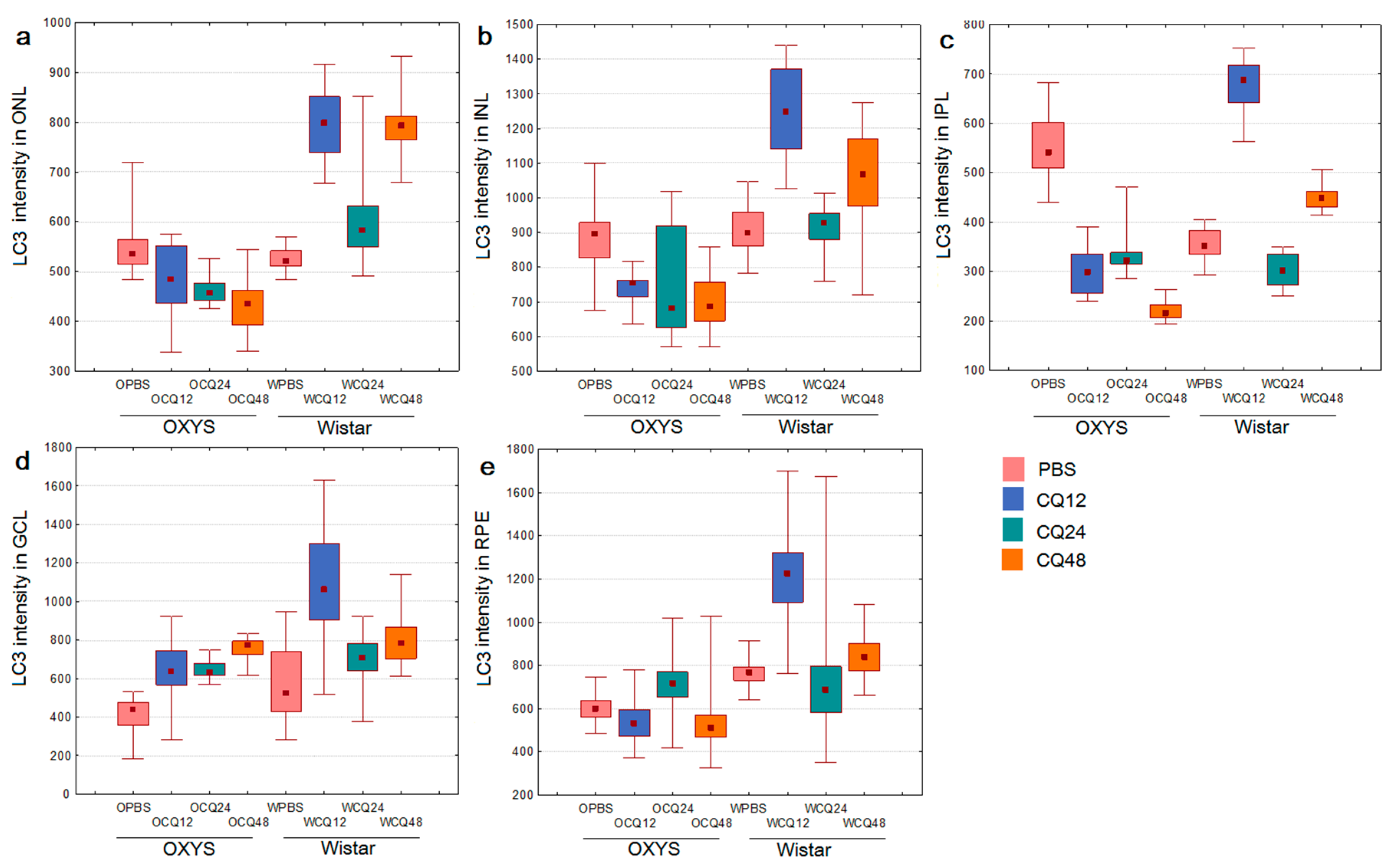
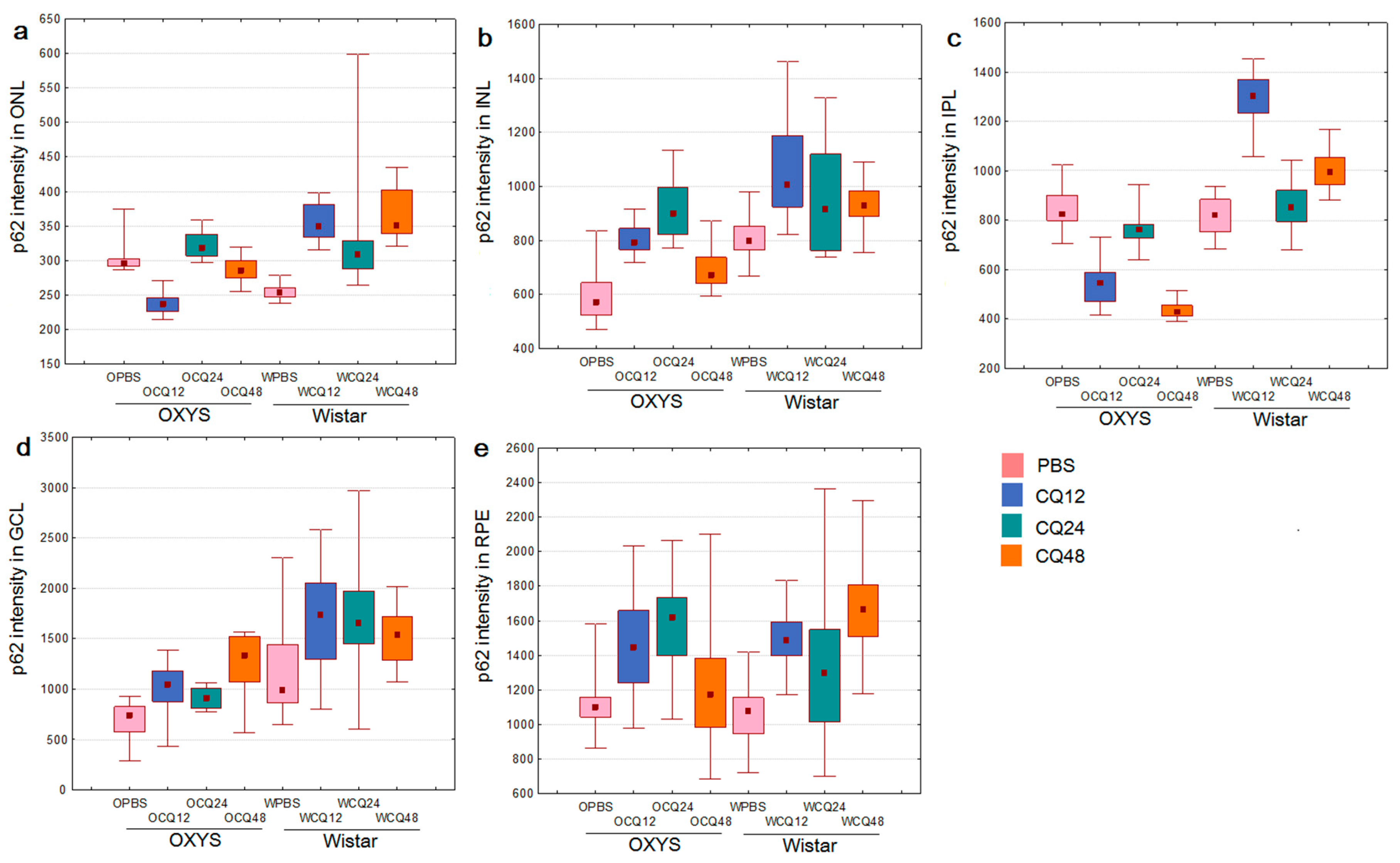
2.2. The Effects of Autophagy Modulation on the Expression of Autophagy Markers
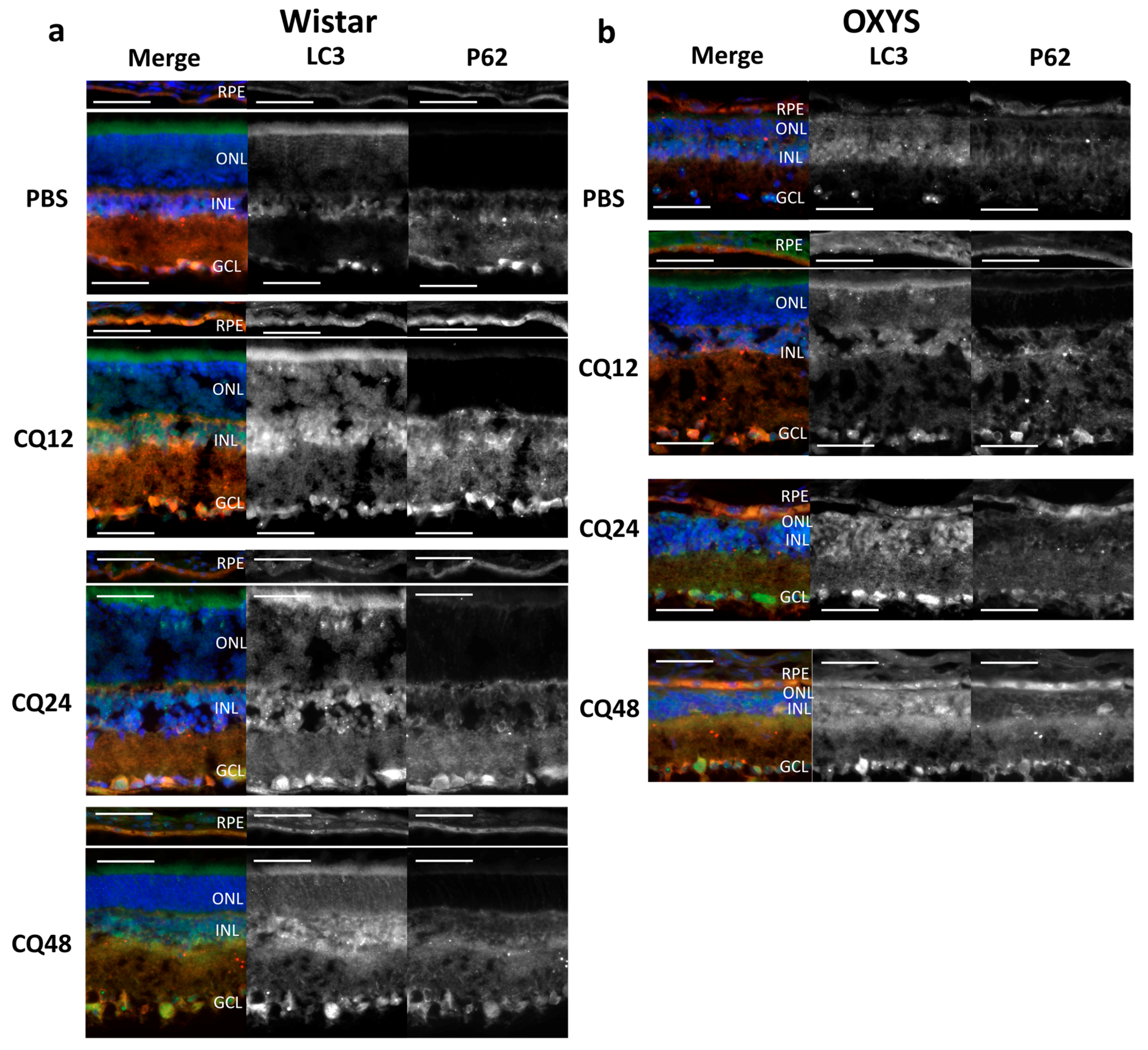
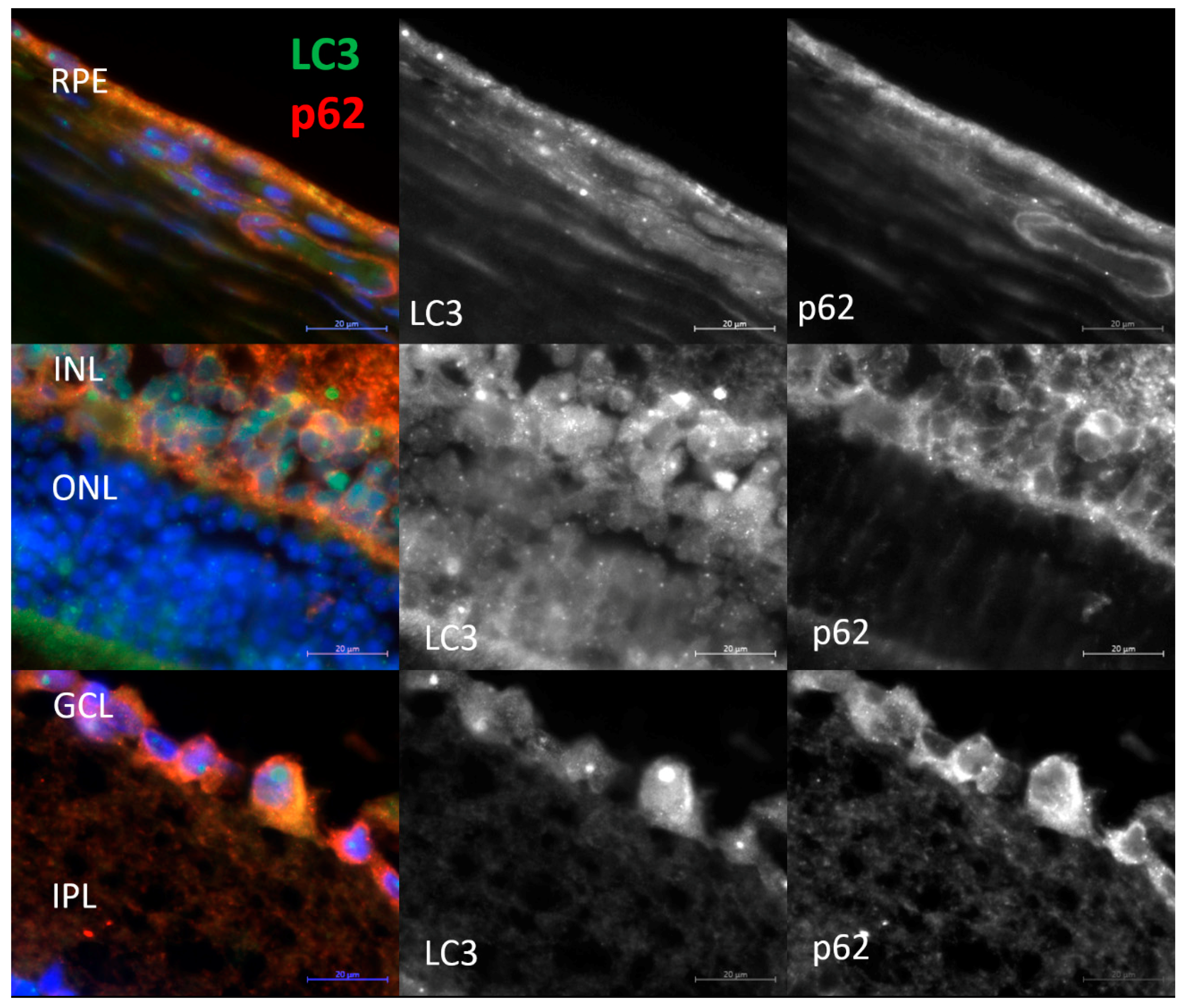
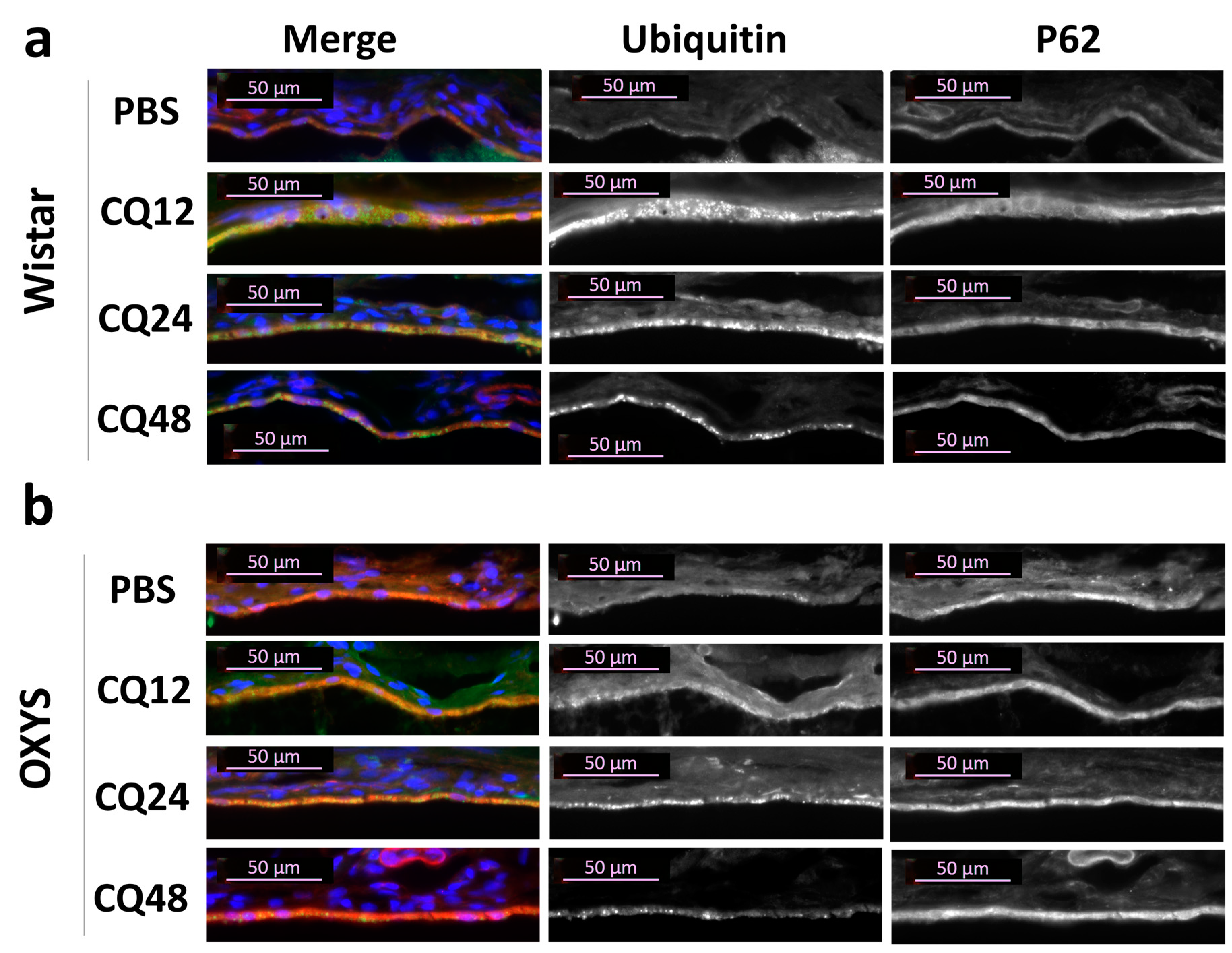
2.3. Decreased Autophagic Reactivity in the Retina of OXYS Rats
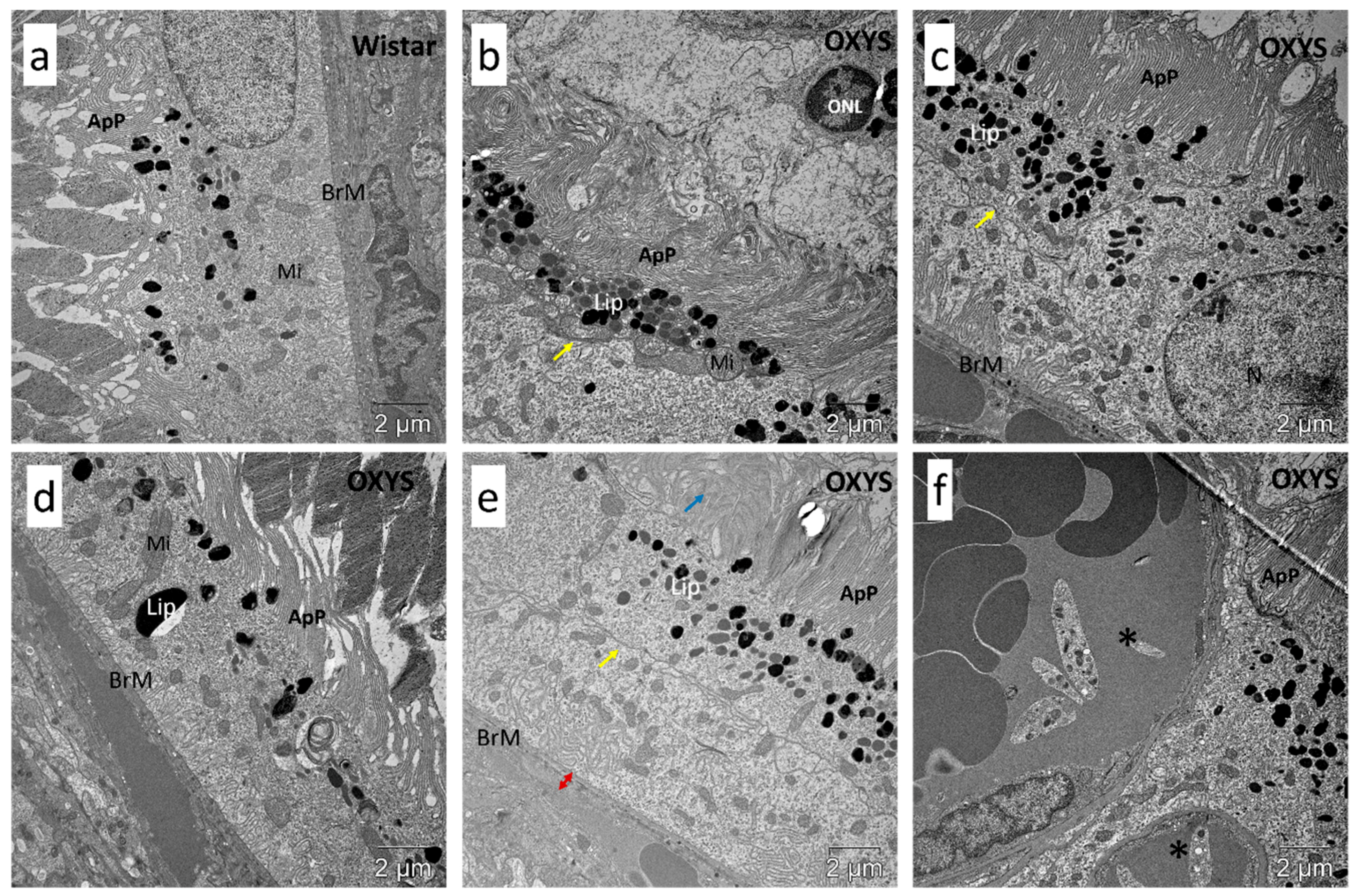
2.4. Impaired Autophagic Flux in RPE Cells of OXYS Rats Revealed by Transmission Electron Microscopy (TEM)
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Treatments
4.3. Functional Analysis of RNA-Seq Data
4.4. Quantitative Reverse-Transcription PCR (qPCR)
4.5. Immunohistochemistry
4.6. TEM
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| ALIS | Aggresome-like induced structures |
| BSA | Bovine serum albumin |
| CQ | Chloroquine |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| DEGs | Differentially expressed genes |
| GCL | Ganglion cell layer |
| GO | Gene Ontology |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ONL | Outer nuclear layer |
| PBS | Phosphate-buffered saline |
| POS | Photoreceptors outer segments |
| RNA-Seq | High-throughput transcriptome sequencing |
| RPE | Retinal pigment epithelium |
| UPS | Ubiquitin-proteasome system |
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Klettner, A.; Kauppinen, A.; Blasiak, J.; Roider, J.; Salminen, A.; Kaarniranta, K. Cellular and molecular mechanisms of age-related macular degeneration: From impaired autophagy to neovascularization. Int. J. Biochem. Cell Biol. 2013, 45, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Piechota, M.; Pawlowska, E.; Szatkowska, M.; Sikora, E.; Kaarniranta, K. Cellular Senescence in Age-Related Macular Degeneration: Can Autophagy and DNA Damage Response Play a Role? Oxid. Med. Cell. Longev. 2017, 2017, 5293258. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Pawlowska, E.; Szczepanska, J.; Kaarniranta, K. Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017, 33, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.L.; Thompson, D.A.; et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2019, 9, 790. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, S.; Liu, Y.; Gao, K.; Liu, Z.; Zhang, Z. Inhibition of Starvation-Triggered Endoplasmic Reticulum Stress, Autophagy, and Apoptosis in ARPE-19 Cells by Taurine through Modulating the Expression of Calpain-1 and Calpain-2. Int. J. Mol. Sci. 2017, 18, 2146. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Zhao, H.; Martinez, J.; Doggett, T.A.; Kolesnikov, A.V.; Tang, P.H.; Ablonczy, Z.; Chan, C.C.; Zhou, Z.; Green, D.R.; et al. Noncanonical autophagy promotes the visual cycle. Cell 2013, 154, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Egbejimi, A.; Dharmat, R.; Xu, P.; Zhao, Z.; Long, B.; Miao, H.; Chen, R.; Wensel, T.G.; Cai, J.; et al. Phagocytosed photoreceptor outer segments activate mTORC1 in the retinal pigment epithelium. Sci. Signal 2018, 11, 3315. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, N.G.; Stefanova, N.A.; Korbolina, E.E.; Fursova, A.Z.; Kozhevnikova, O.S. Senescence-accelerated OXYS rats: A genetic model of premature aging and age-related diseases. Adv. Gerontol. 2014, 4, 294–298. [Google Scholar] [CrossRef]
- Telegina, D.V.; Kozhevnikova, O.S.; Bayborodin, S.I.; Kolosova, N.G. Contributions of age-related alterations of the retinal pigment epithelium and of glia to the AMD-like pathology in OXYS rats. Sci. Rep. 2017, 7, 41533. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikova, O.S.; Telegina, D.V.; Devyatkin, V.A.; Kolosova, N.G. Involvement of the autophagic pathway in the progression of AMD-like retinopathy in senescence-accelerated OXYS rats. Biogerontology 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Kozhevnikova, O.S.; Fursova, A.Z.; Kolosova, N.G. Suppression of AMD-Like Pathology by Mitochondria-Targeted Antioxidant SkQ1 Is Associated with a Decrease in the Accumulation of Amyloid β and in mTOR Activity. Antioxidants 2019, 8, 177. [Google Scholar] [CrossRef]
- Kozhevnikova, O.S.; Korbolina, E.E.; Stefanova, N.A.; Muraleva, N.A.; Orlov, Y.L.; Kolosova, N.G. Association of AMD-like retinopathy development with an Alzheimer’s disease metabolic pathway in OXYS rats. Biogerontology 2013, 14, 753–762. [Google Scholar] [CrossRef]
- Telegina, D.V.; Kolosova, N.G.; Kozhevnikova, O.S. Immunohistochemical localization of NGF, BDNF, and their receptors in a normal and AMD-like rat retina. BMC Med. Genom. 2019, 12 (Suppl. S2), 48. [Google Scholar] [CrossRef]
- Korbolina, E.E.; Kozhevnikova, O.S.; Stefanova, N.A.; Kolosova, N.G. Quantitative trait loci on chromosome 1 for cataract and AMD-like retinopathy in senescence-accelerated OXYS rats. Aging 2012, 4, 49–59. [Google Scholar] [CrossRef][Green Version]
- Kolosova, N.G.; Kozhevnikova, O.S.; Telegina, D.V.; Fursova, A.Z.; Stefanova, N.A.; Muraleva, N.A.; Venanzi, F.; Sherman, M.Y.; Kolesnikov, S.I.; Sufianov, A.A.; et al. p62/SQSTM1 coding plasmid prevents age related macular degeneration in a rat model. Aging 2018, 10, 2136–2147. [Google Scholar] [CrossRef]
- Kozhevnikova, O.S.; Korbolina, E.E.; Ershov, N.I.; Kolosova, N.G. Rat retinal transcriptome: Effects of aging and AMD-like retinopathy. Cell Cycle 2013, 12, 1745–1761. [Google Scholar] [CrossRef]
- Telegina, D.V.; Korbolina, E.E.; Ershov, N.I.; Kolosova, N.G.; Kozhevnikova, O.S. Identification of functional networks associated with cell death in the retina of OXYS rats during the development of retinopathy. Cell Cycle 2015, 14, 3544–3556. [Google Scholar] [CrossRef]
- Abada, A.; Levin-Zaidman, S.; Porat, Z.; Dadosh, T.; Elazar, Z. SNARE priming is essential for maturation of autophagosomes but not for their formation. Proc. Natl. Acad. Sci. USA 2017, 114, 12749–12754. [Google Scholar] [CrossRef]
- Ylä-Anttila, P.; Eskelinen, E.L. Roles for RAB24 in autophagy and disease. Small GTPases 2018, 9, 57–65. [Google Scholar] [CrossRef]
- Keeling, E.; Lotery, A.J.; Tumbarello, D.A.; Ratnayaka, J.A. Impaired Cargo Clearance in the Retinal Pigment Epithelium (RPE) Underlies Irreversible Blinding Diseases. Cells 2018, 7, 16. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adhihetty, P.J. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Liu, X.D.; Ko, S.; Xu, Y.; Fattah, E.A.; Xiang, Q.; Jagannath, C.; Ishii, T.; Komatsu, M.; Eissa, N.T. Transient aggregation of ubiquitinated proteins is a cytosolic unfolded protein response to inflammation and endoplasmic reticulum stress. J. Biol. Chem. 2012, 287, 19687–19698. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Cheng, Y.; Ouyang, W.; Sang, X.; Liu, J.; Su, Y.; Liu, Y.; Li, C.; Yang, L.; et al. Autophagy Dysfunction, Cellular Senescence, and Abnormal Immune-Inflammatory Responses in AMD: From Mechanisms to Therapeutic Potential. Oxid. Med. Cell. Longev. 2019, 2019, 3632169. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, J.; Zhai, Y.; Sun, J.; Wang, J.; She, X.; Gu, Q.; Liu, Y.; Zhu, H.; Luo, X.; et al. Autophagy activated by SIRT6 regulates Aβ induced inflammatory response in RPEs. Biochem. Biophys. Res. Commun. 2018, 496, 1148–1154. [Google Scholar] [CrossRef]
- Zhang, Y.; Cross, S.D.; Stanton, J.B.; Marmorstein, A.D.; Le, Y.Z.; Marmorstein, L.Y. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7. Mol. Vis. 2017, 23, 228–241. [Google Scholar] [CrossRef]
- Lei, L.; Tzekov, R.; Li, H.; McDowell, J.H.; Gao, G.; Smith, W.C.; Tang, S.; Kaushal, S. Inhibition or Stimulation of Autophagy Affects Early Formation of Lipofuscin-Like Autofluorescence in the Retinal Pigment Epithelium Cell. Int. J. Mol. Sci. 2017, 18, 728. [Google Scholar] [CrossRef]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nazio, F.; Tettamanti, G.; Girardello, R.; Cianfanelli, V.; Cavaliere, F.; Morrone, L.A.; Corasaniti, M.T.; et al. Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis. 2018, 9, 981. [Google Scholar] [CrossRef]
- Esteban-Martínez, L.; Boya, P. Autophagic flux determination in vivo and ex vivo. Methods 2015, 75, 79–86. [Google Scholar] [CrossRef]
- Sinha, D.; Valapala, M.; Shang, P.; Hose, S.; Grebe, R.; Lutty, G.A.; Zigler, J.S., Jr.; Kaarniranta, K.; Handa, J.T. Lysosomes: Regulators of autophagy in the retinal pigmented epithelium. Exp. Eye Res. 2016, 144, 46–53. [Google Scholar] [CrossRef]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.M.T.; Koskela, A.; Chen, M.; Leinonen, H.; Toropainen, E. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Pevzner, I.B.; Andrianova, N.V.; Zorova, L.D.; Popkov, V.A.; Silachev, D.N.; Zorov, D.B. The age-associated loss of ischemic preconditioning in the kidney is accompanied by mitochondrial dysfunction, increased protein acetylation and decreased autophagy. Sci. Rep. 2017, 7, 44430. [Google Scholar] [CrossRef]
- Korbolina, E.E.; Zhdankina, A.A.; Fursova, A.Z.; Kozhevnikova, O.S.; Kolosova, N.G. Genes of susceptibility to early neurodegenerative changes in the rat retina and brain: Analysis by means of congenic strains. BMC Genet. 2016, 17 (Suppl. S3), 153. [Google Scholar] [CrossRef][Green Version]
- Fedoseeva, L.A.; Klimov, L.O.; Ershov, N.I.; Efimov, V.M.; Markel, A.L.; Orlov, Y.L.; Redina, O.E. The differences in brain stem transcriptional profiling in hypertensive ISIAH and normotensive WAG rats. BMC Genom. 2019, 20 (Suppl. S3), 297. [Google Scholar] [CrossRef]
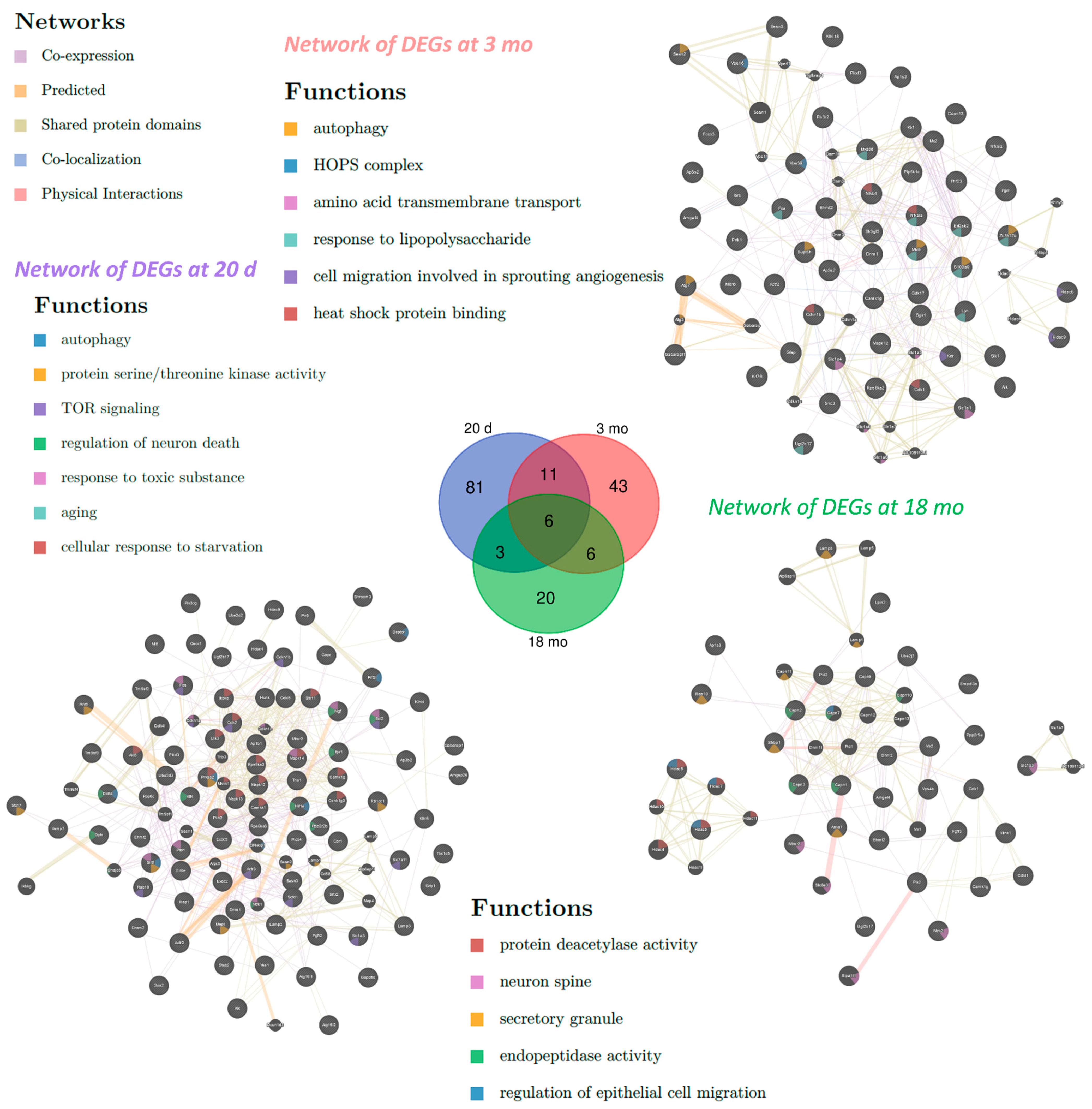

| Category | 20 Days | 3 Months | 18 Months |
|---|---|---|---|
| Biological processes | Autophagy, macroautophagy, regulation of autophagy, intracellular signal transduction, protein phosphorylation, response to oxygen-containing compound, response to organic substance, cellular response to stress, cell death, regulation of apoptotic process | Protein phosphorylation, response to organic substance | |
| TOR signaling, apoptotic process, autophagosome assembly, vesicle-mediated transport, neurogenesis | Response to lipid, peptidyl-amino acid modification, innate immune response, response to lipopolysaccharide, response to oxidative stress | Regulation of organelle organization, neurogenesis, response to nitrogen compound, response to light stimulus, Golgi to plasma membrane transport, histone H3 deacetylation | |
| Molecular functions | Protein binding, protein serine/threonine kinase activity, ATP binding, protein kinase binding | ||
| Protein deacetylase activity | Transcription factor binding | Calcium-dependent cysteine-type endopeptidase activity | |
| Cell compartments | Vacuolar membrane, cytoplasmic, membrane-bounded vesicle, vesicle membrane, Golgi apparatus | ||
| Autophagosome, endosome membrane, protein complex, TORC2 complex | Autophagosome, membrane coat, TOR complex, lysosome, endosome, AP-type membrane coat adaptor complex | Extracellular exosome | |
| KEGG pathway | FoxO signaling, neurotrophin signaling, Toll-like receptor signaling, TNF signaling | ||
| mTOR signaling, PI3K-AKT signaling, MAPK signaling, AMPK signaling, regulation of autophagy | Lysosome, B cell receptor signaling, T cell receptor signaling, chemokine signaling | Endocytosis | |
| Title 1 | Forward | Reverse |
|---|---|---|
| Atg5 | ACCTCGGTTTGGCTTGGTTG | AGTATGGCTCTGCTTCTCGTT |
| Atg7 | AGCCTGTTCATCCAAAGTTCT | CTGTGGTTGCTCAGACGGT |
| Becn1 | GCGTCGGGGCCTAAAGAATG | CTCCTGGCTCTCTCCTGGTT |
| Gabarapl1 | CCTCCGACCTCACTGTTGG | TGCCTCATTTCCCGTAGACAC |
| Map1lc3b | GGAGCTTCGAACAAAGAGTGG | TGCAGGCGCCTTCTAATTATCT |
| p62 | CTGAGTCGGCTTCTGCTCCAT | ATCTTCTGTGCCTGTGCTGGA |
| Nbr1 | TAGTCCCAGAAGTGGCAGGA | ATTGTGGTGCCTTGAGTGGT |
| Rpl30 | ATGGTGGCTGCAAAGAAGAC | CAAAGCTGGACAGTTGTTGG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhevnikova, O.S.; Telegina, D.V.; Tyumentsev, M.A.; Kolosova, N.G. Disruptions of Autophagy in the Rat Retina with Age During the Development of Age-Related-Macular-Degeneration-like Retinopathy. Int. J. Mol. Sci. 2019, 20, 4804. https://doi.org/10.3390/ijms20194804
Kozhevnikova OS, Telegina DV, Tyumentsev MA, Kolosova NG. Disruptions of Autophagy in the Rat Retina with Age During the Development of Age-Related-Macular-Degeneration-like Retinopathy. International Journal of Molecular Sciences. 2019; 20(19):4804. https://doi.org/10.3390/ijms20194804
Chicago/Turabian StyleKozhevnikova, Oyuna S., Darya V. Telegina, Mikhail A. Tyumentsev, and Nataliya G. Kolosova. 2019. "Disruptions of Autophagy in the Rat Retina with Age During the Development of Age-Related-Macular-Degeneration-like Retinopathy" International Journal of Molecular Sciences 20, no. 19: 4804. https://doi.org/10.3390/ijms20194804
APA StyleKozhevnikova, O. S., Telegina, D. V., Tyumentsev, M. A., & Kolosova, N. G. (2019). Disruptions of Autophagy in the Rat Retina with Age During the Development of Age-Related-Macular-Degeneration-like Retinopathy. International Journal of Molecular Sciences, 20(19), 4804. https://doi.org/10.3390/ijms20194804





