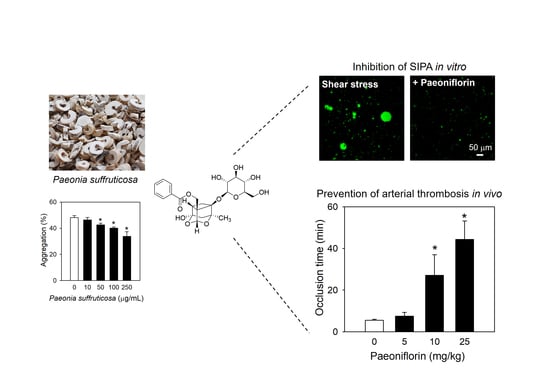
Antithrombotic Effects of Paeoniflorin from Paeonia suffruticosa by Selective Inhibition on Shear Stress-Induced Platelet Aggregation
Abstract
1. Introduction
2. Results
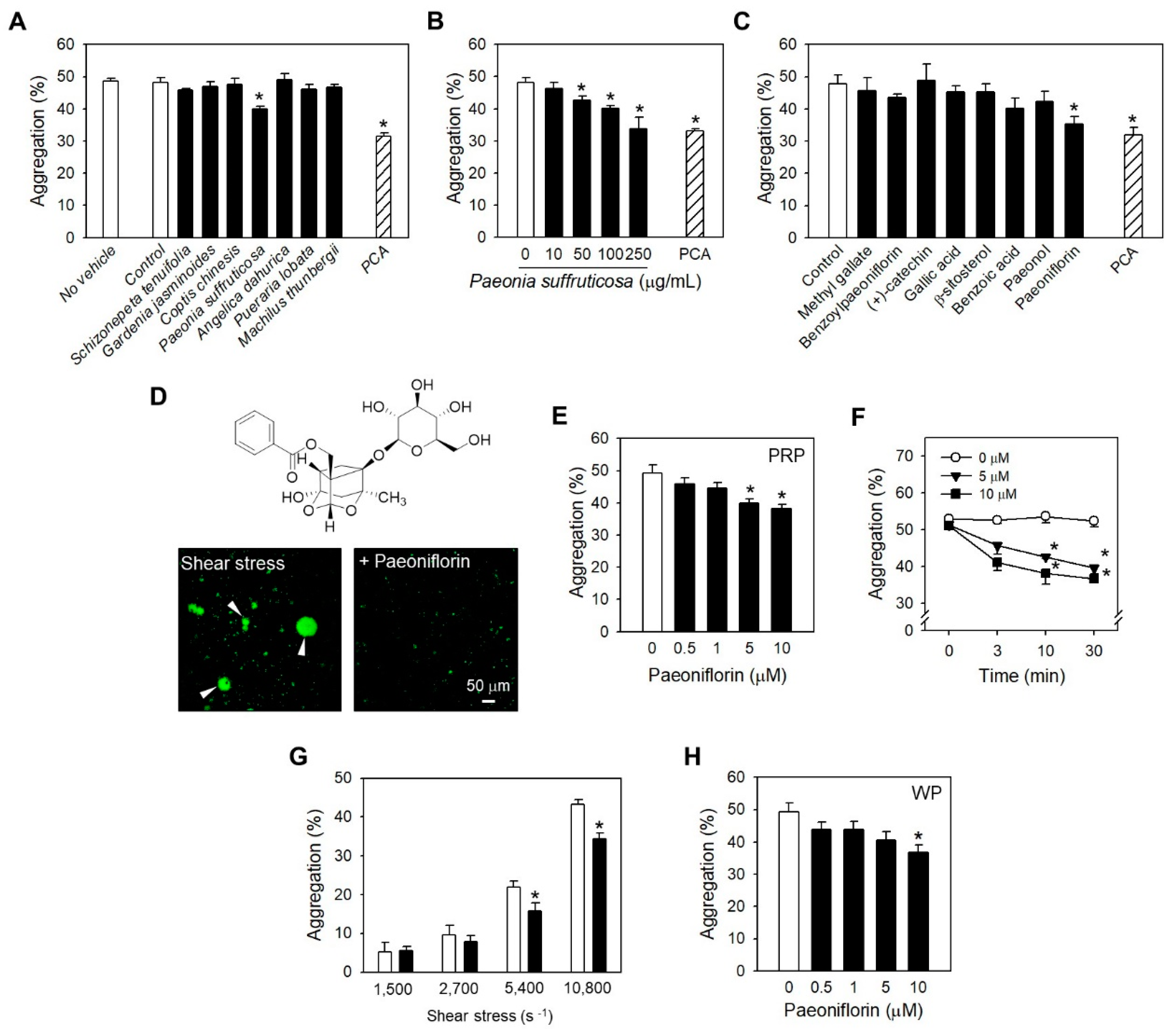
2.1. Paeoniflorin from Paeonia Suffruticosa Extract Significantly Inhibited Shear Stress-Induced Platelet Aggregation
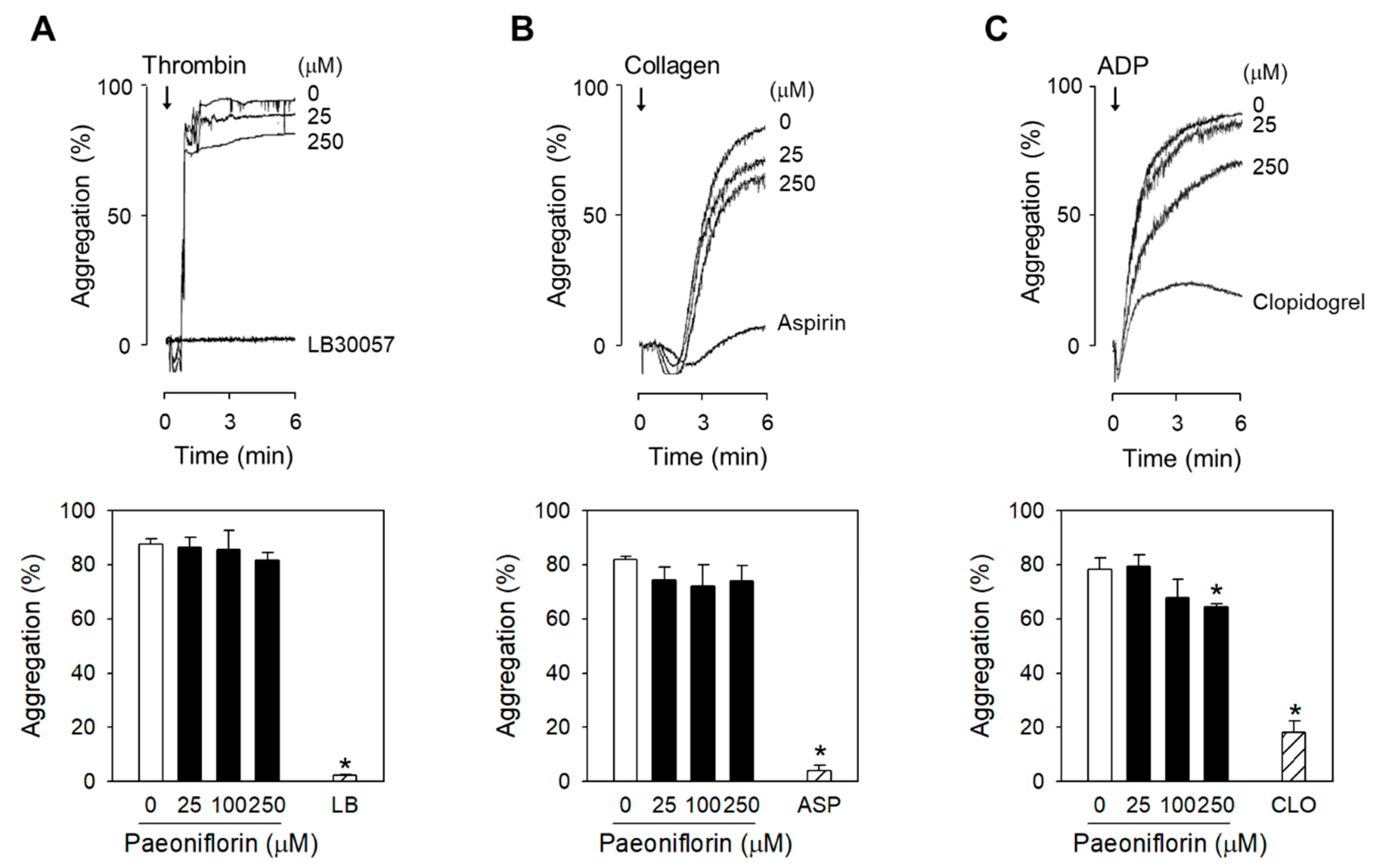
2.2. Paeoniflorin Selectively Inhibits Platelet Aggregation-Induced by Shear Stress
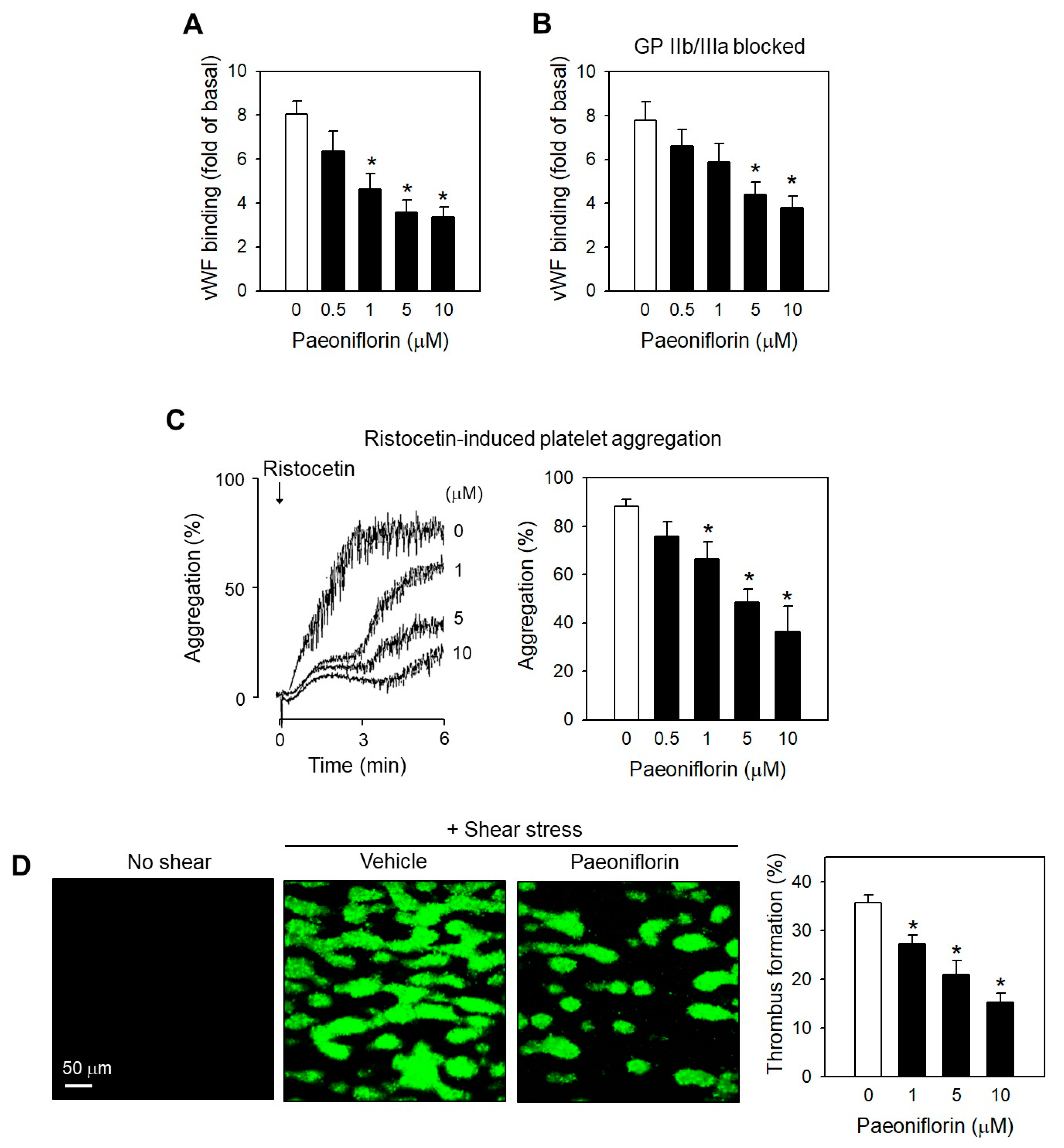
2.3. Paeoniflorin Inhibits Platelet Activation Events in High Shear Stress Condition
2.4. Paeoniflorin Inhibited SIPA through Modulating vWF-GP Ib Interaction
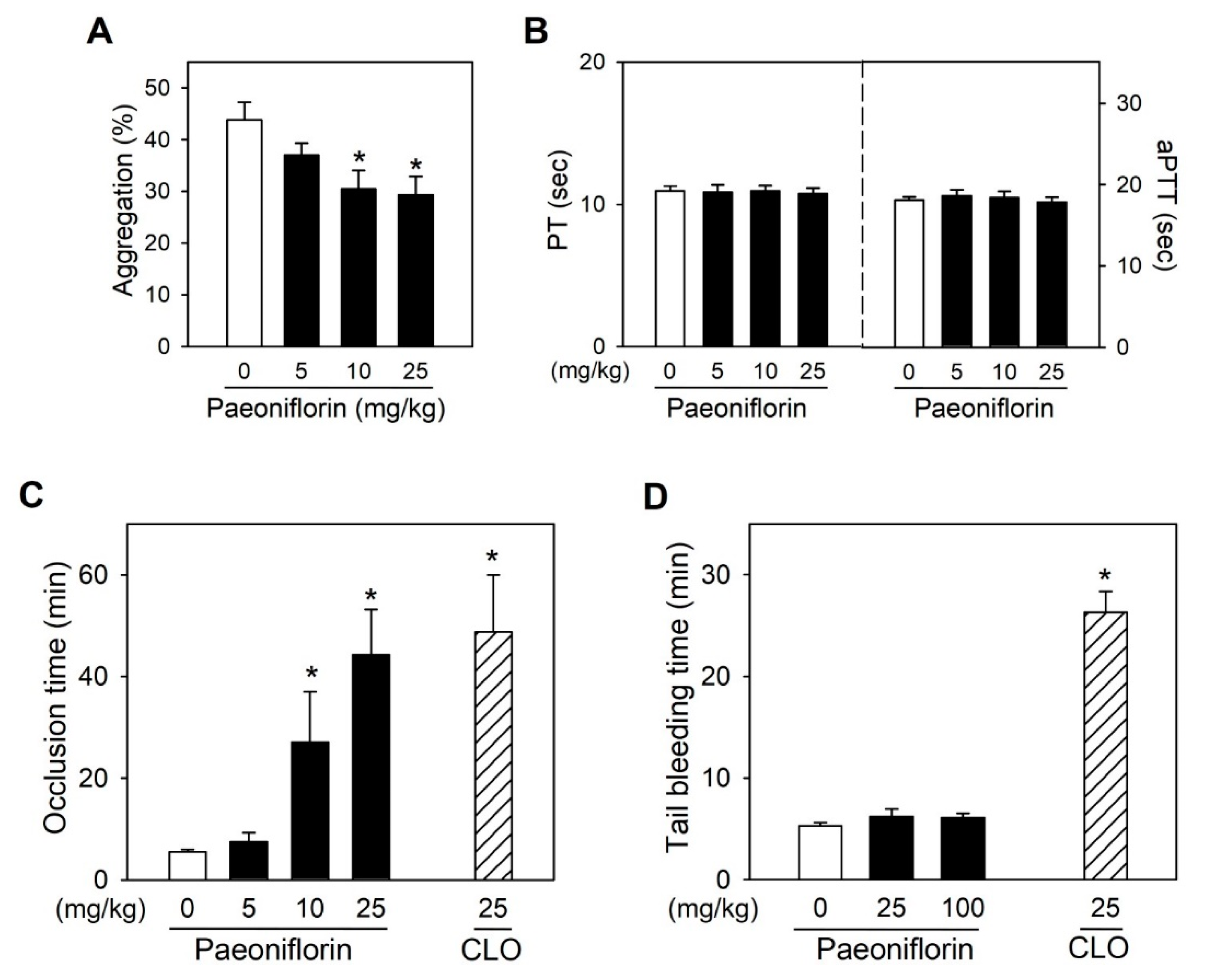
2.5. Paeoniflorin Significantly Prevented Thrombus Formation without Increased Bleeding Risk
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Platelet Preparation
4.3. Measurement of Shear Stress-Induced Platelet Aggregation
4.4. Assessment of Cytoplasmic Calcium Levels
4.5. Assessment of P-Selectin Expression, GP IIb/IIIa Activation, and Fibrinogen Binding
4.6. Assessment of Platelet Cytotoxicity
4.7. Measurement of Agonist-Induced Platelet Aggregation in human PRP
4.8. Determination Effects on Thrombus Formation under Flow Conditions
4.9. Determination of Serotonin Release
4.10. Ex Vivo Determination of Anti-SIPA Activity
4.11. In Vivo Arterial Thrombosis Model
4.12. In Vivo Tail Bleeding Time Measurement
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ab | antibody |
| ADP | adenosine diphosphate |
| AM | acetoxymethyl ester |
| aPTT | activated partial thromboplastin time |
| CLO | clopidogrel |
| DMSO | dimethyl sulfoxide |
| FITC | fluorescein isothiocyanate |
| GP | glycoprotein |
| LDH | lactate dehydrogenase |
| PBS | phosphate-buffered saline |
| PE | phycoerythrin |
| PF | paeoniflorin |
| PGE1 | prostaglandin E1 |
| PRP | platelet-rich plasma |
| WP | washed platelet |
| PT | prothrombin time |
| SIPA | shear stress-induced platelet aggregation |
| vWF | von Willebrand factor |
References
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. American Heart Association Statistics, C.; Stroke Statistics, S., Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar]
- McFadyen, J.D.; Schaff, M.; Peter, K. Current and future antiplatelet therapies: Emphasis on preserving haemostasis. Nat. Rev. Cardiol. 2018, 15, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M. Platelets in atherothrombosis. Nat. Med. 2002, 8, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 2004, 61, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Michelson, A.D. Antiplatelet therapies for the treatment of cardiovascular disease. Nat. Rev. Drug Discov. 2010, 9, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Jawad, M.; Kamal, M.A.; Baldi, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Evidence and prospective of plant derived flavonoids as antiplatelet agents: Strong candidates to be drugs of future. Food Chem. Toxicol. 2018, 119, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133–148. [Google Scholar] [CrossRef]
- Kroll, M.H.; Hellums, J.D.; McIntire, L.V.; Schafer, A.I.; Moake, J.L. Platelets and shear stress. Blood 1996, 88, 1525–1541. [Google Scholar]
- Kamada, H.; Imai, Y.; Nakamura, M.; Ishikawa, T.; Yamaguchi, T. Shear-induced platelet aggregation and distribution of thrombogenesis at stenotic vessels. Microcirculation 2017, 24. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Jackson, S.P. The growing complexity of platelet aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Pleines, I.; Bender, M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 2011, 9, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Firbas, C.; Siller-Matula, J.M.; Jilma, B. Targeting von Willebrand factor and platelet glycoprotein Ib receptor. Expert Rev. Cardiovasc. Ther. 2010, 8, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 1, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Terano, T.; Hamazaki, T.; Sajiki, J.; Saito, H.; Tahara, K.; Tamura, Y.; Kumagai, A. Studies on the mechanism of antiaggregatory effect of Moutan Cortex. Thromb. Res. 1983, 31, 29–40. [Google Scholar] [CrossRef]
- Jeon, B.R.; Irfan, M.; Kim, M.; Lee, S.E.; Lee, J.H.; Rhee, M.H. Schizonepeta tenuifolia inhibits collagen stimulated platelet function via suppressing MAPK and Akt signaling. J. Biomed. Res. 2019. [Google Scholar]
- Liu, H.; Chen, Y.F.; Li, F.; Zhang, H.Y. Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J. Asian Nat. Prod. Res. 2013, 15, 94–110. [Google Scholar] [CrossRef]
- Tan, H.L.; Chan, K.G.; Pusparajah, P.; Duangjai, A.; Saokaew, S.; Mehmood Khan, T.; Lee, L.H.; Goh, B.H. Rhizoma Coptidis: A Potential Cardiovascular Protective Agent. Front. Pharmacol. 2016, 7, 362. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Wang, N.; He, L. Antioxidant effect of imperatorin from Angelica dahurica in hypertension via inhibiting NADPH oxidase activation and MAPK pathway. J. Am. Soc. Hypertens 2014, 8, 527–536. [Google Scholar] [CrossRef]
- Tam, W.Y.; Chook, P.; Qiao, M.; Chan, L.T.; Chan, T.Y.; Poon, Y.K.; Fung, K.P.; Leung, P.C.; Woo, K.S. The efficacy and tolerability of adjunctive alternative herbal medicine (Salvia miltiorrhiza and Pueraria lobata) on vascular function and structure in coronary patients. J. Altern Complement. Med. 2009, 15, 415–421. [Google Scholar] [CrossRef]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- Stalker, T.J.; Newman, D.K.; Ma, P.; Wannemacher, K.M.; Brass, L.F. Platelet signaling. Handb. Exp. Pharmacol. 2012, 210, 59–85. [Google Scholar]
- Ikeda, Y.; Murata, M.; Goto, S. Von Willebrand factor-dependent shear-induced platelet aggregation: Basic mechanisms and clinical implications. Ann. N. Y. Acad. Sci. 1997, 811, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.F.; Berndt, M.C.; Schade, A.; McIntire, L.V.; Andrews, R.K.; Lopez, J.A. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood 2001, 97, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Nesbitt, W.S.; Westein, E. Dynamics of platelet thrombus formation. J. Thromb. Haemost. 2009, 7, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nor, N.H.; Othman, F.; Mohd Tohit, E.R.; Md Noor, S. Medicinal Herbals with Antiplatelet Properties Benefit in Coronary Atherothrombotic Diseases. Thrombosis 2016, 2016, 5952910. [Google Scholar] [CrossRef]
- Chen, C.; Yang, F.Q.; Zhang, Q.; Wang, F.Q.; Hu, Y.J.; Xia, Z.N. Natural Products for Antithrombosis. Evid Based Complement. Alternat. Med. 2015, 2015, 876426. [Google Scholar] [CrossRef]
- Ain, Q.U.; Khan, H.; Mubarak, M.S.; Pervaiz, A. Plant Alkaloids as Antiplatelet Agent: Drugs of the Future in the Light of Recent Developments. Front. Pharmacol. 2016, 7, 292. [Google Scholar] [CrossRef]
- Vilahur, G.; Badimon, L. Antiplatelet properties of natural products. Vascul. Pharmacol. 2013, 59, 67–75. [Google Scholar] [CrossRef]
- Mueller, R.L.; Scheidt, S. History of drugs for thrombotic disease. Discovery, development, and directions for the future. Circulation 1994, 89, 432–449. [Google Scholar] [CrossRef]
- Huang, E.S.; Strate, L.L.; Ho, W.W.; Lee, S.S.; Chan, A.T. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am. J. Med. 2011, 124, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Gadi, D.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Legrand, C.; Lafeve, F.F.; Mekhfi, H. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J. Ethnopharmacol. 2009, 125, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Lee, D.U.; Kwak, J.H.; Lee, S.M.; Kim, Y.S.; Jung, Y.S. Antiplatelet effects of Cyperus rotundus and its component (+)-nootkatone. J. Ethnopharmacol. 2011, 135, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bae, O.N.; Lim, K.M.; Noh, J.Y.; Kang, S.; Chung, K.Y.; Chung, J.H. Novel antiplatelet activity of protocatechuic acid through the inhibition of high shear stress-induced platelet aggregation. J. Pharmacol. Exp. Ther. 2012, 343, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Duan, H.; Yang, X.; Yan, W.; Zheng, X. Anti-thrombosis effect of paeoniflorin: Evaluated in a photochemical reaction thrombosis model in vivo. Planta Med. 2001, 67, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Stegner, D.; Nieswandt, B. Platelet receptor signaling in thrombus formation. J. Mol. Med. 2011, 89, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Asazuma, N.; Satoh, K.; Yatomi, Y.; Takafuta, T.; Berndt, M.C.; Ozaki, Y. Interaction between von Willebrand factor and glycoprotein Ib activates Src kinase in human platelets: Role of phosphoinositide 3-kinase. Blood 2003, 101, 3469–3476. [Google Scholar] [CrossRef]
- Scharf, R.E. Platelet Signaling in Primary Haemostasis and Arterial Thrombus Formation: Part 1. Hamostaseologie 2018, 8, 203–210. [Google Scholar]
- Ma, Y.Q.; Qin, J.; Plow, E.F. Platelet integrin alpha(IIb) beta (3): Activation mechanisms. J. Thromb Haemost 2007, 5, 1345–1352. [Google Scholar] [CrossRef]
- Jesty, J.; Bluestein, D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: Elimination of the feedback activation of platelets by thrombin. Anal. Biochem. 1999, 272, 64–70. [Google Scholar] [CrossRef]
- Lof, A.; Muller, J.P.; Brehm, M.A. A biophysical view on von Willebrand factor activation. J. Cell Physiol. 2018, 233, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Li, Y.S.; Chen, J.C.; Chen, Y.W. Effects of exercise training and deconditioning on platelet aggregation induced by alternating shear stress in men. Arter. Thromb. Vasc. Biol. 2005, 25, 454–460. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abelson, J.N.; Simon, M.I.; Hawiger, J.J. (Eds.) Platelets: Receptors, adhesion, secretion. Part A. In Methods Enzymol, 1st ed.; Academic Press: Cambridge, MA, USA, 1989; Volume 169, pp. 1–512. [Google Scholar]
- Hussein, H.M.; Emiru, T.; Georgiadis, A.L.; Qureshi, A.I. Assessment of platelet inhibition by point-of-care testing in neuroendovascular procedures. AJNR Am. J. Neuroradiol 2013, 34, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wang, R.; Cheng, X.M.; He, Y.Q.; Wang, Z.T.; Wu, C.; Cao, J. Comparative pharmacokinetic study of paeoniflorin after oral administration of decoction of Radix Paeoniae Rubra and Radix Paeoniae Alba in rats. J. Ethnopharmacol. 2008, 117, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Isono, T.; Wakui, Y.; Matsuzaki, Y.; Sasaki, H.; Amagaya, S.; Maruno, M. Absorption and excretion of paeoniflorin in rats. J. Pharm. Pharmacol. 1995, 47, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, Z.; Niu, S.; Zhang, J.; Mondal, N.K.; Griffith, B.P.; Wu, Z.J. Quantification of Shear-Induced Platelet Activation: High Shear Stresses for Short Exposure Time. Artif. Organs 2015, 39, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Slepian, M.J.; Sheriff, J.; Hutchinson, M.; Tran, P.; Bajaj, N.; Garcia, J.G.N.; Scott Saavedra, S.; Bluestein, D. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J. Biomech. 2017, 50, 20–25. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, T.; Kim, K.; Bian, Y.; Noh, H.; Lim, K.-M.; Chung, J.-H.; Bae, O.-N. Antithrombotic Effects of Paeoniflorin from Paeonia suffruticosa by Selective Inhibition on Shear Stress-Induced Platelet Aggregation. Int. J. Mol. Sci. 2019, 20, 5040. https://doi.org/10.3390/ijms20205040
Ngo T, Kim K, Bian Y, Noh H, Lim K-M, Chung J-H, Bae O-N. Antithrombotic Effects of Paeoniflorin from Paeonia suffruticosa by Selective Inhibition on Shear Stress-Induced Platelet Aggregation. International Journal of Molecular Sciences. 2019; 20(20):5040. https://doi.org/10.3390/ijms20205040
Chicago/Turabian StyleNgo, Thien, Keunyoung Kim, Yiying Bian, Hakjun Noh, Kyung-Min Lim, Jin-Ho Chung, and Ok-Nam Bae. 2019. "Antithrombotic Effects of Paeoniflorin from Paeonia suffruticosa by Selective Inhibition on Shear Stress-Induced Platelet Aggregation" International Journal of Molecular Sciences 20, no. 20: 5040. https://doi.org/10.3390/ijms20205040
APA StyleNgo, T., Kim, K., Bian, Y., Noh, H., Lim, K.-M., Chung, J.-H., & Bae, O.-N. (2019). Antithrombotic Effects of Paeoniflorin from Paeonia suffruticosa by Selective Inhibition on Shear Stress-Induced Platelet Aggregation. International Journal of Molecular Sciences, 20(20), 5040. https://doi.org/10.3390/ijms20205040