Cloning, Expression and Effects of P. americana Thymosin on Wound Healing
Abstract
1. Introduction
2. Results
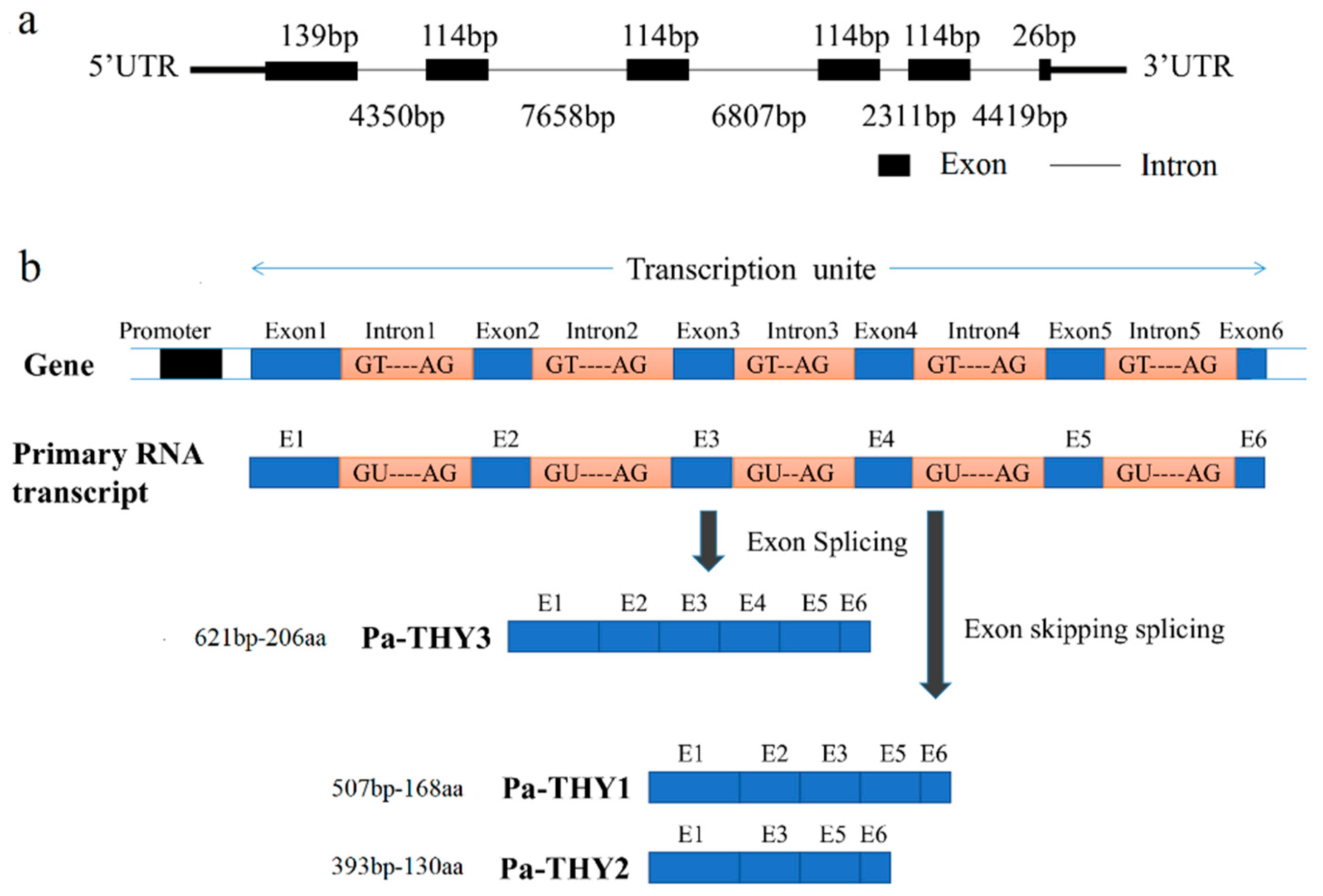
2.1. Bioinformatics Analysis of P. americana Thymosin
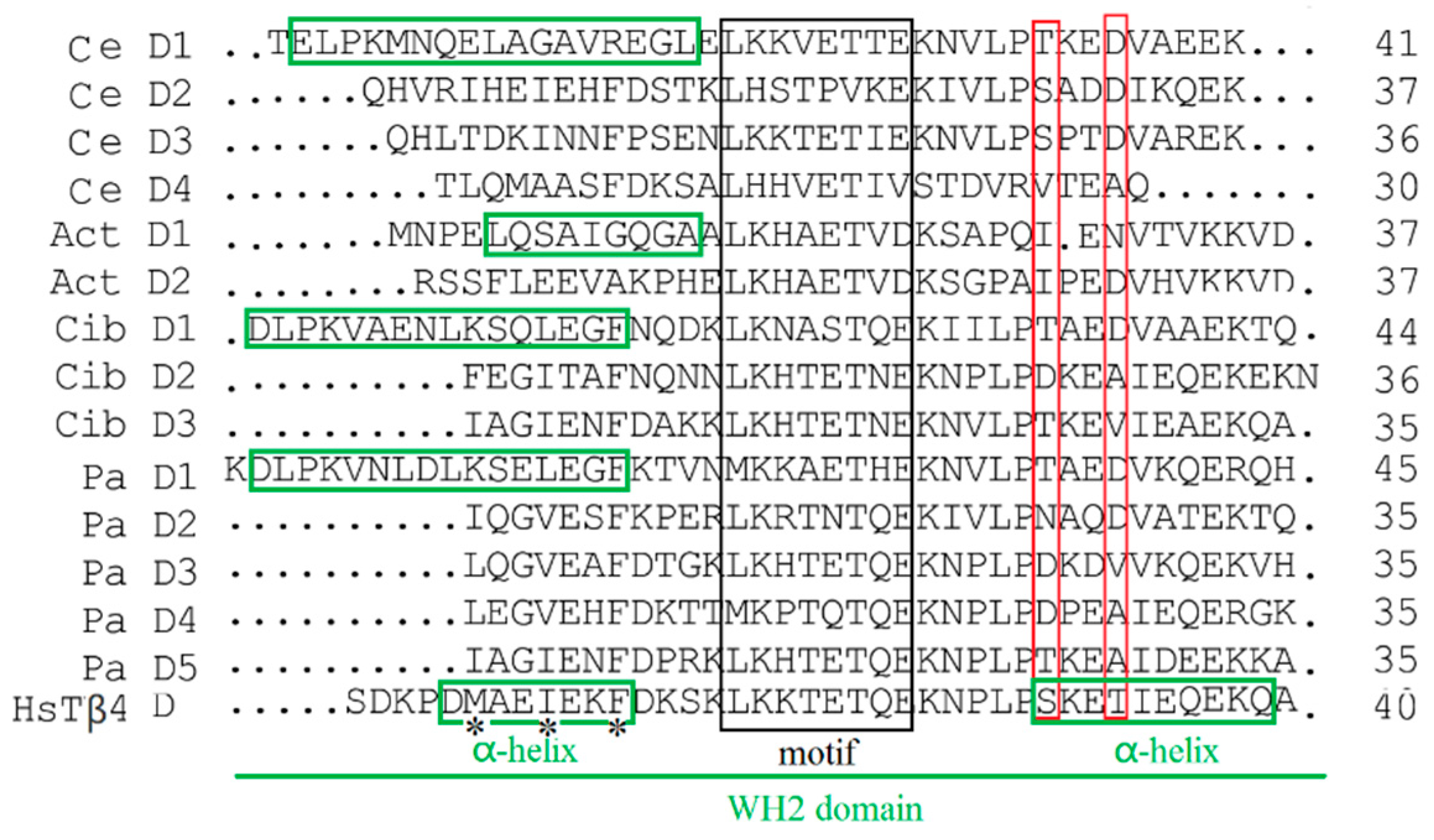
2.2. Function Domain Analysis of P. americana Thymosin
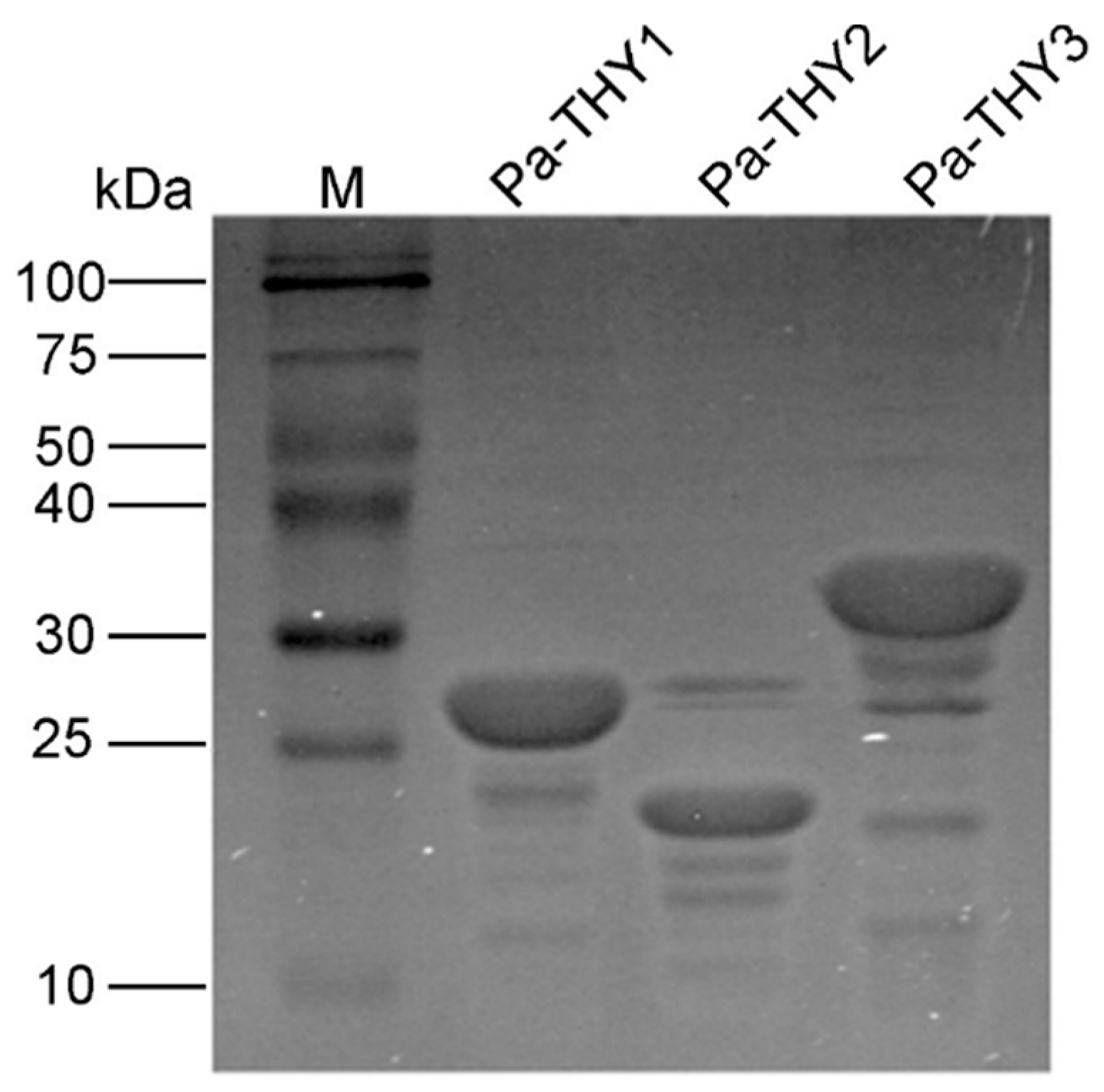
2.3. Expression and Purification of Recombinant Protein Pa-THYs
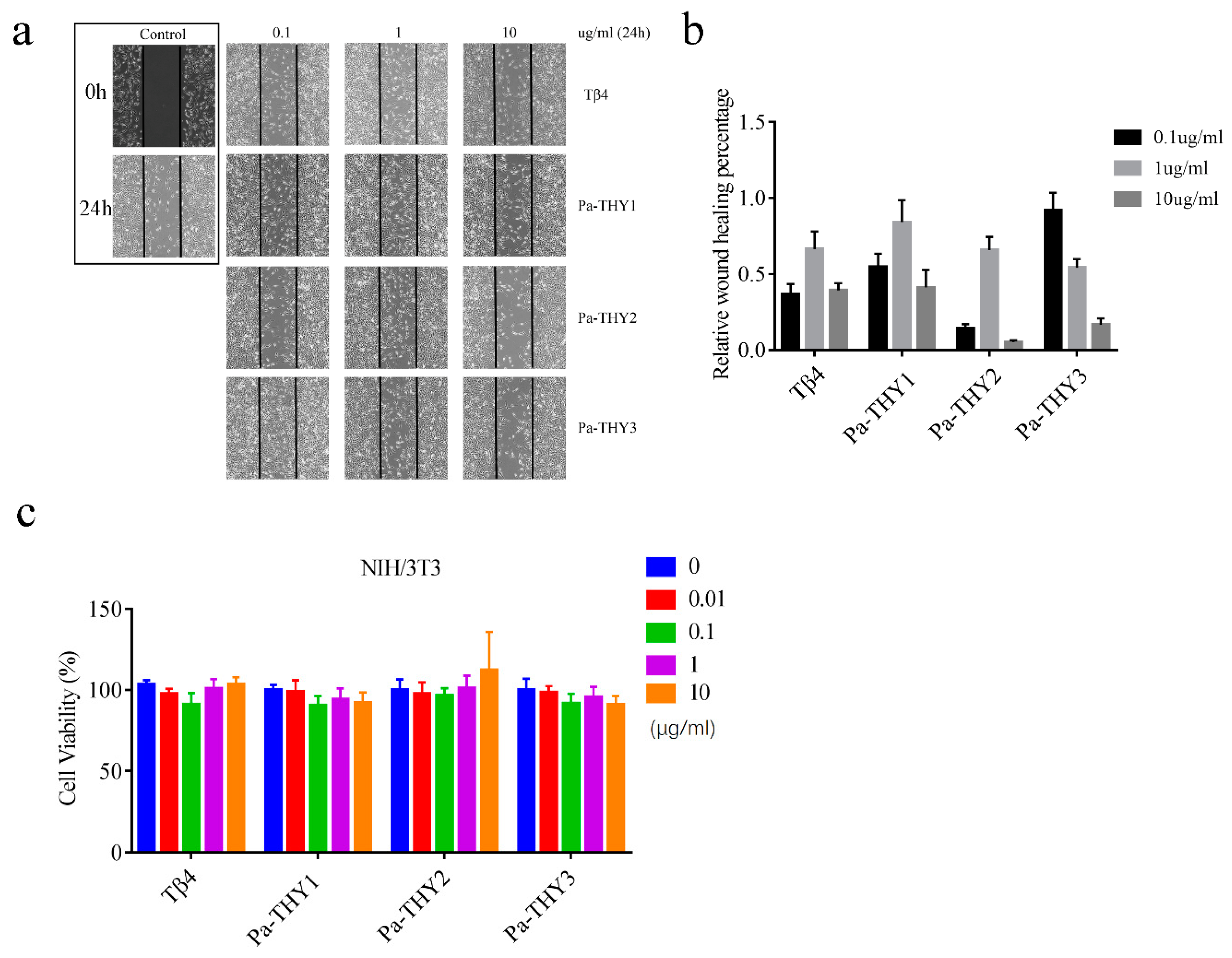
2.4. Pa-THYs Promoted Cell Migration of Fibroblasts
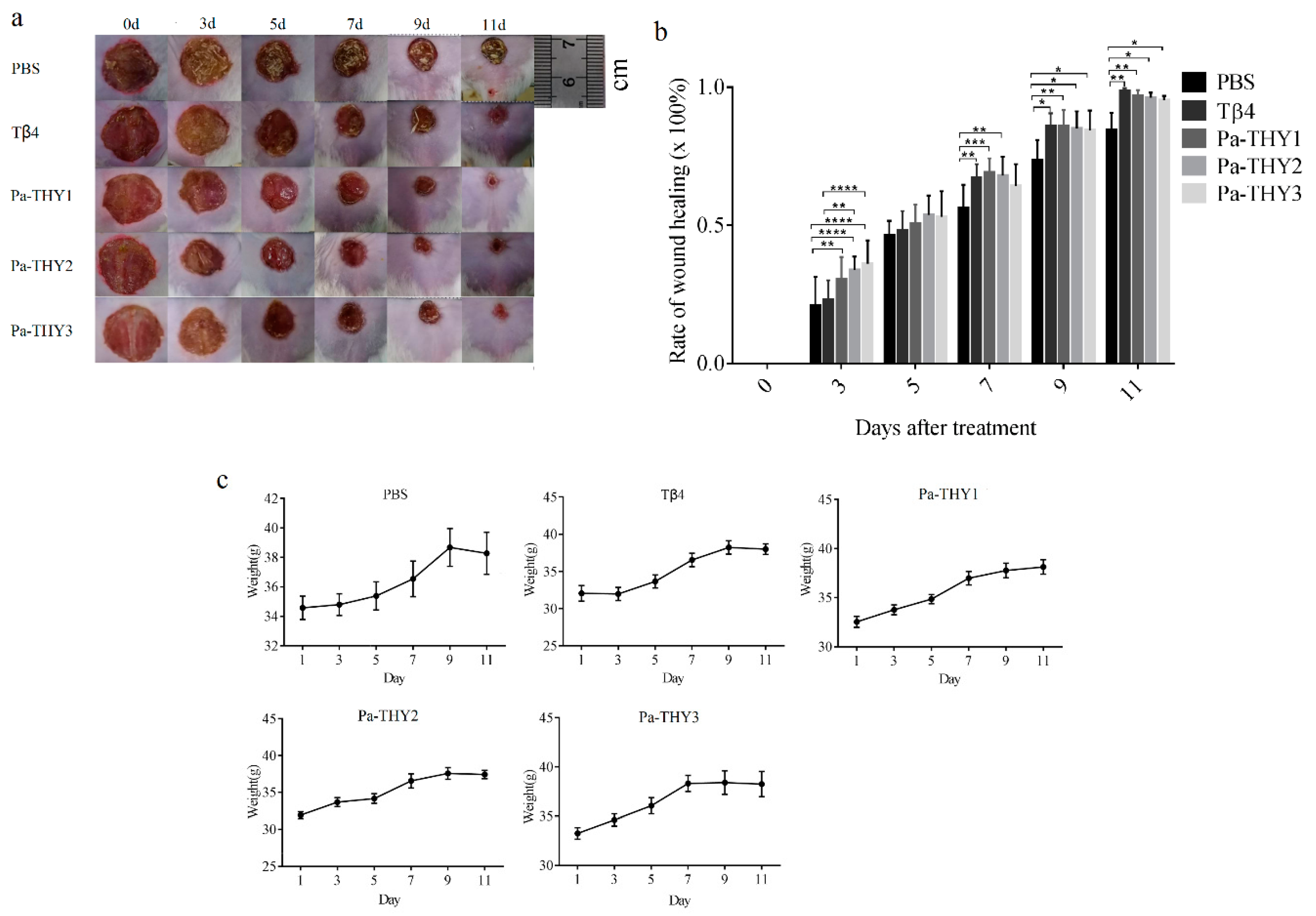
2.5. Recombinant Protein Pa-THYs Promoted Wound Healing
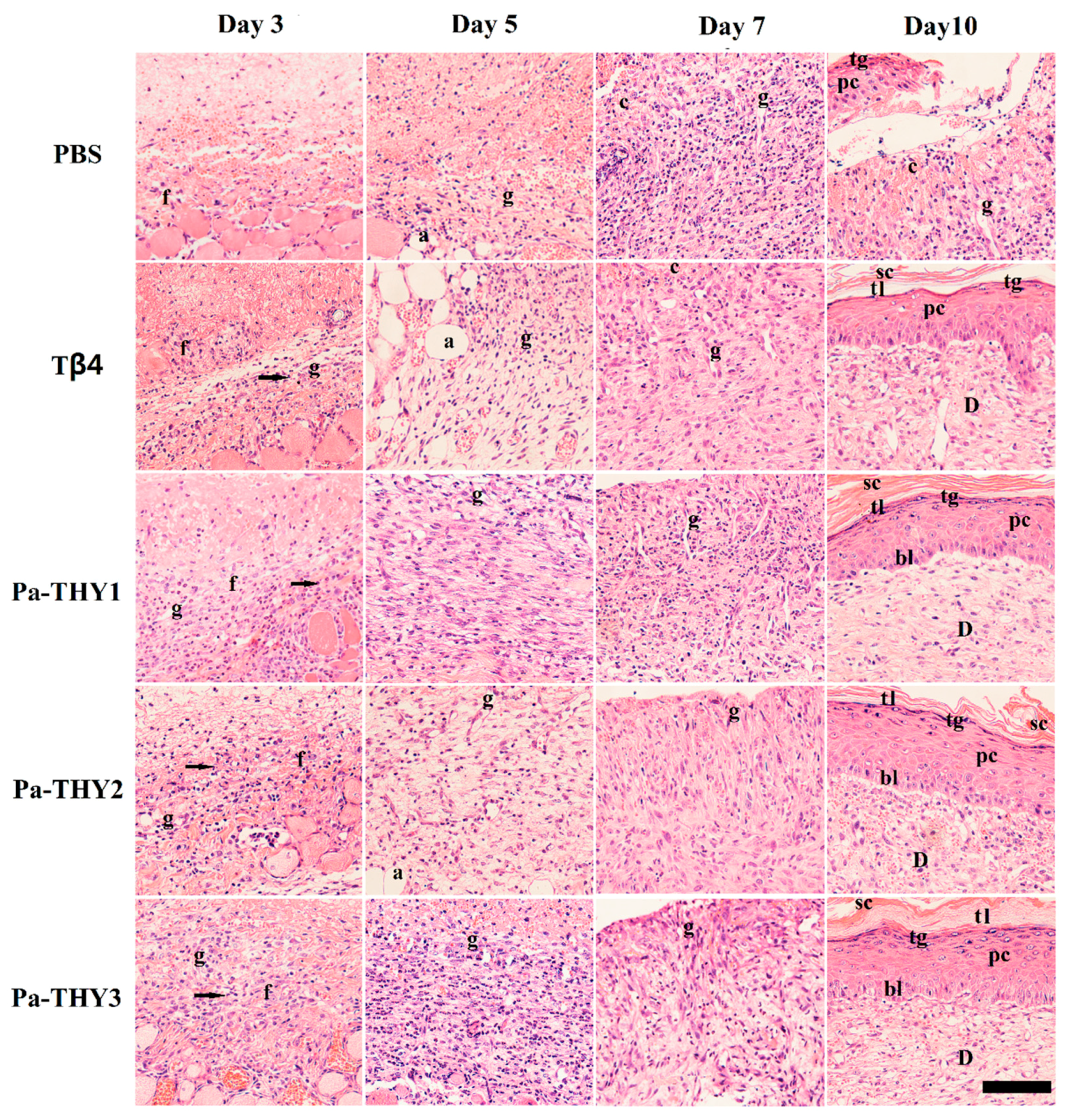
2.6. Pa-THYs Promoted Wound Healing by Accelerating Dermal Regeneration
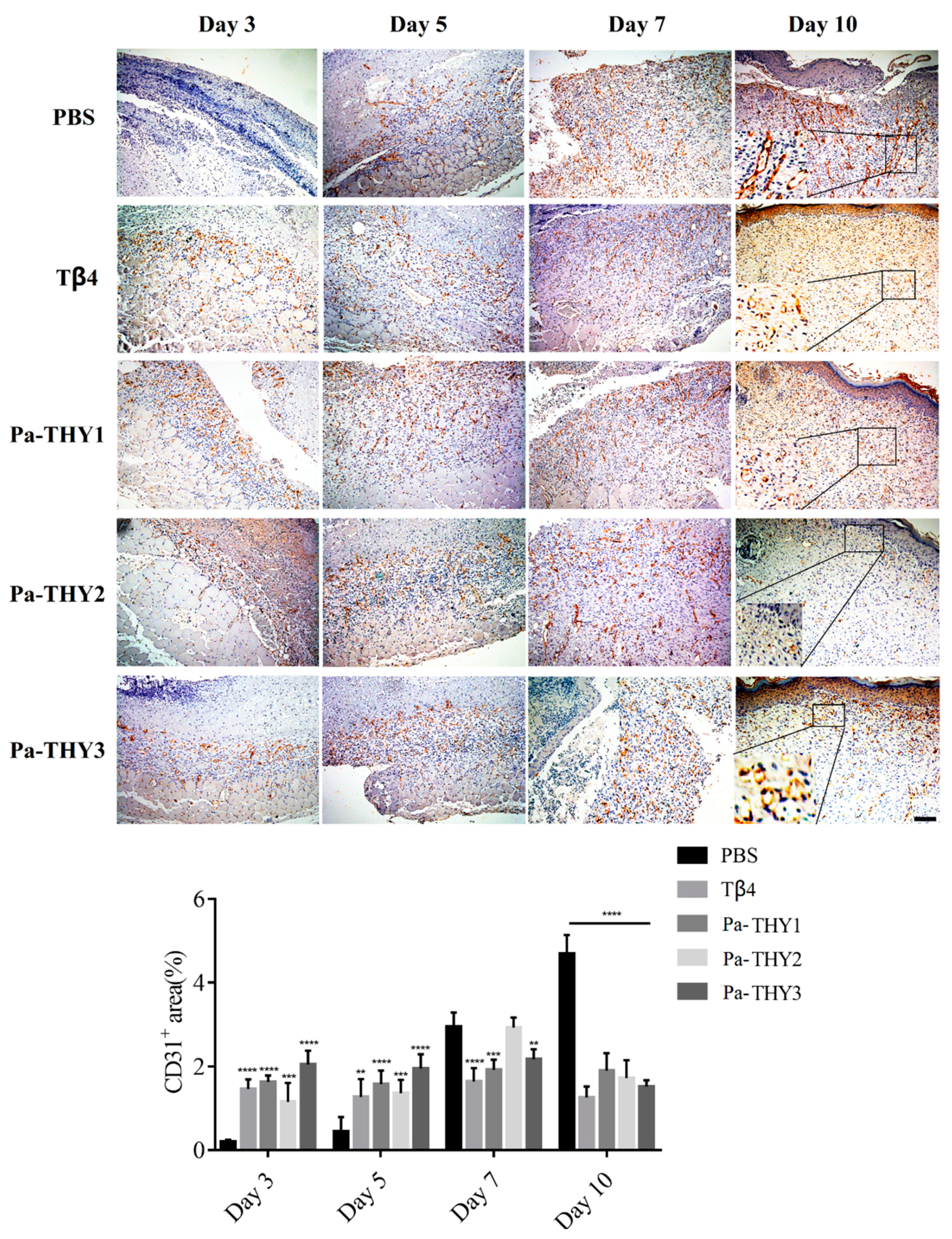
2.7. Pa-THYs Promoted Wound Healing Through Stimulating Angiogenesis
2.8. Pa-THYs Promoted Wound Healing Through Stimulating Collagen Deposition
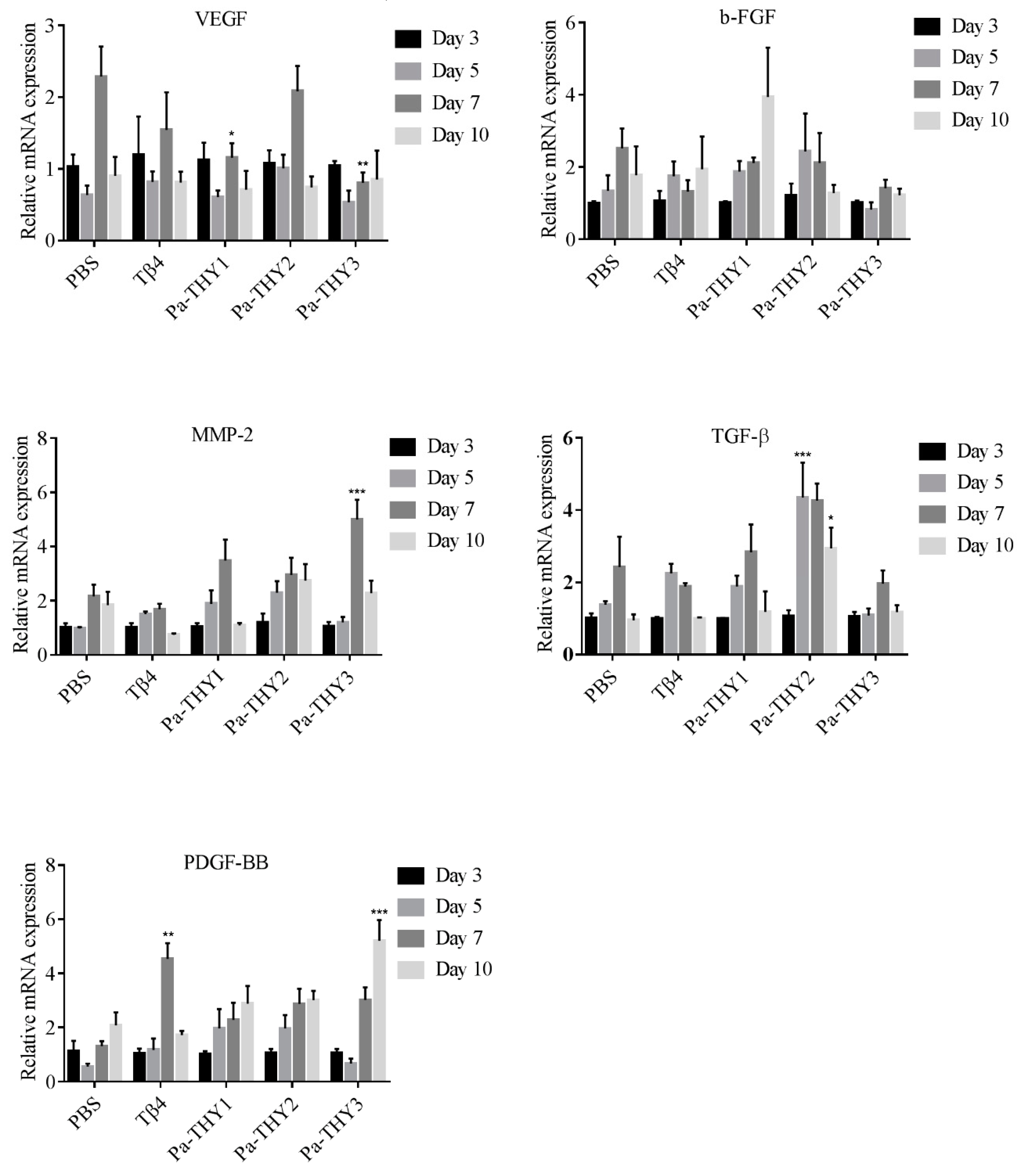
2.9. Pa-THYs Stimulating the Expression of Cytokines and Growth Factors
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis for DNA Sequences of P. americana Thymosin
4.2. Cloning, Expression, and Purification of Pa-THYs
4.3. Cell Culture
4.4. Cell Migration and Proliferation Assays
4.5. Animal Model
4.6. Macroscopic Evaluation
4.7. Sample Collection and Histological Analysis
4.8. RNA Extraction and qRT-PCR
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IPTG | isopropyl β-D-thiogalactoside |
| Tβ4 | human thymosin β4 |
| THY | P. americana thymosin |
| VEGF | vascular endothelial growth factor |
| TGF-β | transforming growth factor-β |
| b-FGF | fibroblast growth factor |
| MMP-2 | matrix metallopeptidase 2 |
| PDGF-BB | platelet derived growth factor-BB |
References
- Sosne, G.; Qiu, P.; Goldstein, A.L.; Wheater, M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010, 24, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Zou, Z.; Yang, M.; Wu, C.; Su, Y.; Tang, J.; Yang, X. OM-LV20, a novel peptide from odorous frog skin, accelerates wound healing in vitro and in vivo. Chem. Biol. Drug Design 2018, 91, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, J.; Schmaljohann, D.; Boyce, D.; Thomas, D. Epidermal growth factor therapy and wound healing—Past, present and future perspectives. Surgeon 2008, 6, 172–177. [Google Scholar] [CrossRef]
- Yong, L.; Weichang, L.; Yanpeng, J.; Rui, G.; Yi, Z.; Wei, X.; Yuanming, Z. Therapeutic efficacy of antibiotic-loaded gelatin microsphere/silk fibroin scaffolds in infected full-thickness burns. Acta Biomater. 2014, 10, 3167–3176. [Google Scholar]
- Huff, T.; Otto, A.M.; Müller, C.S.; Meier, M.; Hannappel, E. Thymosin beta4 is released from human blood platelets and attached by factor XIIIa (transglutaminase) to fibrin and collagen. FASEB J. 2002, 16, 691. [Google Scholar] [CrossRef]
- Hertzog, M.; van Heijenoort, C.; Didry, D.; Gaudier, M.; Coutant, J.; Gigant, B.; Didelot, G.; Preat, T.; Knossow, M.; Guittet, E.; et al. The beta-thymosin/WH2 domain: Structural basis for the switch from inhibition to promotion of actin assembly. Cell 2004, 117, 611–623. [Google Scholar] [CrossRef]
- Malinda, K.M.; Sidhu, G.S.; Mani, H.; Banaudha, K.; Maheshwari, R.K.; Goldstein, A.L.; Kleinman, H.K. Thymosin beta 4 accelerates wound healing. J. Investig. Dermatol. 1999, 113, 364–368. [Google Scholar] [CrossRef]
- Philp, D.; Goldstein, A.L.; Kleinman, H.K. Thymosin β 4 promotes angiogenesis, wound healing, and hair follicle development. Mech. Ageing Dev. 2004, 125, 113–115. [Google Scholar] [CrossRef]
- Marks, E.D.; Kumar, A. Thymosin β4: Roles in Development, Repair, and Engineering of the Cardiovascular System. Vitam. Horm. 2016, 102, 227. [Google Scholar]
- Kim, C.E.; Kleinman, H.K.; Sosne, G.; Ousler, G.W.; Kim, K.; Kang, S.; Yang, J. RGN-259 (thymosin β4) improves clinically important dry eye efficacies in comparison with prescription dr μgs in a dry eye model. Sci. Rep. 2018, 8, 10500. [Google Scholar] [CrossRef]
- Sosne, G.; Szliter, E.A.; Barrett, R.; Kernacki, K.A.; Kleinman, H.; Hazlett, L.D. Thymosin Beta 4 Promotes Corneal Wound Healing and Decreases Inflammation in Vivo Following Alkali Injury. Exp. Eye Res. 2002, 74, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hee-Jae, C.; Deborah, P.; Soo-Hyun, L.; Hye-Sung, M.; Kleinman, H.K.; Takashi, N. Over-expression of thymosin beta 4 promotes abnormal tooth development and stimulation of hair growth. Int. J. Dev. Biol. 2010, 54, 135–140. [Google Scholar]
- Mohamad, S.; Pascal, P.; Christine, P.; Catherine, G.J.; Joelle, D.; Pascal, M.; Svetlana, U. Thymosins β-4 and β-10 are expressed in bovine ovarian follicles and upregulated in cumulus cells during meiotic maturation. Reprod. Fertil. Dev. 2010, 22, 1206. [Google Scholar]
- Troys, M.V.; Dhaese, S.; Vandekerckhove, J.; Ampe, C. Multirepeat β-Thymosins; Lappalainen, P., Ed.; Springer: New York, NY, USA, 2007; pp. 71–81. [Google Scholar]
- Ma, S.; Kang, Z.; Peng, L.; Yang, Y.; Yao, Q.; Xia, H.; Chen, K. Molecular and Physiological Characterization of Two Novel Multirepeat β-Thymosins from Silkworm, Bombyx mori. PLoS ONE 2015, 10, e0140182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gai, Y.; Zhao, J.; Song, L.; Wang, L.; Qiu, L.; Ning, X.; Zheng, X.; Zhang, Y.; Mu, C.; Zhang, Y.; et al. Two thymosin-repeated molecules with structural and functional diversity coexist in Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2009, 33, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Aguda, A.H.; Xue, B.; Irobi, E.; Préat, T.; Robinson, R.C. The Structural Basis of Actin Interaction with Multiple WH2/β-Thymosin Motif-Containing Proteins. Structure 2006, 14, 469–476. [Google Scholar] [CrossRef]
- Zhang, F.X.; Shao, H.L.; Wang, J.X.; Zhao, X.F. beta-thymosin is upregulated by the steroid hormone 20-hydroxyecdysone and microorganisms. Insect Mol. Biol. 2011, 20, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Gou, Q.; Xie, Y.; Zhang, Z.; Fu, C. Periplaneta americana Extracts Promote Skin Wound Healing via Nuclear Factor Kappa B Canonical Pathway and Extracellular Signal-Regulated Kinase Signaling. Evid. -Based Complement. Altern. Med. 2017, 2017, 5821706. [Google Scholar] [CrossRef]
- Bo, X.; Cedric, L.; Grimes, J.M.; Robinson, R.C. Structural basis of thymosin-β4/profilin exchange leading to actin filament polymerization. Proc. Natl. Acad. Sci. USA 2014, 111, 4596–4605. [Google Scholar]
- Xue, B.; Robinson, R.C. Chapter Three–Actin-Induced Structure in the Beta-Thymosin Family of Intrinsically Disordered Proteins. Vitam. Horm. 2016, 102, 55. [Google Scholar]
- Knighton, D.R.; Silver, I.; Hunt, T.K. Regulation of wound-healing angiogenesis—Effect of oxygen gradients and inspired oxygen concentration. Surgery 1981, 90, 262–270. [Google Scholar] [PubMed]
- Lei, C.; Qi, X.; Qiyi, Z.; Mitchell, T.; Fei, Z.; Lili, C.; Yingbin, X.; Shaohai, Q.; Feng, Z. Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics 2017, 7, 117–131. [Google Scholar]
- Boquet, I.; Boujemaa, R.; Carlier, M.F.; Préat, T. Ciboulot Regulates Actin Assembly during Drosophila Brain Metamorphosis. Cell 2000, 102, 797–808. [Google Scholar] [CrossRef]
- Shigeyuki, K.; Richard, C.; Tadao, M.; Toru, M. The homolog of Ciboulot in the termite (Hodotermopsis sjostedti): A multimeric β-thymosin involved in soldier-specific morphogenesis. BMC Dev. Biol. 2010, 10, 63. [Google Scholar]
- Tompa, P. Intrinsically unstructured proteins evolve by repeat expansion. Bioessays 2003, 25, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Troys, M.; Van Dewitte, D.; Goethals, M.; Carlier, M.F.; Vandekerckhove, J.; Ampe, C. The actin binding site of thymosin beta 4 mapped by mutational analysis. EMBO J. 1996, 15, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Simenel, C.; Van Troys, M.; Vandekerckhove, J.; Ampe, C.; Delepierre, M. Structural requirements for thymosin β4 in its contact with actin. FEBS J. 2000, 267, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Didry, D.; Cantrelle, F.X.; Husson, C.; Roblin, P.; Moorthy, A.M.E.; Perez, J.; Clainche, C.L.; Hertzog, M.; Guittet, E.; Carlier, M.F. How a single residue in individual β-thymosin/WH2 domains controls their functions in actin assembly. EMBO J. 2014, 31, 1000–1013. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors Affecting Wound Healing. Otolaryngol. Clin. North Am. 1984, 17, 243. [Google Scholar] [CrossRef]
- Arul, V.; Kartha, R.; Jayakumar, R. A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices. Life Sci. 2007, 80, 275–284. [Google Scholar] [CrossRef]
- Blain, E.J.; Mason, D.J.; Duance, V.C. The effect of thymosin beta4 on articular cartilage chondrocyte matrix metalloproteinase expression. Biochem. Soc. Trans 2002, 30, 879–882. [Google Scholar] [CrossRef]
- Philp, D.; Badamchian, M.; Scheremeta, B.; Nguyen, M.; Goldstein, A.L.; Kleinman, H.K. Thymosin beta(4) and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound Repair Regen. 2003, 11, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, L.; Peng, F.; Qi, C.; Zhang, X.; Zhou, A.; Liu, Z.; Wu, S. Recombinant thymosin beta 4 can promote full-thickness cutaneous wound healing. Protein Expr. Purif. 2007, 56, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sosne, G.; Dunn, S.; Crockford, D.; Kim, C.; Dixon, E. Thymosin Beta 4 Eye Drops Significantly Improve Signs and Symptoms of Severe Dry Eye in a Physician-Sponsored Phase 2 Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6033. [Google Scholar]
- Janarthini, R.; Wang, X.; Chen, L.; Lei, G.; Zhao, L. A Tobacco-Derived Thymosinβ4 Concatemer Promotes Cell Proliferation and Wound Healing in Mice. Biomed. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 2007, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Ilan, N.; Mahooti, S.; Madri, J.A. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J. Cell Sci. 1998, 111, 3621–3631. [Google Scholar]
- Long, K.B.; Burgwin, C.M.; Huneke, R.; Artlett, C.M.; Blankenhorn, E.P. Tight Skin 2 Mice Exhibit Delayed Wound Healing Caused by Increased Elastic Fibers in Fibrotic Skin. Adv. Wound Care 2014, 3, 573–581. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Peters, K.G.; De Vries, C.; Williams, L.T. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair s μggests a role in endothelial differentiation and blood vessel growth. Proc. Natl. Acad. Sci. USA 1993, 90, 8915–8919. [Google Scholar] [CrossRef]
- Nissen, N. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445. [Google Scholar] [PubMed]
- Heike, H. Modified fibrin hydrogel matrices: Both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis. Curr. Pharm. Des. 2007, 13, 3597–3607. [Google Scholar]
- Smiell, J.M. Clinical safety of becaplermin (rhPDGF-BB) gel. Am. J. Surg. 1998, 176, 68S–73S. [Google Scholar] [CrossRef]
- Hao, X.; Månsson-Broberg, A.; Gustafsson, T.; Grinnemo, K.H.; Blomberg, P.; Siddiqui, A.J.; Wärdell, E.; Sylvén, C. Angiogenic effects of dual gene transfer of bFGF and PDGF-BB after myocardial infarction. Biochem. Biophys. Res. Commun. 2004, 315, 1058. [Google Scholar] [CrossRef] [PubMed]
- Philp, D.; Scheremeta, B.; Sibliss, K.; Zhou, M.I.N.; Fine, E.L.; Nguyen, M.; Wahl, L.; Hoffman, M.P.; Kleinman, H.K. Thymosin b 4 Promotes Matrix Metalloproteinase Expression During Wound Repair. J. Cell. Physiol. 2010, 208, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ma, R.J.; Zhang, S.Y. Thymosin beta 4 regulates the expression of VEGF and laminin-5 in accelerating skin wound healing in diabetic rat. Chin. J. Diabetes 2011, 19, 60–63. [Google Scholar]
- Gabriel, S.; Christopherson, P.L.; Barrett, R.P.; Rafael, F. Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2388–2395. [Google Scholar]
- Jin, J.; Wujiao, L.I.; Mou, B.; Shen, Y.; Geng, F.; Yue, B.; Fan, Z. Whole Genome Sequencing and Analysis of Medicinal Periplaneta americana. Sichuan J. Zool. 2018, 37, 121–126. [Google Scholar]

| Pa-THY1 | Pa-THY2 | Pa-THY3 | |
|---|---|---|---|
| Amino acid | 168 | 130 | 206 |
| Molecular weight (kDa) | 19,039.55 | 14,582.52 | 23,435.50 |
| Isoelectric point (PI) | 6.15 | 5.70 | 5.95 |
| Signal peptide | none | none | none |
| Transmembrane | no | no | no |
| Hydrophilic/Hydrophobic | Hydrophilic | Hydrophilic | Hydrophilic |
| Subcellular locations | plasma membrane and nucleus | plasma membrane and nucleus | plasma membrane and nucleus |
| Alpha helix (%) | 52.38% | 49.23% | 51.% |
| Random coil (%) | 45.24% | 47.69% | 42.72% |
| Beta turn (%) | 2.38% | 3.08% | 3.40% |
| Extended strand (%) | 0% | 0% | 2.43% |
| “THY” domains | 4 | 3 | 5 |
| Primer Name | Sequence F (5′–3′) | Sequence R (5′–3′) |
|---|---|---|
| VEGF | F:CTACTGCCGTCCGATTGA | R:TCTCCGCTCTGAACAAGG |
| TGF-β | F:AATACGTCAGACATTCGGGAAGCA | R:GTCAATGTACAGCTGCCGTACACA |
| b-FGF | F:TGCTTCCACCTCGTCTGTCT | R:GAGGCAAAGTGAAAGGGACC |
| MMP-2 | F:GAACTTGCGATTATGCCATGATGAC | R:TCTGAGGGATGCCATCAAAGAC |
| PDGF-BB | F:CCAGGACGGTCATTTACG | R:TGGTCTGGGTTCAGGTTG |
| β-actin | F:CATCCGTAAAGATCTATGCCAAC | R:ATGGAGCCACCGATCCACA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, J.; Sun, X.; Zhou, C.; Zhang, Y.; Shen, Y.; Zeng, X.; Yue, B.; Zhang, X. Cloning, Expression and Effects of P. americana Thymosin on Wound Healing. Int. J. Mol. Sci. 2019, 20, 4932. https://doi.org/10.3390/ijms20194932
Jing J, Sun X, Zhou C, Zhang Y, Shen Y, Zeng X, Yue B, Zhang X. Cloning, Expression and Effects of P. americana Thymosin on Wound Healing. International Journal of Molecular Sciences. 2019; 20(19):4932. https://doi.org/10.3390/ijms20194932
Chicago/Turabian StyleJing, Jie, Xiaohong Sun, Chuang Zhou, Yifan Zhang, Yongmei Shen, Xiaomao Zeng, Bisong Yue, and Xiuyue Zhang. 2019. "Cloning, Expression and Effects of P. americana Thymosin on Wound Healing" International Journal of Molecular Sciences 20, no. 19: 4932. https://doi.org/10.3390/ijms20194932
APA StyleJing, J., Sun, X., Zhou, C., Zhang, Y., Shen, Y., Zeng, X., Yue, B., & Zhang, X. (2019). Cloning, Expression and Effects of P. americana Thymosin on Wound Healing. International Journal of Molecular Sciences, 20(19), 4932. https://doi.org/10.3390/ijms20194932





